Introduction
Liver cancer is the fifth most prevalent type of
malignant tumor in the world and its incidence has been increasing
to its current rate of 50 out of 100,000 people. Liver cancer is
also the third leading cause of cancer-related death among
malignant tumors (1). By sex, it has
become the second and the sixth leading cause of cancer-related
death in men and women, respectively (2). At present, the main clinical treatment
of liver cancer is surgery, but the early clinical symptoms of
liver cancer are not obvious and are often overlooked; moreover,
when patients have clinical symptoms, such as weight loss,
jaundice, abdominal mass and even liver pain, most of them are
already in the advanced or terminal stage. Therefore, at present,
only 10% of patients have a favourable probability for complete
resection of the liver cancer. Although another 90% of patients are
treated with radiotherapy, chemotherapy, radiofrequency ablation,
and other intensive treatments, it is difficult to achieve expected
clinical results due to low efficacy and high rates of side
effects. Therefore, the treatment of liver cancer is still in an
abysmal state due to the absence of effective treatment options
(3,4).
Traditional Chinese medicine has many advantages and
the active ingredients of its herbs often have properties that
impart high efficacy and low toxicity. Scientists worldwide have
been giving increasing attention to Chinese herbal medicines due to
their irreplaceable advantages. In recent years, with the study of
anti-carcinogenic mechanisms of Chinese herbal medicine, more
active ingredients have been extracted. Naringin belongs to a
family of natural flavonoids and is derived from Rutaceae
Citrus Pomelo. Studies have shown that naringin not only has
anti-viral effects, but can also support cancer prevention
(5). In addition, Birt et al
(6), further demonstrated that
flavonoids could induce apoptosis and enhance tumor suppressor gene
expression, thereby inhibiting tumor cells.
MicroRNA (miRNA) is a single-stranded RNA with a
molecular chain length of ~22 nucleotides and does not encode for a
protein. miRNA can regulate post-transcriptional protein expression
via interaction with the 3′UTR region of the target gene mRNA
(7). miRNA-19b is one of the major
members of the miRNA-17-92 family (8). It has been found that miR-19b is highly
expressed in breast, cervical, colon and pancreatic carcinomas and
participates in the apoptosis of tumor cells (9,10).
While the relationship between the mechanisms of
action of naringin and miRNAs had not yet been reported, the
results of our previous study suggested that naringin could
increase the expression of miR-19b in HepG2 cells. Therefore, this
study aimed to investigate the potential effects of naringin on the
proliferation of HepG2 cells and to compare the expression of
miR-19b before and after naringin treatment. We also sought to
explore the possible mechanisms of naringin in the process of
apoptosis, thereby laying a scientific foundation for the treatment
of hepatocellular carcinoma with naringin.
Materials and methods
Materials and reagents
Naringin, MTT assay kit (Sigma, St. Louis, MO, USA),
human hepatocellular carcinoma cell line HepG2 (Cell Bank of
Chinese Academy of Sciences, Shanghai, China), DMEM (Gibco Life
Technologies, Carlsbad, CA, USA), RNA extraction kit, reverse
transcriptase kit, RT-PCR kit (Invitrogen Life Technologies,
Carlsbad, CA, USA), BCA protein quantification kit, cell lysis
buffer (Biyuntian Biotechnology Research Institute, Nantong, China)
and primer synthesis materials (Takara Bio, Dalian, China) were all
sourced for this study.
Cell culture
HepG2 cells were cultured at 37°C in a 5%
CO2 incubator until reaching 85% confluence, then were
digested with trypsin and the cell suspension was diluted with DMEM
containing 10% fetal bovine serum until the cell concentration was
adjusted to 2×108/l. Cells were then counted and seeded
in culture plates for the following experiments.
Determination of cell proliferation
inhibition rate
Cell proliferation was measured by MTT assay after
the cells were treated with naringin. The cells were seeded into
96-well plates at a concentration of 1×105/ml, with each
well containing 100 µl. After 24 h, naringin was added until final
concentrations reached 10, 20 and 40 µM, respectively. Each
concentration was repeated for 5 wells and the experiment was
repeated 6 times, while the control group was not treated with
naringin. The cells were incubated for 24, 48 and 72 h at 37°C in a
5% CO2 incubator and then the cell culture medium
containing naringin was changed. A total of 10 µl MTT was added to
each well until a final concentration of 5 mg/ml was obtained.
After 4 h, the optical density (OD) at 570 nm was measured by a
microplate reader. The inhibition rate was calculated according to
the following formula: inhibition rate (%) = (OD value of normal
control group - OD value of experimental group/OD value of normal
control group) × 100%.
Morphological observation
Cells were treated with 10, 20 or 40 µM naringin for
24 h, respectively, and the morphological changes were observed and
recorded with an inverted microscope (Nikon, Tokyo, Japan).
DAPI staining
Cells were seeded in 6-well plates at a density of
104 cells/well. After 24 h, the supernatant was
aspirated and cells were cultured in medium containing 10, 20 or 40
µM naringin for another 24 h, then washed with precooled PBS 3
times. DAPI solution (1 µg/ml) was added to each well and cells
were incubated in a 37 °C incubator for 5 min and washed again with
precooled PBS. Cells were then observed and photographed using a
fluorescence microscope in the dark (Nikon).
RT-PCR detection
Cells were seeded in 6-well plates at a density of
104 cells/well. After 24 h, the supernatant was
aspirated and cells were cultured in medium containing 10, 20 or 40
µM naringin for another 24 h. The cells were then collected and
total RNA was extracted according to the instructions of the RNA
extraction kit. Total RNA concentration and purity (A260/A280
>1.8 indicating pure RNA) were determined by UV-Vis
spectrophotometer (Hitachi, Tokyo, Japan). cDNA was obtained via
reverse transcription from mRNA according to the instructions of
the reverse transcription kit. The expression of miR-19b mRNA was
detected by RT-PCR assay, according to the instructions of the
RT-PCR kit, and the U6 RNA was used as the internal control. The
primer sequences of the miR-19b and U6 are shown in Table I, with the reaction conditions as
follows: 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min, with
a total of 40 cycles of amplification. The Cq value was calculated
by pplied Biosystems 7500 (Applied Biosystems, Foster City, CA,
USA) and the relative quantification of gene expression was
calculated by the 2-ΔCq method, according to the following formula:
ΔCq (target gene) = Cq (target gene) - Cq (control gene).
 | Table I.The primer sequences of RT-PCR. |
Table I.
The primer sequences of RT-PCR.
| Gene | Primer sequence |
|---|
| miR-19b | F:
5-UGUGCAAAUCCAUGCAAAACUGA-3 |
|
| R:
5-GCTCACTGCAACCCTCCTCCTCC-3 |
| U6 | F:
5-GCTTCGGCAGCACATATACTAAAAT-3 |
|
| R:
5-CGCTTCACGAATTTGCGTGTCAT-3 |
Western blotting
Cells were seeded in 6-well plates at a density of
1×104 cells/well. After 24 h, the supernatant was
aspirated and cells were cultured in medium containing 10, 20 or 40
µM naringin for another 24 h. Cells were collected and lysed with
cell lysis buffer, then centrifuged for 15 min at a high speed at
4°C. After centrifugation, the supernatant was collected. The
extracted protein concentrations were determined by a BCA kit. A
total of 50 µg of protein was separated by SDS-PAGE and the
separated protein was transferred to a PVDF membrane. The membrane
was incubated in blocking buffer for 1 h at room temperature and
then rabbit monoclonal Bcl-2 antibody (dilution, 1:500; cat. no.
ab32124) and rabbit monoclonal Bax antibody (dilution, 1:500; cat.
no. ab32503) (both from Abcam, Cambridge, MA, USA) were added and
the membrane was incubated overnight at 4°C. After washing membrane
with TTBS, the goat anti-rabbit secondary antibody (dilution,
1:2,000; cat. no. ab6721; Abcam) was added and the membrane was
incubated at room temperature for 1 h. ECL was added to the
membrane and the blots were developed in the dark, with images
recorded using a gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GADPH was used as the internal reference and
the gray-scale values were analyzed and compared.
Statistical analysis
Data are presented as means ± standard deviations
and analyzed with SPSS 17.0 (IBM Corp., Armonk, NY, USA), using
one-way ANOVA. P<0.05 was considered statistically
significant.
Results
The effect of naringin on HepG2 cell
proliferation inhibition
After cells were cultured in the medium containing
10, 20 and 40 µM naringin for 24 h, the proliferation of HepG2
cells was significantly inhibited in all groups. The proliferation
inhibition rates were significantly increased with the increase of
concentration and time (P<0.05), showing an obvious
dose-dependent pattern. The inhibitory rate of proliferation was
53.13% when 20 µM of naringin was used for 24 h (Table II).
 | Table II.The effects of different
concentrations of naringin on HepG2 cell proliferation
inhibition. |
Table II.
The effects of different
concentrations of naringin on HepG2 cell proliferation
inhibition.
|
| Cell proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Concentration
(µM) | 24 h | 48 h | 72 h |
|---|
| Control group
(0) | 0 | 0 | 0 |
| 10 |
30.25±0.12a |
40.32±4.13a |
45.92±5.33a |
| 20 |
52.13±0.31a |
62.32±5.31a |
70.25±8.12a |
| 40 |
70.67±1.32a |
80.22±7.28a |
86.43±10.21a |
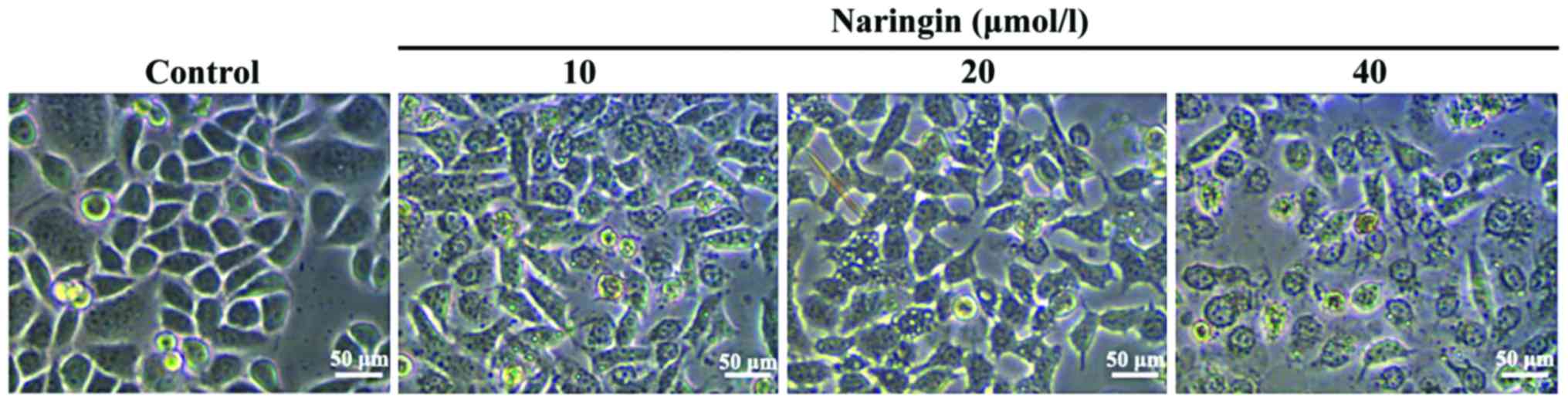
The effects of naringin on HepG2 cell
morphology
To explore possible regulatory mechanisms, 10, 20
and 40 µM naringin were chosen as the drug concentrations and
incubation time was 24 h. After cells were cultured for 24 h with
10, 20 and 40 µM naringin, compared with the control group, the
morphology of the cells had obvious changes, such as cell
shrinkage, decreased cell adherence, reduced cell number and
increased cell death number. These changes of morphology exhibited
a notable dose-dependent pattern (Fig.
1).
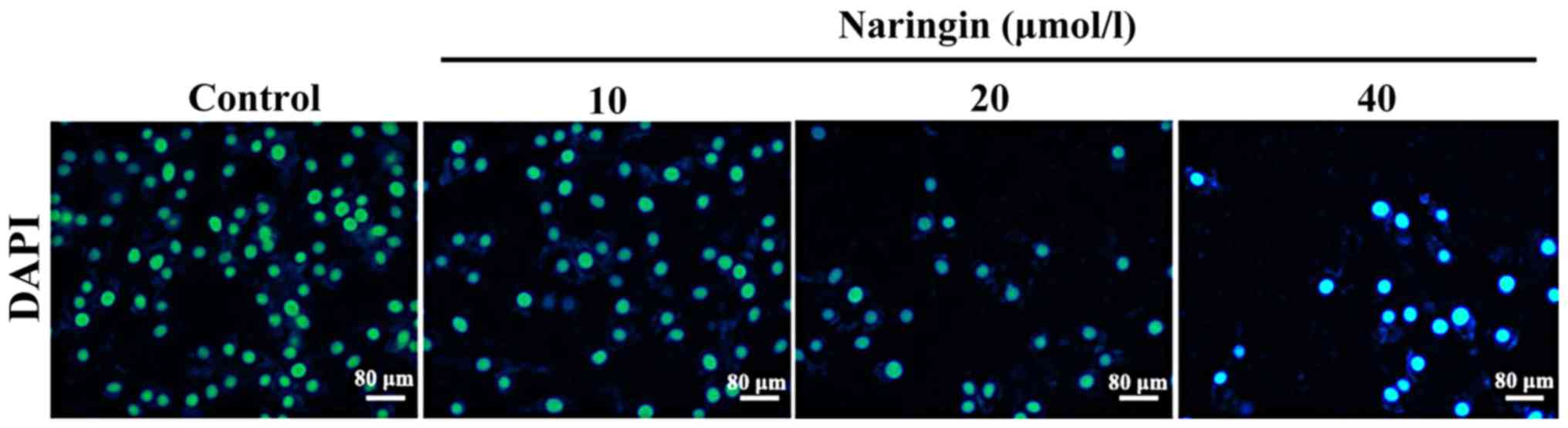
The effects of naringin on cell
apoptosis
After cells were cultured for 24 h with 10, 20 and
40 µM naringin, compared with the control group, DAPI staining
showed cell shrinkage and nuclear chromatin condensation,
suggesting the occurrence of cell apoptosis; moreover, the number
of apoptotic cells increased accordingly with an increase in the
concentration of naringin (Fig.
2).
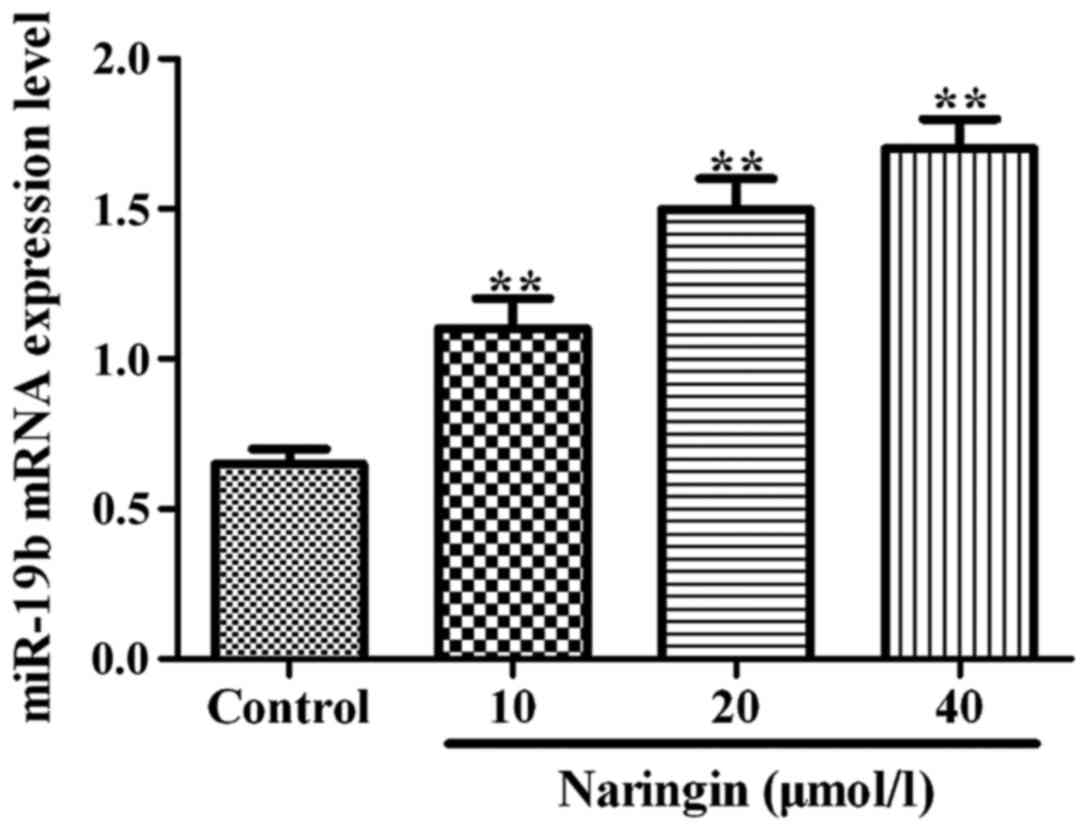
The effects of naringin on miR-19b
mRNA expression levels
As shown in Fig. 3,
compared with the control group, the expression of miR-19b mRNA was
significantly increased (P<0.01) after cells were cultured for
24 h with 10, 20 and 40 µM naringin.
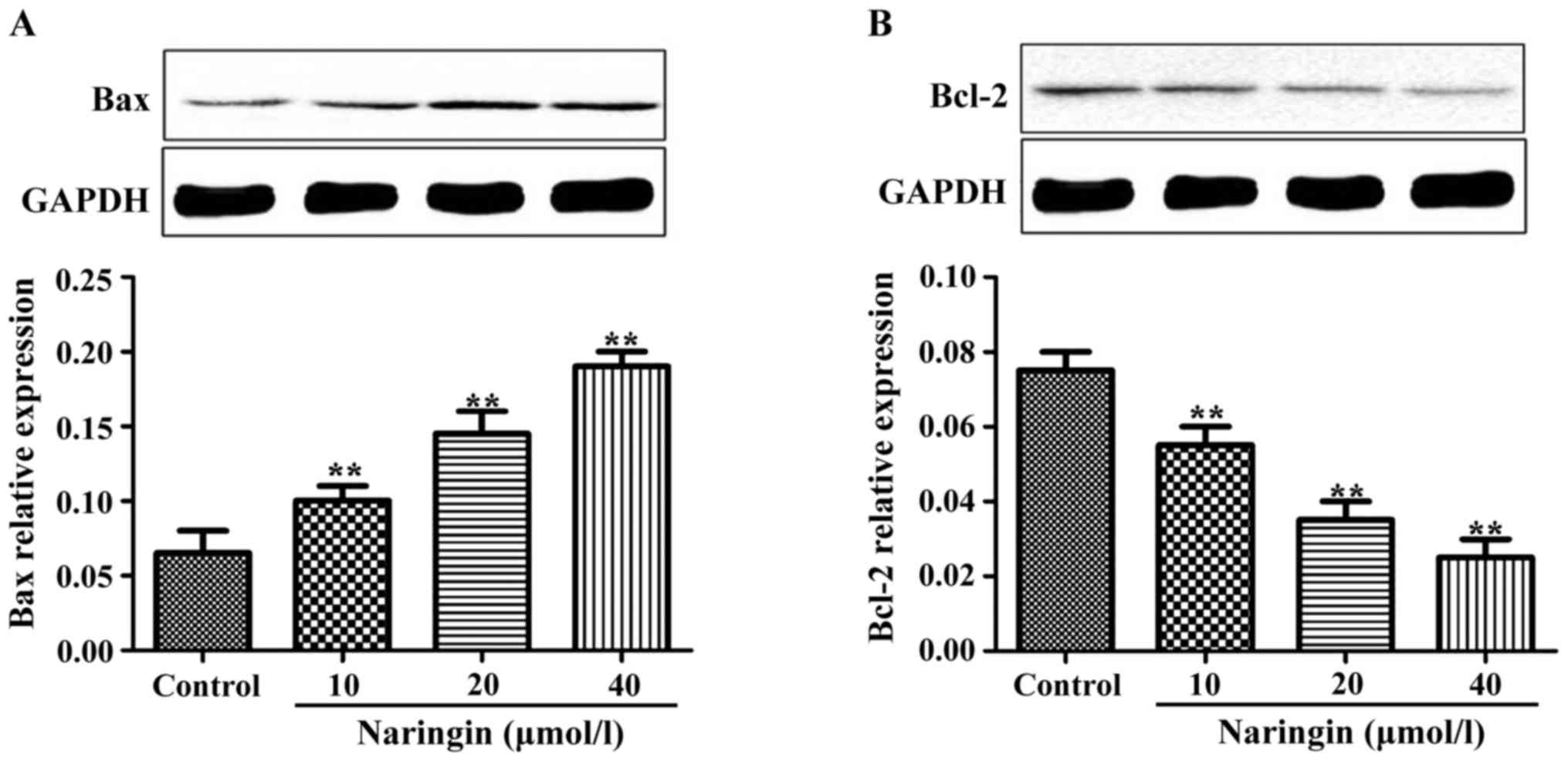
The effects of naringin on Bax and
Bcl-2 protein expression levels
After cells were cultured for 24 h with 10, 20 and
40 µM naringin, compared with the control group, the expression
levels of Bax protein were significantly increased (P<0.01). The
expression levels of Bcl-2 were significantly decreased in the
treated groups compared to the control group, showing an inverse
dose-dependent pattern to some extent (Fig. 4).
Discussion
Hepatocellular carcinoma (HCC) is a common malignant
tumor and currently has a high mortality rate due to a lack of
effective early diagnosis and therapy (11). A variety of factors can lead to the
occurrence of liver carcinoma, including chronic liver inflammation
caused by excessive drinking, viral hepatitis and non-alcoholic
liver fatty degeneration (12).
Traditional Chinese medicine and Chinese herbal
medicine are conventional forms of medicine that are unique to
China. Traditional Chinese medicine focuses on the overall
treatment of the individual and Chinese herbal medicine is
characterized by treatments with reduced side effects. Chinese
herbal medicine cannot only inhibit the growth of tumor cells, but
can also enhance the patients immune function in the treatment of
cancer (13). Natural compounds
extracted from Chinese herbal medicines as a novel method for drug
discovery, is becoming increasingly accepted and applied in
clinical research. This method cannot only obtain compounds with
better activity, but can also better control the quality of Chinese
herbal medicines. Therefore, the extraction of active chemical
ingredients from herbs used in traditional Chinese medicine has
become a widely used and promising avenue in antitumor research
(14).
Studies have shown that natural products can
regulate the proliferation and differentiation of tumor cells by
regulating the expression of related miRNAs in tumor cells, thereby
inducing tumor cell apoptosis (15).
miRNAs are small non-coding RNA molecules that regulate the
expression of related genes by specifically binding to the 3′ end
untranslated region of the targeted mRNA chain, leading to
degradation of the target mRNA or reduction of its stability,
thereby regulating the gene transcription and expression (10). Different miRNAs have been found to be
differentially expressed in many tumor cells; moreover, further
studies have shown that abnormal expressions of miRNAs play key
roles in the proliferation, differentiation and apoptosis of tumor
cells (16).
It has been found that miR-19b is highly expressed
in bladder carcinoma, vesicular rhabdomyosarcoma and colon cancer,
and its high expression is closely related to tumor angiogenesis,
disease prognosis and survival rate (17–19).
Another study reported that miR-19b expression in prostate and
cervical cancers has a role in promoting tumorigenesis (20). However, miR-19 could inhibit the
proliferation, invasion and metastasis of hepatocellular carcinoma
(21).
Bcl-2 is an apoptosis inhibitory protein and plays a
key role in the signaling pathway of apoptosis. It protects cells
from apoptosis induced by many factors and increases the
proliferation and viability of cells. When Bcl-2 protein expression
is upregulated, the apoptosis rate of tumor cells decreases
significantly, suggesting that Bcl-2 protein and apoptosis of tumor
cells have a very close relationship (22). The effects of Bax protein on apoptosis
are contrary to Bcl-2, as it functions as a pro-apoptotic protein.
Bax and Bcl-2 genes belong to the same gene family and Bax can both
antagonize the inhibitory effect of Bcl-2 on cell apoptosis as well
as act directly on the cells to promote tumor cell apoptosis
(23). Studies have shown that, when
Bcl-2 exists as a homodimer, it acts to inhibit apoptosis. In
contrast, when Bax exists as a homodimer or polymerizes with Bcl-2
into a dimer, it plays a role in promoting apoptosis (24).
In the present study, the hepatocellular carcinoma
cell line, HepG2, was treated with different concentrations of
naringin and then observed at progressive time points of 24, 48 and
72 h. The results of the MTT assay showed that naringin
significantly inhibited the proliferation of HepG2 cells compared
with the control group. Notable morphological changes of apoptotic
HepG2 cells after treatment with naringin were found under inverted
microscope. The results of DAPI staining showed cell shrinkage and
nuclear chromatin condensation. The results of RT-PCR showed that
naringin could upregulate the expression of miR-19b mRNA.
Comparable studies have demonstrated that the expression of
miRNA-19b was significantly increased after HepG2 cells were
treated with total alkaloid extracts of Rhizoma Corydalis,
another herb from Chinese herbal medicine. These results support
the theory that the expression of miRNA-19b may be an important
mechanism against tumorigenesis (25,26).
Western blotting showed an increase in the
expression of Bax protein and a decrease in the expression of Bcl-2
protein with an increase in naringin concentration. This implies
that naringin could upregulate Bax and downregulate Bcl-2 protein
expression, thereby inducing cell apoptosis. Xi et al
(27), indicated that carnosol
increased Bax expression and decreased Bcl-2 protein expression by
34–53% in leukemic cells. Although carnasol is derived from a
different botanical source, its mechanism of action is comparable
to that of naringin. Collectively, the existing evidence supports a
theoretical framework for the therapeutic effects of naringin
against liver carcinoma. However, the required therapeutic drug
concentrations and efficacy in vivo need to be further
explored with animal experiments and clinical trials.
In conclusion, the results of this study demonstrate
that naringin can inhibit the proliferation of hepatocellular
carcinoma HepG2 cell lines and induce apoptosis. The induction of
apoptosis may be achieved by upregulating the expression of
miRNA-19b mRNA and Bax protein and downregulating the expression of
Bcl-2 protein. Through these mechanisms, naringin has potential as
an effective medicinal therapy against liver carcinoma in clinical
applications.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma LW and Jia TZ: Optimal management for
hepatocellular carcinoma. China J Cancer Prev Treat. 12:471–473.
2005.
|
|
4
|
Kettenbach J, Stadler A, Katzler IV,
Schernthaner R, Blum M, Lammer J and Rand T: Drug-loaded
microspheres for the treatment of liver cancer: review of current
results. Cardiovasc Intervent Radiol. 31:468–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanno S, Shouji A, Asou K and Ishikawa M:
Effects of naringin on hydrogen peroxide-induced cytotoxicity and
apoptosis in P388 cells. J Pharmacol Sci. 92:166–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: Flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung YJ, Kim JW, Park SJ, Min BY, Jang ES,
Kim NY, Jeong SH, Shin CM, Lee SH, Park YS, et al: c-Myc-mediated
overexpression of miR-17-92 suppresses replication of hepatitis B
virus in human hepatoma cells. J Med Virol. 85:969–978. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu M, Wang Z, Yang S, Zhang W, He S, Hu
C, Zhu H, Quan L, Bai J and Xu N: TNF-α is a novel target of
miR-19a. Int J Oncol. 38:1013–1022. 2011.PubMed/NCBI
|
|
11
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa H, Umemura A, Taniguchi K,
Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E,
Hidalgo J, et al: ER stress cooperates with hypernutrition to
trigger TNF-dependent spontaneous HCC development. Cancer Cell.
26:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
14
|
le Sage C and Agami R: Immense promises
for tiny molecules: Uncovering miRNA functions. Cell Cycle.
5:1415–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kusenda B, Mraz M, Mayer J and Pospisilova
S: MicroRNA biogenesis, functionality and cancer relevance. Biomed
Pap Med Fac Univ Palacky Olomouc Czech Repub. 150:205–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu SP, Sun GP, Shen YX, Wei W, Peng WR and
Wang H: Antiproliferation and apoptosis induction of paeonol in
HepG2 cells. World J Gastroenterol. 13:250–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de la Peña F Ayala, Kanasaki K, Kanasaki
M, Tangirala N, Maeda G and Kalluri R: Loss of p53 and acquisition
of angiogenic microRNA profile are insufficient to facilitate
progression of bladder urothelial carcinoma in situ to invasive
carcinoma. J Biol Chem. 286:20778–20787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reichek JL, Duan F, Smith LM, Gustafson
DM, Oconnor RS, Zhang C, Dunlevy MJ, Gastier-Foster JM and Barr FG:
Genomic and clinical analysis of amplification of the 13q31
chromosomal region in alveolar rhabdomyosarcoma: A report from the
Childrens Oncology Group. Clin Cancer Res. 17:1463–1473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YX, Lang F, Liu YX, Yang CQ and Gao
HJ: In situ hybridization analysis of the expression of miR-106b in
colonic cancer. Int J Clin Exp Pathol. 8:786–792. 2015.PubMed/NCBI
|
|
20
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu XM, Wang XB, Chen MM, Liu T, Li YX, Jia
WH, Liu M, Li X and Tang H: MicroRNA-19a and −19b regulate cervical
carcinoma cell proliferation and invasion by targeting CUL5. Cancer
Lett. 322:148–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Packham G and Cleveland JL: c-Myc and
apoptosis. Biochimica et Biophysica Acta. 1242:11–28.
1995.PubMed/NCBI
|
|
23
|
Brady HJ and Gil-Gómez G: Bax. The
pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol.
30:647–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu QL, Abel P, Foster CS and Lalani EN:
bcl-2: Role in epithelial differentiation and oncogenesis. Hum
Pathol. 27:102–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae S, Lee EM, Cha HJ, Kim K, Yoon Y, Lee
H, Kim J, Kim YJ, Lee HG, Jeung HK, et al: Resveratrol alters
microRNA expression profiles in A549 human non-small cell lung
cancer cells. Mol Cells. 32:243–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Law PT and Wong N: Emerging roles of
microRNA in the intracellular signaling networks of hepatocellular
carcinoma. J Gastroenterol Hepatol. 26:437–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xi S, Dyer KF, Kimak M, Zhang Q, Gooding
WE, Chaillet JR, Chai RL, Ferrell RE, Zamboni B, Hunt J, et al:
Decreased STAT1 expression by promoter methylation in squamous cell
carcinogenesis. J Natl Cancer Inst. 98:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|