Introduction
Hepatocellular carcinoma (HCC), the fifth most
common cancer worldwide, is also the third leading cause of
cancer-associated mortalities (1,2). It
accounts for ~695,900 mortalities per year, half of which occur in
China, as a result of the high chronic hepatitis B virus infection
incidence (3). At present, surgical
resection remains the primary approach for treating HCC. However,
<20% of patients receive timely radical surgical resection
mainly due to advanced cancer, which includes high degrees of
malignancy, early metastasis and the lack of effective therapies
(4,5).
Therefore, it is desirable to reveal the molecular mechanisms of
carcinogenesis, to identify novel prognostic markers that may
detect HCC earlier or facilitate the development of effective
therapeutic strategies, including antibody or immune-based
treatments for advanced cancer.
Numerous transcription factors or signaling
pathways, including P53, Wnt signaling pathway key members
[including glycogen synthase kinase-3β (Gsk-3β) and β-catenin] and
nuclear factor (NF)-κB signaling, contribute to the proliferation
of cancer cells (6–8). Particularly, NF-κB signaling, including
NF-κB transcription factor, inhibitor of κ-light polypeptide gene
enhancer in B-cells kinase γ (Ikbkg) and TRAF family member
associated NF-κB activator (Tank), is involved in several aspects
of tumorigenesis, including the prevention of apoptosis, an
increase in the metastatic potential of tumor cells and cancer cell
survival and proliferation (9,10).
Although these observations demonstrated that NF-κB signaling had
an important role in liver cancer tumorigenesis and cells
proliferation, its upstream regulators remain to be elucidated.
In comparison with non-transformed cells, carcinoma
cells exhibit increased metabolic autonomy in taking up and
metabolizing nutrients, which support cell growth and proliferation
(11). In the plasma membrane,
sphingolipids, together with cholesterol, form lipid microdomains,
which are hypothesized to function as structural scaffolds and
platforms for signal transduction. The biosynthesis of this plasma
membrane is essential for cell growth and proliferation (12,13).
Sphingolipids, particularly ceramides, have also been implicated in
regulating physiological activity, including apoptosis induced by
stress stimuli, such as radiation and chemotherapeutic drugs
(14).
Ceramide synthase-4 (CERS4), one of the six
mammalian CERSs, catalyzes an amide bond between asphingoid base
and a fatty acyl-coenzyme A (15,16).
Mutations in this gene are involved in human diseases, including
the disease of sphingolipidoses, which are characterized by the
accumulation of specific sphingolipid subtypes (14). It was shown that ceramide synthase is
involved in tumorigenesis (17,18);
however, its roles in HCC have not been studied. The present study
revealed that CERS4 was highly expressed in HCC cells, and the
functions and mechanisms of CERS4 in HCC were investigated.
Materials and methods
Cell line and human samples
The human 293T cell line and liver cancer HepG2 and
Huh7 cell lines were purchased from the Cell Bank of the Type
Culture Collection of the Chinese Academy of Sciences, Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). Human samples were obtained from the People's Hospital of
Zhengzhou (Zhengzhou, China) by surgery between March 2014 and May
2014, according to procedures approved by the Ethics Committee at
the Chinese Academy of Medical Sciences and Peking Union Medical
College (Beijing, China). Informed consent was obtained from all of
the three patients that participated in the present study.
Cell viability assay
The cell proliferation rate was evaluated by MTT
assay. Briefly, HepG2 and Huh7 cells were seeded onto 96-well
plates (1×103 cells/well), and cell proliferation was
documented at 12, 24, 48, 72 and 96 h. The number of viable cells
was assessed by measuring the absorbance at 490 nm using a
microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Colony forming assay
Various groups of HepG2 cells were maintained in
RPMI-1640 complete media (10% fetal bovine serum and 1%
penicillin/streptomycin). The HepG2 cells were then plated on
6-well plates (500 cells/well), followed by incubation at 37°C
overnight. The media was changed every 2 days and the cells were
cultured for ~2 weeks to form colonies. After 14 days, each well
was washed with 1 ml PBS, and 1 ml of crystal violet solution (1%
crystal violet and 10% ethanol; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was added to each well, followed by incubation
for 10 min at room temperature. The excess crystal violet was
washed out with PBS and the colonies were counted using NIH Image J
software (NIH, Bethesda, MD, USA).
Cell cycle assay
Cell cycle analysis of HepG2 cancer cells was
performed according to the manufacturer's protocol (BD Cycle Test
Plus DNA Reagent kit; BD Biosciences, Franklin Lakes, CA, USA).
Different groups of HepG2 cells were seeded on T25 flasks and
incubated at 37°C for 48 h. Following incubation, the cells of each
group were harvested. Methanol (90%) was then added to the
harvested cells for re-suspending and fixing for 30 min. After 30
min, the cells were centrifuged at 400 × g for 5 min at room
temperature and washed twice with PBS. The pellets were then
re-suspended in propidium iodide and incubated at 37°C for 1 h. A
fluorescence-activated cell sorting machine (FACS Calibur flow
cytometer; BD Biosciences) was utilized to analyze the data.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR was performed to detect mRNA expression
levels of CERS4, P53, Gsk-3β, β-catenin1, Ikbkg and Tank, according
to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). Total cellular RNA was extracted from HepG2 or
Huh7 cells from different groups by lysing cells with TRIzol®
reagent (Gibco; Thermo Fisher Scientific, Inc.), followed by
centrifugation at 10,000 × g for 15 min at 4°C with
chloroform (in the ratio of 5:1). The supernatant was centrifuged
with isopropanol (in the ratio of 1:1) at 8,000 × g for 10
min at 4°C. The RNA pellet was washed with 75% ethanol and
solubilized with DNase and RNase free water. The RNA was quantified
by measuring absorbance at 260 nm using NanoDrop ND-1000 (Thermo
Fisher Scientific, Inc.). Single stranded cDNA was prepared using
the Prime-Script RT Reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China). The mRNA expression of the target gene was
determined by SYBR-Green assays. The SYBR-Green qPCR kit was
purchased from Roche (Roche Diagnostics GmbH, Mannheim, Germany).
qPCR was performed using an Applied BioSystems 7300 sequence
detection system (Thermo Fisher Scientific, Inc.). All experiments
were performed in triplicate. The relative gene expression levels
were calculated using the 2−ΔΔCq analysis tool (19). Primers were as follows: CERS4 forward,
5′-TCGGTCCTGTACCACGAGTC-3′ and reverse,
5′-GCCTGATTAGCAGTGAGAGGTAG-3′; β-catenin forward,
5′-CATCTACACAGTTTGATGCTGCT-3′ and reverse,
5′-GCAGTTTTGTCAGTTCAGGGA-3′; Gsk-3β forward,
5′-AGACGCTCCCTGTGATTTATGT-3′ and reverse,
5′-CCGATGGCAGATTCCAAAGG-3′; P53 forward,
5′-GAGGTTGGCTCTGACTGTACC-3′ and reverse,
5′-TCCGTCCCAGTAGATTACCAC-3′; Ikbkg forward,
5′-CGGCAGAGCAACCAGATTCT-3′ and reverse,
5′-CCTGGCATTCCTTAGTGGCAG-3′; and Tank forward,
5′-AGCAGAGAATACGTGAACAACAG-3′ and reverse,
5′-CAGAAGCAATGTCTACCTTTGGT-3′. The GAPDH internal control primers
were GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blot analysis
The standard western blot analysis procedure was
utilized to detect the protein expression levels of CERS4, NF-κB
and GAPDH in HepG2 or Huh7 cells. The cells were washed twice with
ice-cold PBS and lysed using a protein sample buffer kit (Beyotime
Institute of Biotechnology, Shanghai, China), according to the
manufacturer's protocol. Total cell lysates were then centrifuged
(10,000 × g 15 min; 4°C), and the supernatants were used for
additional processing. The bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology) was used to determine the
protein concentration. Total protein (20 µg) was separated by
SDS-PAGE and electro-blotted to a polyvinylidene fluoride membrane
(Merck Millipore). The membranes were blocked with 5% bovine serum
albumin for 2 h at room temperature, and incubated overnight at 4°C
with different primary antibodies: Anti-CERS4 antibody (dilution,
1:2,000; cat. no. ab118379; Abcam, Cambridge, UK); anti-NF-κB
(dilution, 1:2,000; cat. no. ab32360; Abcam); and anti-GAPDH
antibody (dilution, 1:5,000; cat. no. ab181602; Abcam). Following
incubation with primary antibodies, the horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(dilution, 1:5,000; cat. no. SC-2004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) were utilized and incubated for 1 h at room
temperature. Protein bands were visualized using an electro
chemiluminescence assay kit (Beyotime Institute of Biotechnology)
and a luminescent image analyzer (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA).
Animal studies
Male Balb/c nude mice (5 weeks old) were purchased
from the SLAC Laboratory Animal Company (Shanghai, China). The
total number of mice used is the present study was 21 and the
average weight of these mice was ~15 g. All mice were maintained in
the specific-pathogen-free conditions at 22±2°C and 40–70% relative
humidity throughout the experiments according to animal welfare
regulations and protocols approved by the Institutional Animal Care
and Use Committee of Sichuan University (Sichuan, China). Different
groups of HepG2 liver cancer cells (1×107 cells/mouse)
were injected subcutaneously into the right forelimb axillaries of
Balb/c nude mice to generate tumors in mice. The mice were
sacrificed by decapitation following 4 weeks and the weight and
volume of the tumors from each mouse were evaluated.
Statistical analysis
All the results represented three or more
independent experiments, with the data expressed as the mean ±
standard deviation. Differences between the control and treatment
groups were analyzed using Student's t-test with SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
CERS4 is highly expressed in liver
cancer tissues
To investigate the function of CERS4 in liver
cancer, the expression levels of CERS4 in liver cancer tissues and
paired normal liver tissues were evaluated. Firstly, a RT-qPCR
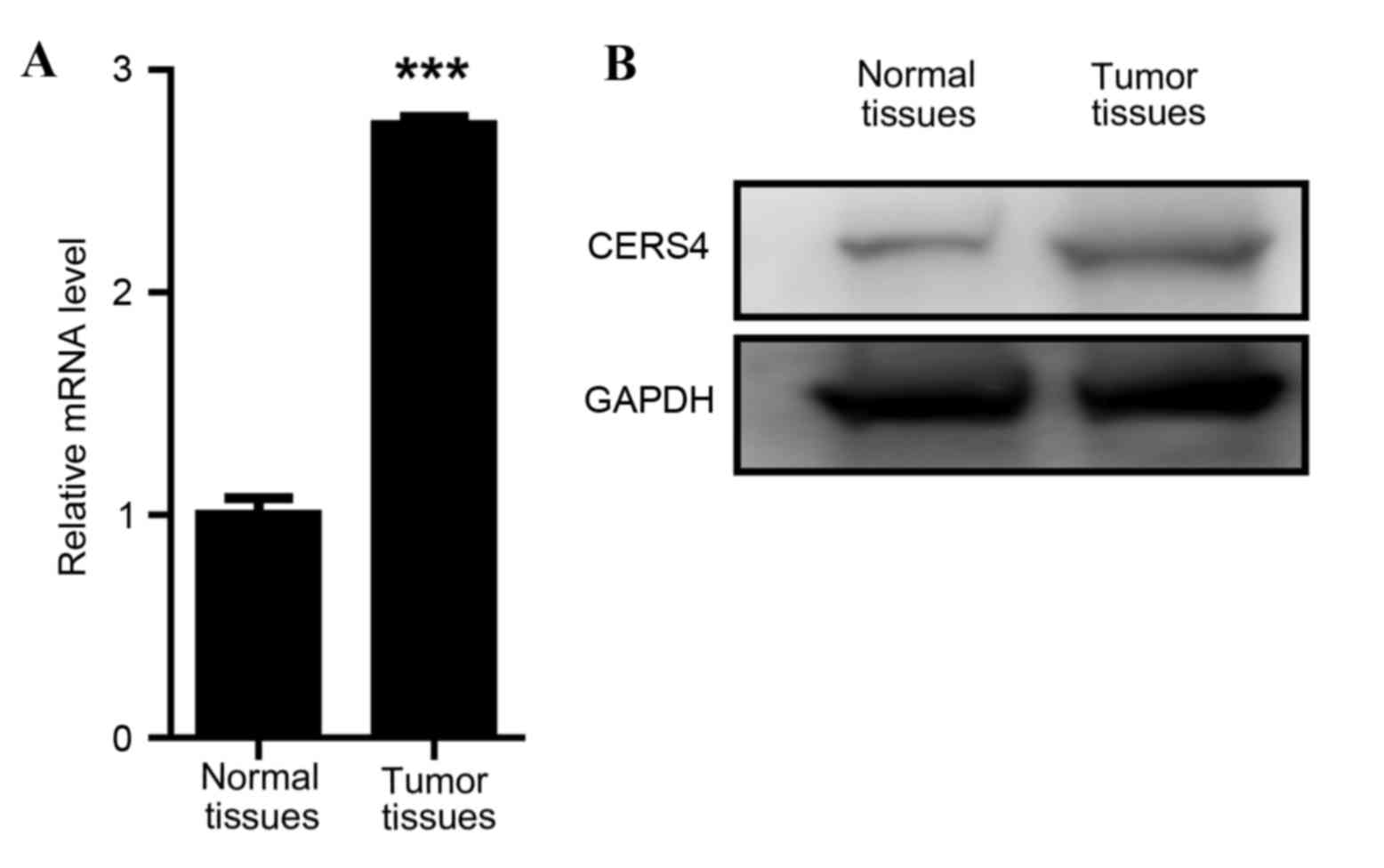
assay was applied to determine the mRNA levels of CERS4. The data
revealed that the mRNA relative level of CERS4 in liver cancer
tissue was ~3 times that of the paired normal liver tissue,
indicating a high expression level of CERS4 in liver cancer tissue
(Fig. 1A). Western blot analysis was
also performed to evaluate the protein expression level of CERS4,
and this reconfirmed that the expression level of CERS4 in liver
cancer tissue was high compared with paired normal liver tissue
(Fig. 1B). The data demonstrated that
CERS4 was highly expressed in liver cancer, indicating that CERS4
may serve an important role in the regulation of liver cancer
cells.
CERS4 is effectively silenced by
lentivirus-mediated RNA interfere (RNAi)
Due to the high expression of CERS4 in liver cancer
tissue, lentivirus-mediated RNAi technology was applied to
knockdown the CERS4 expression in HepG2 and Huh7 liver cancer cell
lines, in order to reveal the function of CERS4 in liver cancer
cells. CERS4 short hairpin RNA (shRNA) targets were cloned into
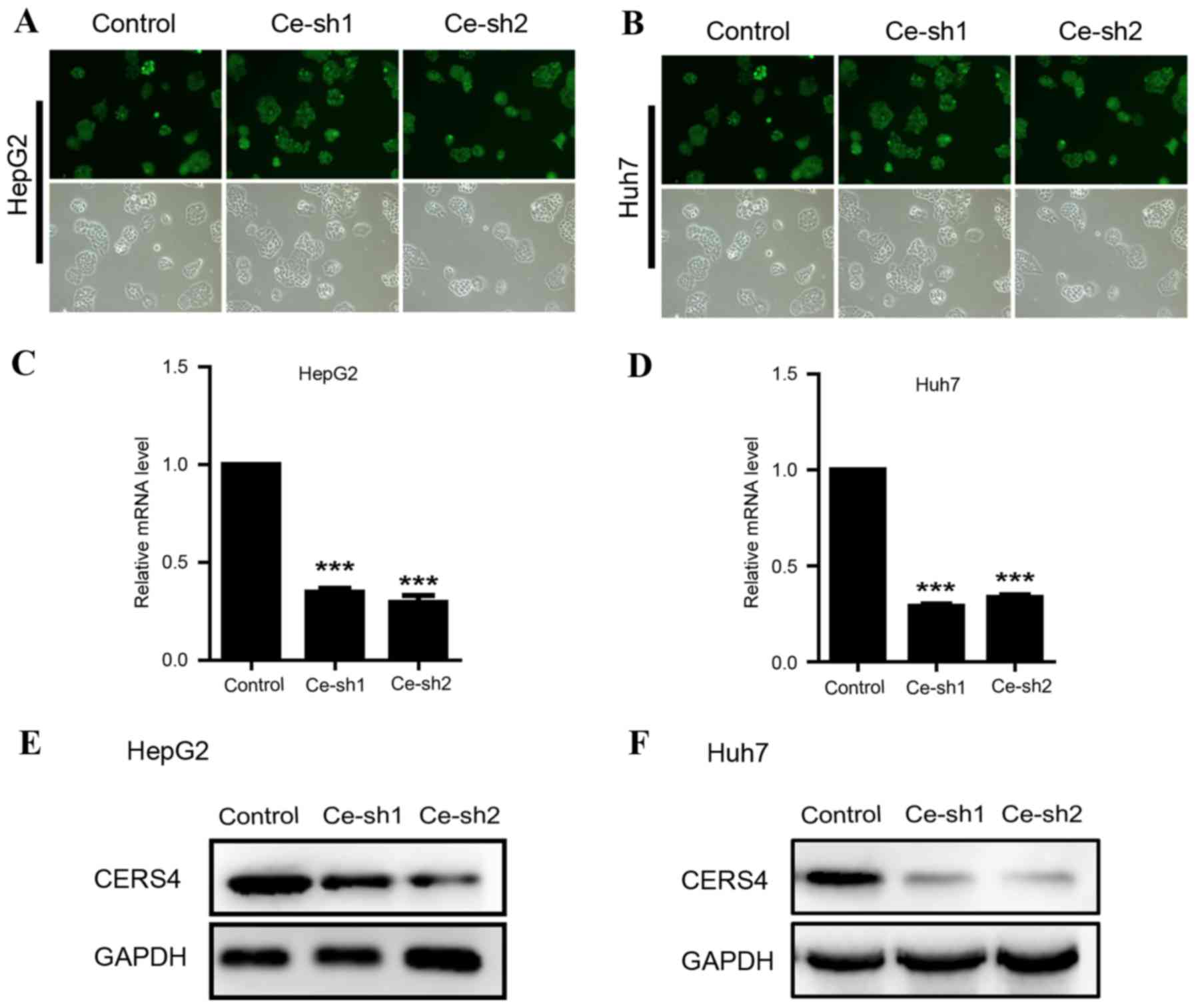
lentivirus vectors and the lentivirus was packaged to infect HepG2
and Huh7 cells. The infecting efficiency was >90%, as assessed
with green fluorescent protein, indicating successful lentivirus
infection in liver cancer cells (Fig. 2A
and B). Quantification analysis by RT-qPCR revealed that
lentivirus-mediated RNAi evidently reduced (P<0.001) CERS4 mRNA
expression levels by 70% in liver cancer HepG2 cells (Fig. 2C). Similarly, CERS4 mRNA levels were
markedly decreased (P<0.001) following lentivirus-mediated RNAi
in liver cancer Huh7 cells (Fig. 2D).
The protein levels of CERS4 were also decreased in HepG2 and Huh7
liver cancer cells infected by CERS4 shRNA lentivirus (Fig. 2E and F). In conclusion, the results
demonstrated that the expression of CERS4 was effectively silenced
by CERS4 shRNA lentivirus in HepG2 and Huh7 liver cancer cells.
CERS4 knockdown inhibits the
proliferation of liver cancer cells
To investigate whether CERS4 affected the
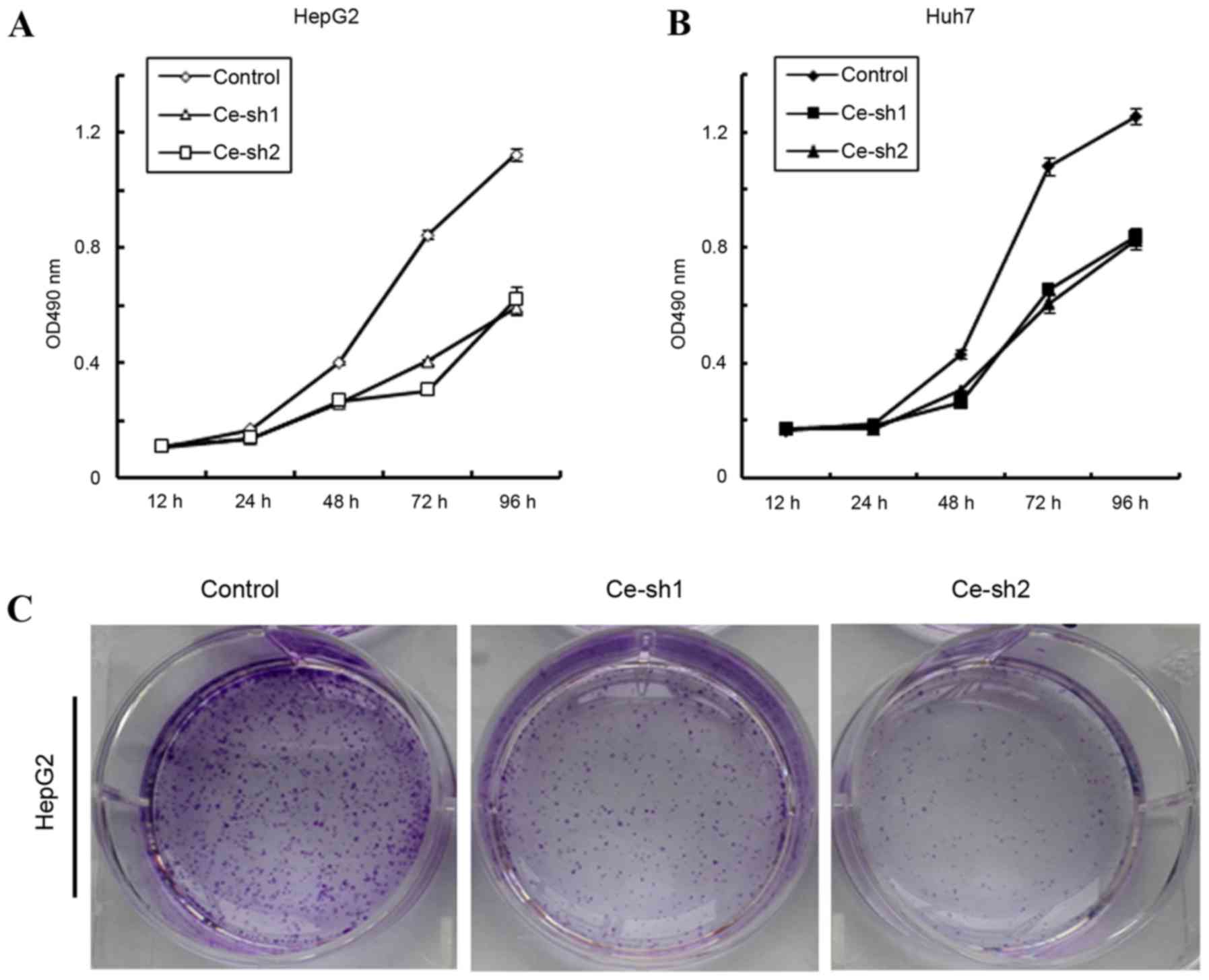
proliferation of liver cancer cells, MTT assay was performed. The
proliferation of HepG2 and Huh7 liver cancer cells was documented
at 12, 24, 48, 72 and 96 h. According to the results, the
proliferation rates of CERS4 silenced HepG2 liver cancer cells were
markedly reduced (P<0.001) compared with that of the scramble
control group (Fig. 3A). For another
established liver cancer cell line, Huh7, the proliferation rates
also decreased markedly (P<0.001) following knockdown of CESR4
(Fig. 3B). Furthermore, the colony
formation assay was also performed to evaluate the effect of CERS4
knockdown on the colony formation ability of HepG2 liver cancer
cells. Compared with the scramble control group, the number of cell
colonies by crystal violet staining in the CERS4 silenced groups
was reduced (Fig. 3C). Therefore, the
present results indicated that CERS4 performs an important role in
the regulation of liver cancer cells proliferation.
CERS4 suppression affects the cell
cycle of liver cancer cells
As CERS4 performed an important role in the
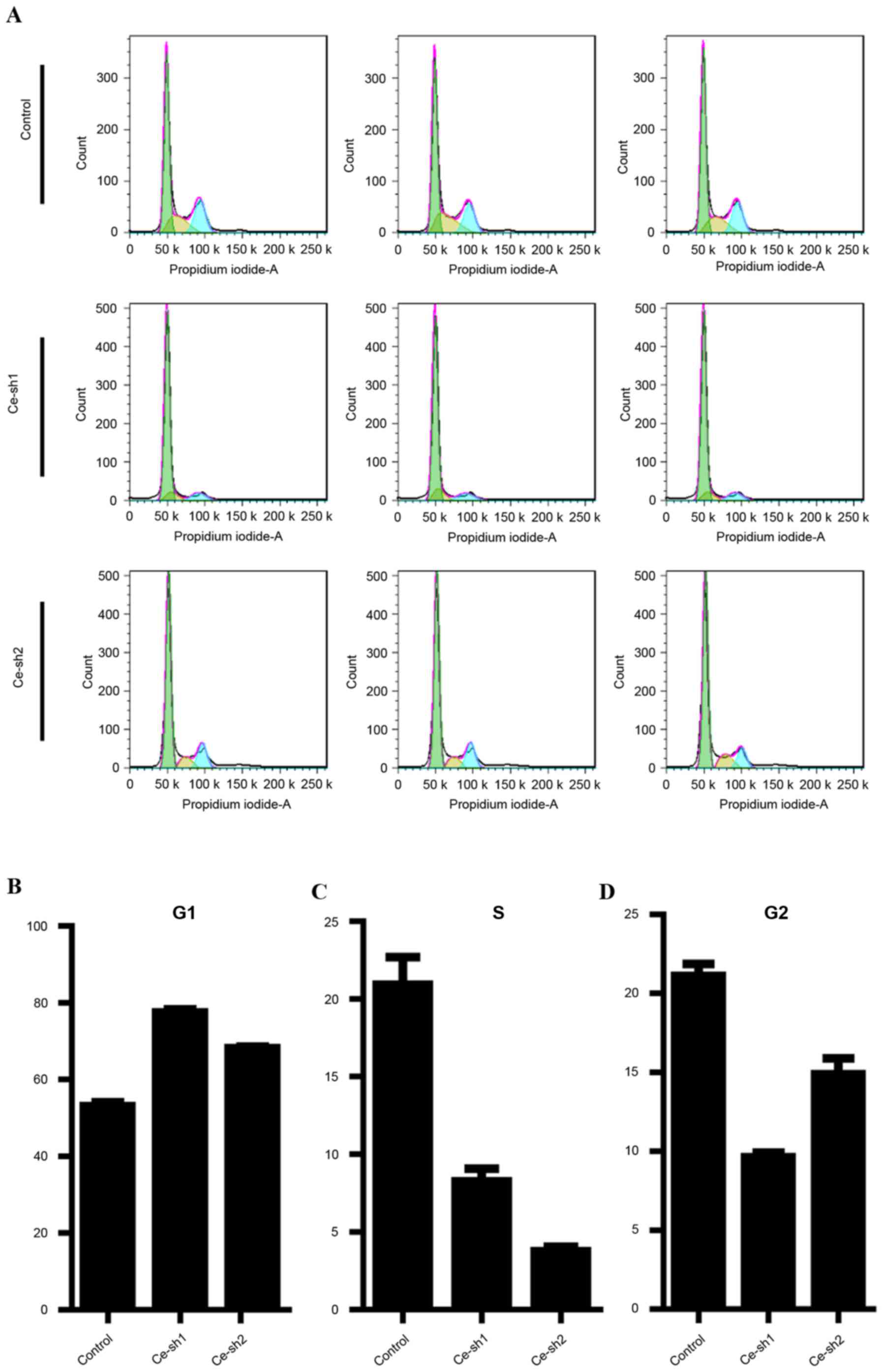
regulation of liver cancer cells proliferation, flow cytometry was
performed to analyze the cell cycle distribution of the HepG2 liver
cancer cells following CERS4 shRNA lentivirus infection (Fig. 4A). In the G0/G1 phase of the cell
cycle, a higher percentage of cells accumulated following
suppression of CERS4 by lentivirus-mediated RNAi compared with the
scramble control group (Fig. 4B).
Correspondingly, the percentage of cells in the S phase was
decreased following lentivirus infection (Fig. 4C). Similarly, in the G2/M phase of
cell cycle, the percentage of cells was also reduced following
silencing of CERS4, indicating a G0/G1 phase arrest subsequent to
depletion of CERS4 (Fig. 4D). The
results revealed that knockdown of CERS4 suppressed the growth of
liver cancer cells, possibly via induction of cell cycle
arrest.
Silencing of CERS4 suppresses the
development of liver cancer in vivo
The present results demonstrated that the
proliferation rate was inhibited subsequent to the expression of
CERS4 being silenced by lentivirus-mediated RNAi technology in
vitro. Therefore, an in vivo study using tumor-bearing
nude mice models was then performed to determine whether silence of
CERS4 suppresses the development of liver cancer in vivo.
Tumors were generated by injecting different groups of HepG2 liver
cancer cells, including the scramble control group, Ce-sh1 group
and Ce-sh2 group into subcutaneous tissues of Balb/c nude mice. The
mice were sacrificed by cervical dislocation and the solid tumors
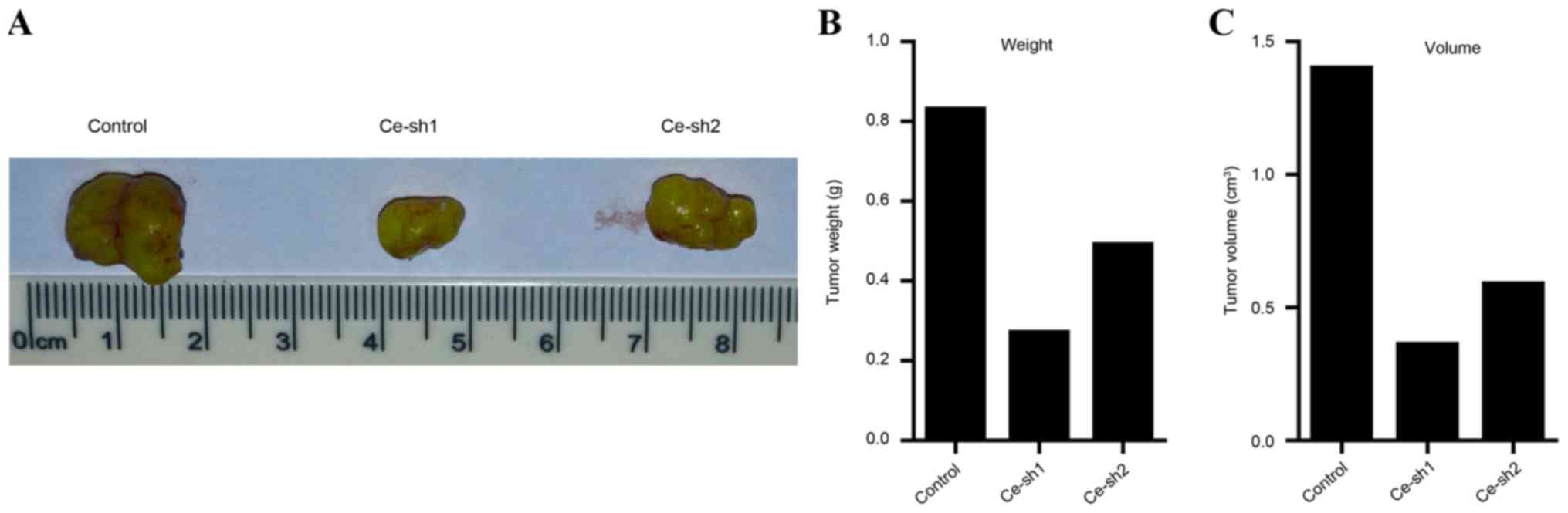
were removed and arranged (Fig. 5A).
Quantification analysis of the weight of tumors suggested that the
weights of the Ce-sh1 and Ce-sh2 groups were decreased compared
with the scramble control group (Fig.
5B). Additionally, the volume of tumors was also measured and
the results demonstrated that the volume of tumors was reduced
following silencing of CERS4 (Fig.
5C). The present results revealed that CERS4 depletion may
suppress the development of liver cancer in vivo.
CERS4 regulates the NF-κB signaling
pathway
In an effort to reveal the essential molecular
mechanism involved in the inhibition of liver cancer cells
proliferation in vitro, and attenuation of tumor development
in vivo induced by CERS4 silencing, a number of known key
genes that perform important roles in regulating cancer cell
proliferation, including P53, Gsk-3β, β-catenin, Ikbkg and Tank
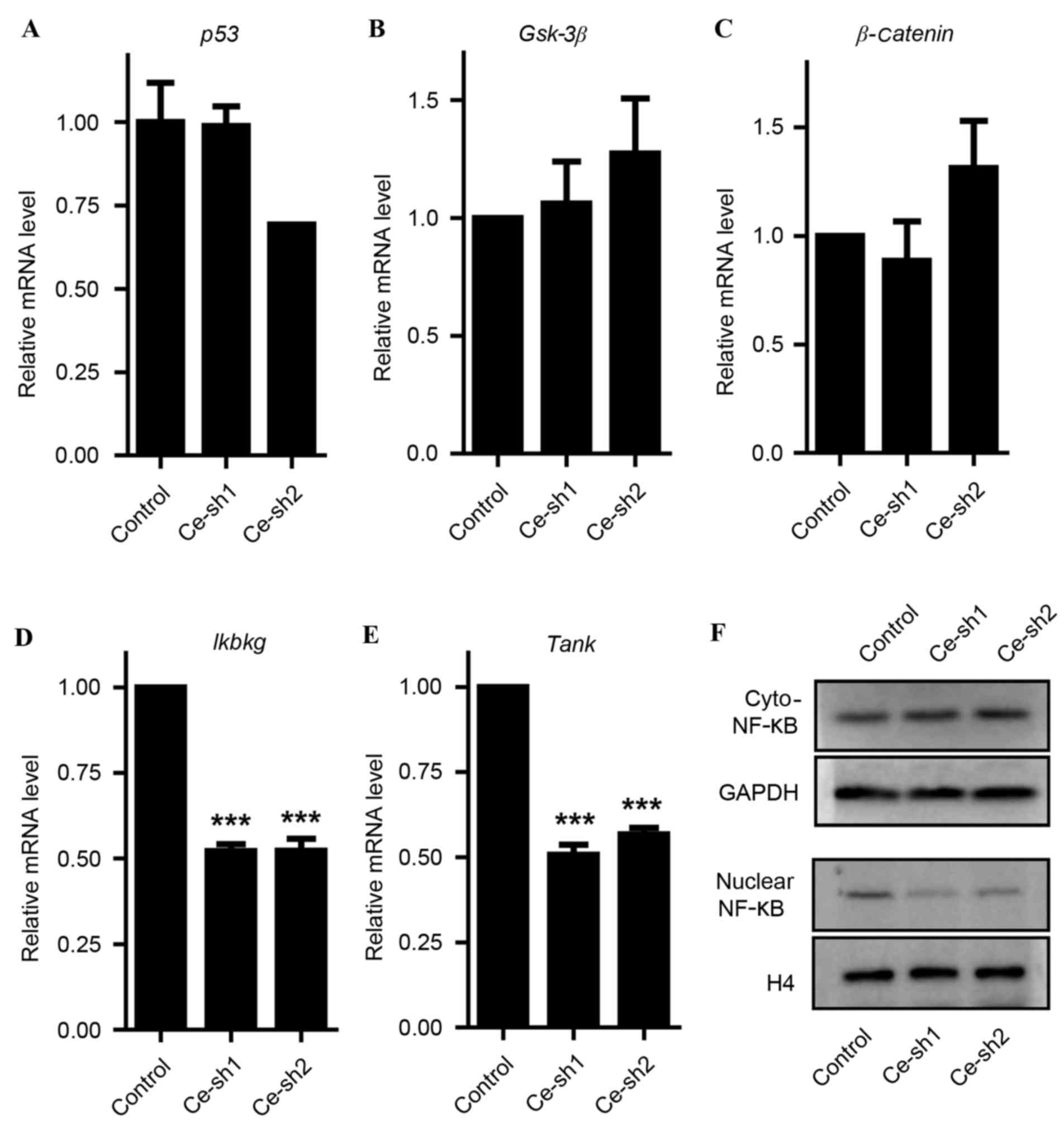
were assessed. Through RT-qPCR analysis, no significant effects of
CERS4 knockdown on P53 and the key genes of the Wnt signaling
pathway (Gsk-3β and β-catenin1) were observed (Fig. 6A-C). However, the mRNA levels of Ikbkg
and Tank involved in the NF-κB signaling pathway decreased
dramatically (P<0.001) subsequent to silencing of CERS4
(Fig. 6D and E). Western blot
analysis reconfirmed that CERS4 knockdown gained the protein levels
of NF-κB in the cytoplasm, but reduced NF-κB in the cell nucleus
(Fig. 6F). These results indicated
that silencing of CERS4 had an essential effect on the NF-κB
signaling pathway in liver cancer cells.
Discussion
CERS4 is an important enzyme that is critical for
ceramide synthase (20). In the
present study, CERS4 was revealed to facilitate HCC formation. The
mRNA and protein expression levels of CERS4 were higher in HCC
tissues compared with normal tissues. Additionally, knockdown of
CERS4 suppressed liver cancer cells proliferation in vivo
and tumor growth in vitro.
Sphingolipids, particularly ceramide, are structural
components of biological membranes. The balance of the key enzymes
in these syntheses not only contributes to the membrane formation,
but also affects numerous cellular processes, including cell
growth, proliferation, differentiation and motility (21). The present results demonstrated that
CERS4 is involved in HCC cell proliferation, which lent support to
the hypothesis. However, the function of CERS4 in cellular
processes remains largely unknown and requires further
investigation.
The NF-κB signaling pathway is extensively involved
in cell proliferation and the development of tumor formation. The
present study revealed that CERS4 affected the NF-κB signaling
pathway. Following CERS4 knockdown, the mRNA expression levels of
Ikbkg and Tank (which were important components of NF-κB signaling)
were downregulated, indicating thatCERS4 is a regulator of NF-κB
signaling. In addition, protein level detection demonstrated that
CERS4 knockdown gained the protein levels of NF-κB in cytoplasm,
but reduced NF-κB in cell nucleus. This reconfirmed that CERS4
performs an important role in regulating NF-κB signaling. In
addition, the mRNA levels of other important cell proliferation
regulators, including P53 and the key genes of Wnt signaling
pathway (Gsk-3β and β-catenin), were also detected. However,
silencing of CERS4 in liver cancer cells did not affect the
expression of P53, Gsk-3β or β-catenin. Although the present study
has provided supporting evidence that CERS4 promotes HCC cell
proliferation by regulating NF-κB signaling, but not Wnt/β-catenin
signaling, other mechanisms may also be involved in the process,
and this requires additional investigation.
In summary, the present study unravels the function
of CERS4 and illustrates the molecular mechanisms by which CERS4 is
involved in HCC cell proliferation. The present finding that CERS4
functions as an important regulator of HCC development maybe a
potential marker for liver tumors and may also facilitate the
utility of the precision medicine in HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31300674 and
31371325).
Glossary
Abbreviations
Abbreviations:
|
CERS4
|
ceramide synthase-4
|
|
RNAi
|
RNA interfere
|
|
HCC
|
hepatocellular carcinoma
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Huang L, Liu CF, Cao J, Yan JJ, Xu
F, Wu MC and Yan YQ: Risk factors and surgical outcomes for
spontaneous rupture of BCLC stages A and B hepatocellular
carcinoma: A case-control study. World J Gastroenterol.
20:9121–9127. 2014.PubMed/NCBI
|
|
4
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teoh NC: Proliferative drive and liver
carcinogenesis: Too much of a good thing? J Gastroenterol Hepatol.
24:1817–1825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI
|
|
9
|
Greten FR and Karin M: The IKK/NF-kappaB
activation pathway-a target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deberardinis RJ, Sayed N, Ditsworth D and
Thompson CB: Brick by brick: Metabolism and tumor cell growth. Curr
Opin Genet Dev. 18:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lingwood D and Simons K: Lipid rafts as a
membrane-organizing principle. Science. 327:46–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simons K and Ikonen E: Functional rafts in
cell membranes. Nature. 387:569–572. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pettus BJ, Chalfant CE and Hannun YA:
Ceramide in apoptosis: An overview and current perspectives.
Biochim Biophys Acta. 1585:114–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizutani Y, Mitsutake S, Tsuji K, Kihara A
and Igarashi Y: Ceramide biosynthesis in keratinocyte and its role
in skin function. Biochimie. 91:784–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reynolds CP, Maurer BJ and Kolesnick RN:
Ceramide synthesis and metabolism as a target for cancer therapy.
Cancer Lett. 206:169–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White-Gilbertson S, Mullen T, Senkal C, Lu
P, Ogretmen B, Obeid L and Voelkel-Johnson C: Ceramide synthase 6
modulates TRAIL sensitivity and nuclear translocation of active
caspase-3 in colon cancer cells. Oncogene. 28:1132–1141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmitggen TD: Analysis of
relative gene expression data using real-time quantitiative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schiffmann S, Sandner J, Birod K, Wobst I,
Angioni C, Ruckhäberle E, Kaufmann M, Ackermann H, Lötsch J,
Schmidt H, et al: Ceramide synthases and ceramide levels are
increased in breast cancer tissue. Carcinogenesis. 30:745–752.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reynolds CP, Maurer BJ and Kolesnick RN:
Ceramide synthesis and metabolism as a target for cancer therapy.
Cancer Lett. 206:169–180. 2004. View Article : Google Scholar : PubMed/NCBI
|