Introduction
Oral cancer is the generic term for a malignant
tumor of the oral cavity. Most of these types of cancers are
considered squamous cell carcinomas, i.e., mucosal variations
(1). Oral cancer generally includes
gingival and tongue cancer, and is commonly seen in the head and
neck (2). Most types of oral cancer
are related to unhealthy living habits, such as the oral use of
tobacco and betel leaf. Since the space of the oral cavity is small
but the blood supply is rich, with more lymph distribution, oral
cancer is easily transferred at an early stage. This may cause
certain challenges regarding treatment (3,4).
In the past, synthetic gibberellin was widely used
as the conditioning agent for plant growth (5). Synthetics of α,β-unsaturated ketone unit
with gibberellin revealed the antitumor activity of the gibberellin
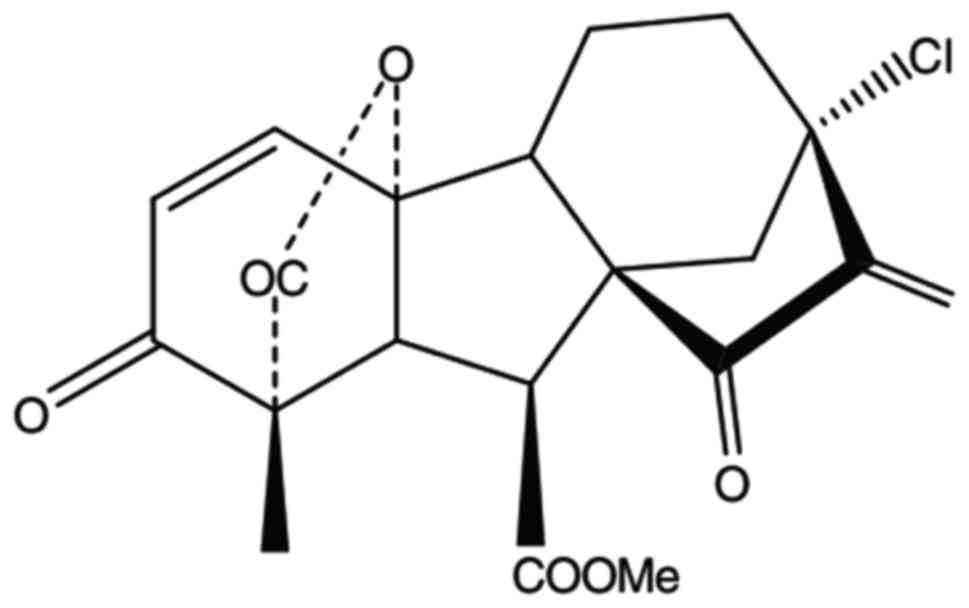
ramification (6). GA-13315 is a
chemical compound that includes α,β-unsaturated ketone (Fig. 1). Some research shows that GA-133315
has antitumor properties in rats with xenotransplantation of tumor
cells for non-small cell lung cancer (A549 cells), but the
antitumor effect of GA-13315 has not been determined yet (7). More recent research shows that GA-13315
offers good treatment for breast cancer in patients with
multidrug-resistant, which is exerted through the expression of
P-glycoprotein (ABCB1). In order to investigate the effect on oral
cancer, we studied the effect mechanism through the use of cancer
cell line H1299 in this study.
Materials and methods
For the preparation of the cell suspension with high
activity, the KB cell concentration was adjusted to
5×106-1×107/ml by 10% FCS RPMI-1640
(Sigma-Aldrich, St. Louis, MO, USA). A 5–50 µl glass tube was
prepared to contain a 40 µl cell suspension after centrifugation at
1,050 × g for 5 min. A scrubbing solution (Keygen, Nanjing, China)
was used to wash the 4°C inactivated rabbit serum twice for 30 min,
at which time a 2 ml scrubbing solution was added. The centrifugal
parameter was set as 850 × g for 5 min; wash at 4°C of 50 µl rabbit
anti-rat fluorescent marker (Biosharp, Hefei, China) for 30 min;
fixing after washing twice; the film was observed under a light
microscope (Labconco, Kansas City, MO, USA).
Detection of MMT cell activity
An MTT experiment was used to detect cell activity.
The suspension of 160 µl with 8×103/µl cells were
inoculated into a 96-well plate for cell contact overnight. In
order to detect the cell toxicity of GA-13315, GA-13315
(Sigma-Aldrich, San Francisco, CA, USA) was added to the cell
culture medium with the total amount of 200 µl.
Cells were cultured for 48 h in a medium with
GA-13315, then washed and placed in the RIPA lysis buffer
(Beyotime, Shanghai, China) with 1 mM PMSF, 1 µg/ml trasylol and
0.5 µg/ml eupeptin for pyrolysis on ice. An equivalent cell lysis
solution (20 µg proteins; Beyotime) was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transfected to the
polyvinylidene fluoride film (KeyGen). TBST (10 mM Tris-HCl, 150 mM
NaCl, and 0.1% Tween-20, pH 8.0) was sealed for 1 h under room
temperature using 5% skim milk. After being incubated overnight
using rabbit polyclonal PPP2R2B antibody (dilution, 1:200; cat. no.
ab137609) under 4°C, it was washed and hatched for 2 h at 25 °C
using secondary goat anti-rabbit (HRP) IgG antibody (dilution,
1:2,000; cat. no. ab6721) (both from Abcam, Cambridge, MA, USA). It
was visually tested by enhanced western blotting and phototope™-HRP
visual kits (Cell Signaling Technology, Danvers, MA, USA).
FluorChem E system (ProteinSimple, San Jose, CA, USA) was used to
analyze imaging. ImageJ sofware (version X; Media Cybernetics,
Silver Springs, MD, USA) was used for quantization of protein
relative expression by referring to β-actin.
Cells were seeded overnight in a 6-well plate
(Corning Inc., Corning, NY, USA) based on the density of
1×105 cells/plate. GA13315 with different doses was used
for simulation, while 0.1% DMSO was used for control. It was
continuously cultured for 48 h under 37°C. TRIzol reagent (Thermo
Fisher Scientific, Waltham, MA, USA) was used for cellular total
RNA extraction. Then, 1 µg of total RNA was used for cDNA first
strand synthesis. M-MLV First Strand kits were used for synthesis.
ABI PRISM® 7500 Real-Time PCR system and
SYBR® Premix Select Master Mix kit (Thermo Fisher
Scientific) were used for amplification of the genes of interest.
β-actin was used as an internal reference. Specific primers were as
follows: β-actin forward, 5′-CACCTTCTACAATGAGCTGCGTGTG-3′ and
reverse, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′; forward,
5′-AGTAGTAGTAGTTGTGAGTGTGT-3′ and reverse,
5′-AAACAACCACAACAAAATAATACC-3′. The thermal cycle program was as
follows: At 50°C for 2 min, at 95°C for 2 min, at 95°C for 15 sec,
45 cycles, at 55°C for 30 sec, at 72°C for 1 min. Specific melting
curves were adopted to describe each reaction. The mean value of
mRNA expression was calculated for the genes of interest in each
group. ΔCq = Cqtarget gene - Cqβ-actin; -ΔΔCq =
-(ΔCqMCF-7/adr-ΔCqMCF-7).
A millipore filtration culture chamber or
double-chamber co-culture system (Corning Inc.) was used with a
cell culture density of 1×106. The experimental
procedures were conducted on built-in standard specifications.
Statistical analysis
SPSS 20.0 statistical analysis software (IBM SPSS,
Armonk, NY, USA) was used to analyze collected data. All
measurement data are presented as mean ± standard deviation. SPSS
17.0 (SPSS, Inc., Chicago, IL, USA) software was used for
processing. Repeatedly measured data via the ANOVA method was
adopted for statistical treatment. The t-test of two independent
samples was used for measurement data between groups, and the
paired t-test was used for intra-group comparison. The
χ2 test was used for enumeration data. P<0.05 was
considered to indicate a statistically significant difference.
Results
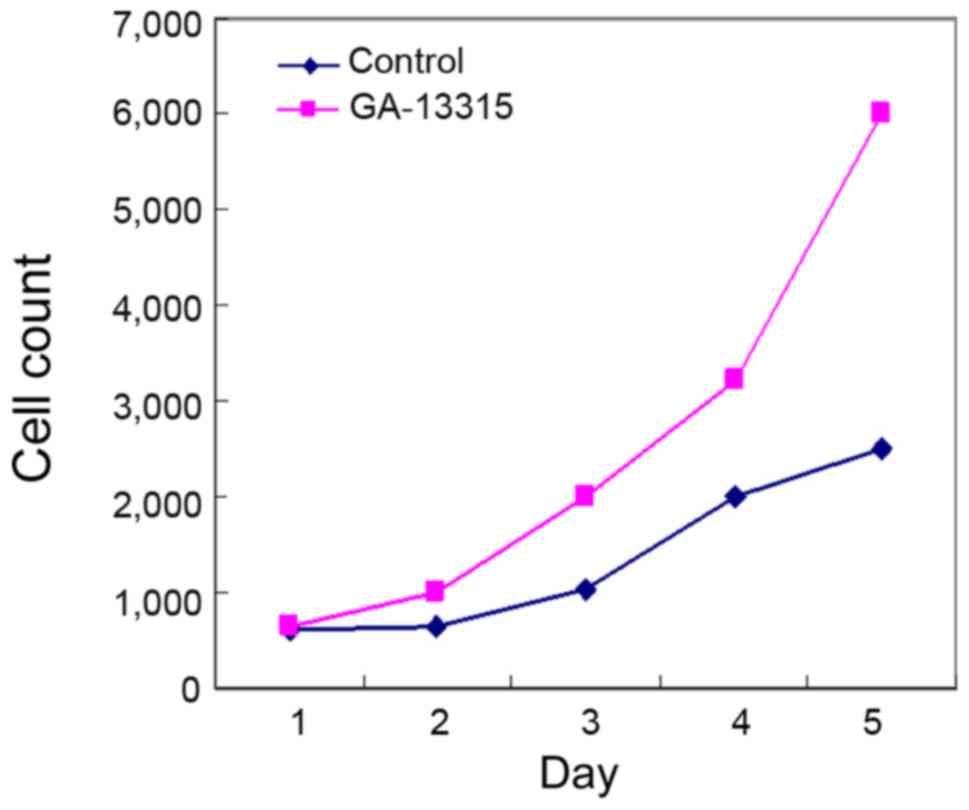
KB cell viability was tested by MMT in the treatment
group. It was found that the cell viability significantly decreased
after being treated by GA-13315 for 48 h. The difference was
statistically significant (P<0.05) as compared to the control
group (Fig. 2).
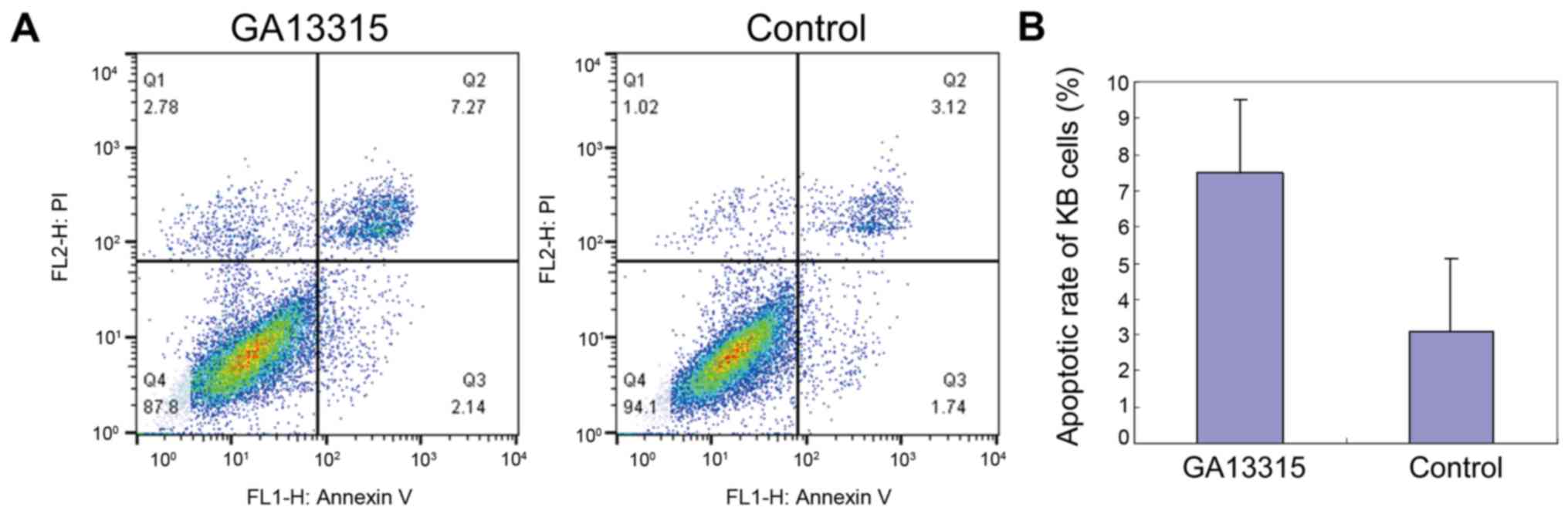
Decreased KB apoptosis after treatment
with GA13315
Flow cytometry was used to test cell apoptosis
between the two groups. The results showed that KB apoptosis
significantly increased after being treated by GA13315. The
difference was statistically significant (P<0.05) as compared to
the control group (Fig. 3).
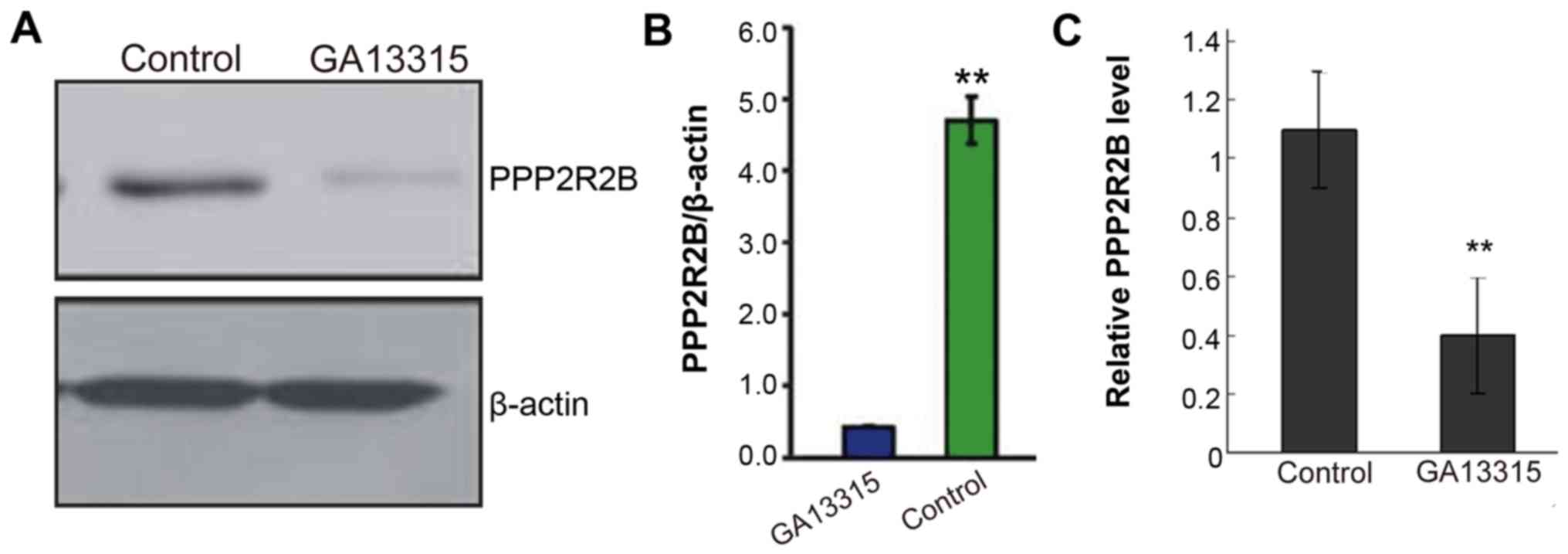
GA-13315 promotes H1299 apoptosis via
downregulating PPP2R2B
Western blotting and quantitative polymerase chain
reaction (qPCR) tests found that PPP2R2B expression among cells in
the treatment group was significantly lower than that of the
control group (Fig. 4). The
difference was statistically significant (P<0.05).
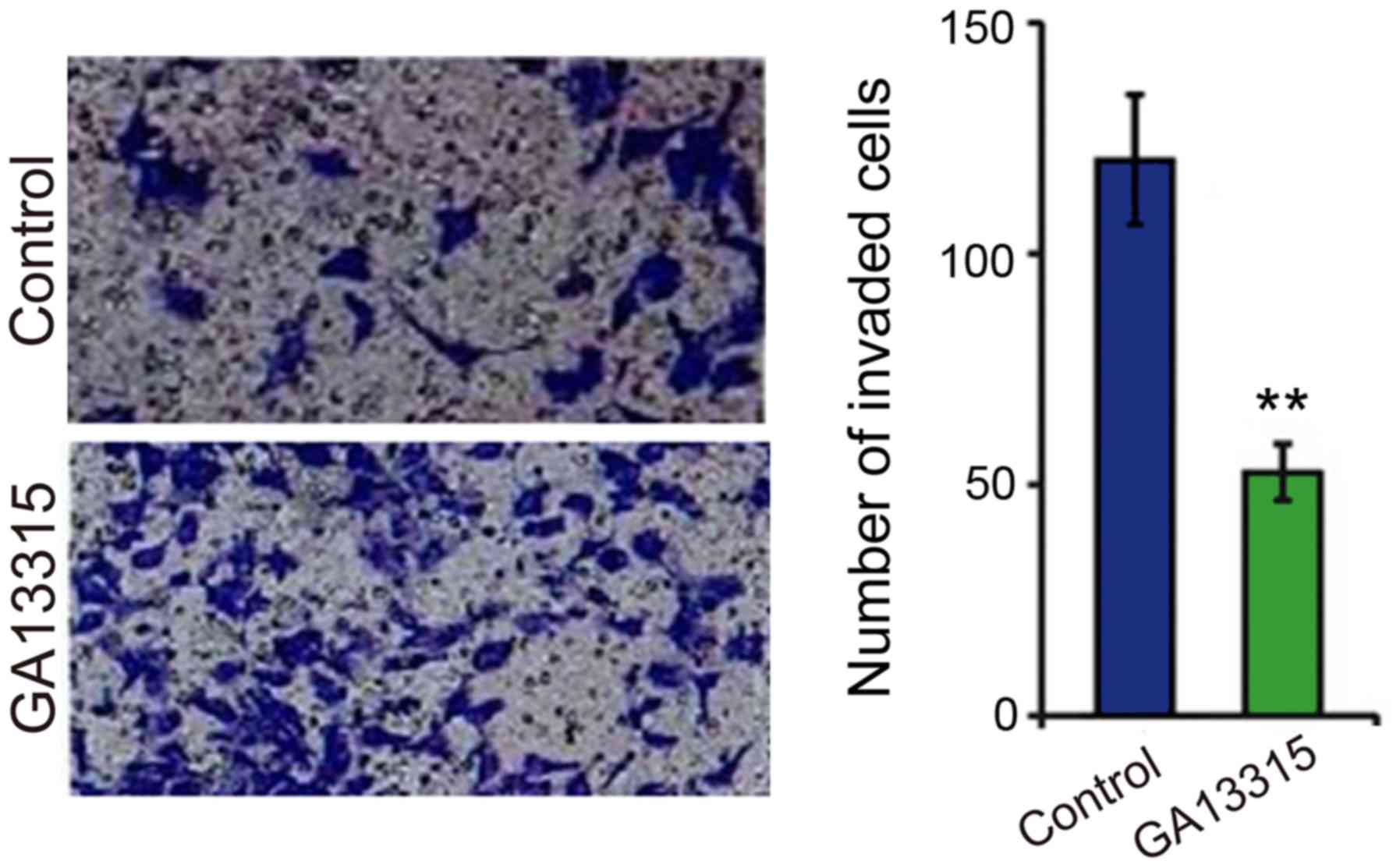
GA13315 lowers the cell invasion
ability
After cell invasion was tested by Transwell
migration assay between the two groups, the results showed that KB
cell invasion ability was significantly decreased after treatment
with GA-13315. The difference was statistically significant
(P<0.05) as compared to the control group (Fig. 5).
Discussion
Oral cancer is a malignant tumor commonly seen in
the head and neck region, which is ranked with oropharyngeal cancer
in the top six of systematic malignant tumors. In some
high-prevalence areas, new cases account for 25% of male malignant
tumors every year (1). Treatments for
patients with oral cancer often cause dysfunction of important
organs, leading to unclear speaking, dysphagia and eating
disorders, as well as changes in facial appearance and impacts on
quality of life. Although oral cancer is not listed in the top 10
of malignant tumors, its morbidity and mortality cannot be ignored.
According to global statistics in 2008, new oral cancer cases were
approximately 274,000 and annual cases of death were 127,000, 2/3
of which were reported in 40–60-year-old adults. Also, 2/3 of the
cases were in developing countries. It should be noted that oral
cancer itself and its treatment course usually cause functional
injuries to vital organs and facial feature damage, thereby
seriously affecting patients' social communications (8–10).
GA-13315 is a chemical compound containing α,β-unsaturated ketone.
In previous research of A549 tumor cells, GA-13315 reduced the
expression of blood coagulation factor VIII, microvessel density
and vascular endothelial growth factors. This indicates that it
plays a role in anti-angiogenesis. These results show that GA-13315
has activities of anti-angiogenesis that contribute to the
development of anticancer characteristics (11,12).
Therefore, we believe that GA-13315 has certain antitumor
effects.
The PPP2R2B gene belongs to phosphatase II
regulation subunit B. Protein phosphatase 2 is one of four major
types of serine/threonine phosphatases, and it plays important
roles in cell growth and division (13). It consists of a common heterogeneous
core enzyme, a catalyst subunit and a constant adjusting subunit.
Various kinds of adjusting subunits play their own roles (14). Some research has found that the
genetic defect in the 5′UTR region may lead to rare type 12
autosomal dominant spinocerebellar ataxias (15). Moreover, PPP2R2B methylation may be
associated with survival and prognosis in patients with gliomas
(16). In tumor cell proliferation
and apoptosis, the mechanism of PPP2R2B remains unclear. In the
present study, we found that PPP2R2B expression of H1299 cells is
significantly decreased after being treated by GA-13315. The
difference is statistically significant (P<0.05) as compared to
the control group. We believe that GA-13315 may increase apoptosis
in oral cancer cells via regulating PPP2R2B and does show a
correlation to dosage. This biological effect is completed via
downregulating PPP2R2B.
References
|
1
|
Warnakulasuriya S: Living with oral
cancer: Epidemiology with particular reference to prevalence and
life-style changes that influence survival. Oral Oncol. 46:407–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haron N, Zain RB, Nabillah WM, Saleh A,
Kallarakkal TG, Ramanathan A, Sinon SH Mohd, Razak I Abdul and
Cheong SC: Mobile phone imaging in low resource settings for early
detection of oral cancer and concordance with clinical oral
examination. Telemed J E Health. Aug 19–2016.(Epub ahead of print).
PubMed/NCBI
|
|
3
|
Bajpai M, Arora M and Chandolia B:
Bioimpedance for Oral Cancer Detection in Clinical Practice and its
Applicability in Developing Nations. J Coll Physicians Surg Pak.
26:7212016.PubMed/NCBI
|
|
4
|
Ishikawa S, Sugimoto M, Kitabatake K,
Sugano A, Nakamura M, Kaneko M, Ota S, Hiwatari K, Enomoto A, Soga
T, et al: Identification of salivary metabolomic biomarkers for
oral cancer screening. Sci Rep. 6:315202016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petersen PE: Oral cancer prevention and
control - the approach of the World Health Organization. Oral
Oncol. 45:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bömke C and Tudzynski B: Diversity,
regulation, and evolution of the gibberellin biosynthetic pathway
in fungi compared to plants and bacteria. Phytochemistry.
70:1876–1893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Sun Z, Zhang Y, Zeng X, Qing C,
Liu J, Li L and Zhang H: Synthesis of gibberellin derivatives with
anti-tumor bioactivities. Bioorg Med Chem Lett. 19:5496–5499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Zhang H, Chen J, Zhao H, Zeng X,
Zhang H and Qing C: Antitumor and antiangiogenic effects of
GA-13315, a gibberellin derivative. Invest New Drugs. 30:8–16.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conway DI, Petticrew M, Marlborough H,
Berthiller J, Hashibe M and Macpherson LM: Socioeconomic
inequalities and oral cancer risk: A systematic review and
meta-analysis of case-control studies. Int J Cancer. 122:2811–2819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petti S: Lifestyle risk factors for oral
cancer. Oral Oncol. 45:340–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo J, Kang M, Ye JX, Chen JB, Zhang HB and
Qing C: Gibberellin derivative GA-13315 sensitizes
multidrug-resistant cancer cells by antagonizing ABCB1 while
agonizes ABCC1. Cancer Chemother Pharmacol. 78:51–61. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zhang H, Chen J, Zhao H, Zeng X,
Zhang H and Qing C: Antitumor and antiangiogenic effects of
GA-13315, a gibberellin derivative. Invest New Drugs. 30:8–16.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mayer RE, Hendrix P, Cron P, Matthies R,
Stone SR, Goris J, Merlevede W, Hofsteenge J and Hemmings BA:
Structure of the 55-kDa regulatory subunit of protein phosphatase
2A: Evidence for a neuronal-specific isoform. Biochemistry.
30:3589–3597. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holmes SE, O'Hearn EE, McInnis MG,
Gorelick-Feldman DA, Kleiderlein JJ, Callahan C, Kwak NG,
Ingersoll-Ashworth RG, Sherr M, Sumner AJ, et al: Expansion of a
novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is
associated with SCA12. Nat Genet. 23:391–392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marmorstein LY, McLaughlin PJ, Stanton JB,
Yan L, Crabb JW and Marmorstein AD: Bestrophin interacts physically
and functionally with protein phosphatase 2A. J Biol Chem.
277:30591–30597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Majchrzak-Celińska A, Słocińska M,
Barciszewska AM, Nowak S and Baer-Dubowska W: Wnt pathway
antagonists, SFRP1, SFRP2, SOX17, and PPP2R2B, are methylated in
gliomas and SFRP1 methylation predicts shorter survival. J Appl
Genet. 57:189–197. 2016. View Article : Google Scholar : PubMed/NCBI
|