Introduction
Telomerase reverse transcriptase (TERT), known as
hTERT in humans, is the catalytic component of telomerase, a type
of nuclear reverse transcriptase responsible for telomere extension
in cells (1). A previous study
demonstrated that abnormal expression of hTERT leads to the
progressive shortening of telomeres, which could induce cell
immortalization and malignant tumor growth (2). The function of hTERT in vivo and
in vitro was studied directly by cloning the open reading
frame (ORF) of the target gene into expression vectors with
different protein tags, a technique used in a previous gene
function study (3).
In the current study, ORF expression cloning
technology was used with the TERT gene as a target to design and
construct a TERT-ORF clone vector and produce virus particles with
the ability to express TERT through viral packaging. The effects of
TERT on fibrosarcoma in vitro were studied.
Materials and methods
Cell lines and cell culture
Human fibrosarcoma HT1080 cells were purchased from
the American Type Culture Collection (Manassas, VA, USA) and were
cultured in Minimal Essential Medium supplemented with 10% fetal
bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C with 5% CO2. Lenti-Pac 293Ta cells (GeneCopoeia,
Inc., Rockville, MY, USA) cultured using Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum and
penicillin/streptomycin in the same conditions. Human non-small
cell lung carcinoma cell line H1299 cells (American Type Culture
Collection) were cultured with RPMI-1640 supplemented with 10%
fetal bovine serum and penicillin/streptomycin, also in the same
conditions.
Transduction of the Lv130 human
immunodeficiency virus (HIV) lentiviral vector
A total of 10 ng of Lv130 HIV lentiviral vector
(GeneCopoeia China Inc., Guangzhou, China) was added to 100 µl
competent Escherichia coli cells (cat no., C7373-03;
Invitrogen; Thermo Fisher Scientific, Inc.) and incubated on ice
for 30 min, then immediately transferred to a 42°C water bath and
heat shocked for 60 sec, then transferred to ice for 2 min. Super
optimal culture medium (200 µl; Sigma-Aldrich China, Beijing,
China) was added and the sample was incubated at 37°C with
agitation for 1 h at 200 rev/min, then 100 µl was cultured on an LB
plate with ampicillin at 37°C overnight. The plasmid was isolated
with a Plasmid Miniprep kit (cat. no., PFM250; Sigma-Aldrich China)
according to the manufacturer's protocol. Restriction endonuclease
digestion and 1% agarose gel electrophoresis with ethidium bromide
were performed to confirm that the TERT sequence had been
incorporated into the plasmid.
Construction of the recombinant
plasmid with lentivirus vector and TERT gene DNA
The TERT gene was obtained by polymerase chain
reaction (PCR) analysis. The total RNA of nude mice brain (supplied
by Laboratory Animal Center of the Harbin Medical University,
Harbin, China) was isolated with RNeasy Plus Universal kits [cat.
no. 73404; QIAGEN (Suzhou) Translational Medicine Co., Ltd.,
Suzhou, China] according to the manufacturer's protocol. Primers
were designed according to the mouse TERT gene sequences in
GenBank. Primer sequences for TERT were as follows: Forward,
5′-GCGGTAGGCGTGTACGGT-3′ and reverse, 5′-CGATCTCGAACTCGTGGC-3′.
Primers contained restriction sites for NheI and
Bsp119I. Reverse transcription (RT)-PCR was performed with a
One Step RT-PCR kit [cat. no. 210212; QIAGEN (Suzhou) Translational
Medicine Co., Ltd.]. The RT-PCR product (TERT gene) and the
lentiviral vector were digested with NheI and Bsp119I
restriction endonucleases. The products of enzyme digestion were
purified with a QIAquick Gel Extraction kit [cat. no. 28704; QIAGEN
(Suzhou) Translational Medicine Co., Ltd.] and ligated with T4 DNA
ligase to construct the recombinant plasmid.
Packaging of the virus
Cells at a confluence of 70–80% were used for
transfection. The lentiviral packaging plasmid mixture (including
0.5 µg recombinant plasmid and 1 µl virus) was co-transfected into
293Ta cells and H1299 cells, which were incubated at room
temperature for 25 min, then added into 6-well plates and cultured
at 37°C with 5% CO2. The medium containing transfection
mixture residues was discarded and fresh medium was added following
12 h incubation. The cell supernatant was collected after 48 h by
centrifugation (1,000 × g at 4°C for 5 min), then a 0.45 µm
polyvinylidene fluoride (PVDF) film was used to filter and harvest
the packaged virus particles.
Overexpression of TERT
The recombinant lentiviral overexpression vector was
transfected into HT1080 cells using Lipofectamine 2000 (cat. no.
11668027; Thermo Fisher Scientific Inc.), according to the
manufacturer's protocol.
RNA interference (RNAi) of TERT
HT1080 cells at confluence of 70–80% were used for
RNAi. TERT-siRNA: AATCAGACAGCACTTGAAGAGGG was used for transfection
of HT1080 cells using Lipofectamine 2000. Fluorescent images were
captured following culture for 48 h to observe the growth status of
the cells. Five images of each culture well were captured in the
visual fields located in the center, upper, lower, left and right
of the well.
RT-quantitative(q) PCR
Total RNA was extracted from cells subjected to the
knockdown or overexpression of TERT after 72 h, using the RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol. Total RNA (1 µg) was subjected to reverse
transcription using a reverse transcription system (cat. no. A3500;
Promega Corporation, Madison, WI, USA). qPCR was performed using
SYBR-Green PCR Master Mix (Promega Corporation). Thermocycler
conditions were as follows: 95°C for 45 sec; 95°C for 5 sec; and
60°C for 30 sec, for 40 cycles. Primers used were as follows: p53
forward, 5′-GCCATGGCCATCTACAAG-3′ and reverse,
5′-CCTTCCACCCGGATAAGAT-3′; survivin forward,
5′-TTCAAGAACTGGCCCTTC-3′ and reverse, 5′-CCTTAAAGCAGAAAAAACACTG-3′;
caspase-3 forward, 5′-TTCTTCAGAGGCGACTACT-3′ and reverse,
5′-TCCCACTGTCTGTCTCAAT-3′; and caspase-7 forward,
5′-TCTTTGCTTACTCCACGGTT-3′ and reverse, 5′-ACCCTGGTCAGGATCTGCAT-3′;
GAPDH (internal control) forward, 5′-TGTGGGCATCAATGGATTTGG-3′ and
reverse, 5′-ACACCATGTATTCCGGGTCAAT-3′. The results were quantified
using the 2−ΔΔCq method (4).
Detection of the expression of protein
kinase B (PKB) with western blotting
Briefly, cells that had been subjected to the
knockdown or overexpression of TERT were lysed 72 h after RNAi in
lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA and protease
inhibitor at 4°C for 2 h. Total protein (50 µg) was separated by
15% SDS-PAGE and transferred to a PVDF membrane. The membrane was
incubated with primary antibodies against PKB (dilution, 1:1,000;
United States Biological, Salem, MA, USA) at 4°C overnight. The
secondary antibodies were added at a dilution of 1:1,000, incubated
at RT for 2 h and the bands were stained with DAB. They were
quantified using β-actin (dilution, 1:1,000; antibody cat. no.
143128; United States Biological) as the control. The protein bands
were quantified using Image J software (version k 1.45; National
Institutes of Health, Bethesda, MA, USA; https://imagej.en.softonic.com/).
Statistical analysis
Statistical analysis was performed using the SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA) and a one-way
analysis of variance was conducted for the comparison between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Successful construction of the
recombinant plasmid with the lentivirus vector and TERT gene
DNA
The recombinant plasmid was isolated and confirmed
to possess the ORF of the TERT gene by enzymatic digestion with
restriction endonucleases NheI and Bsp119I, and
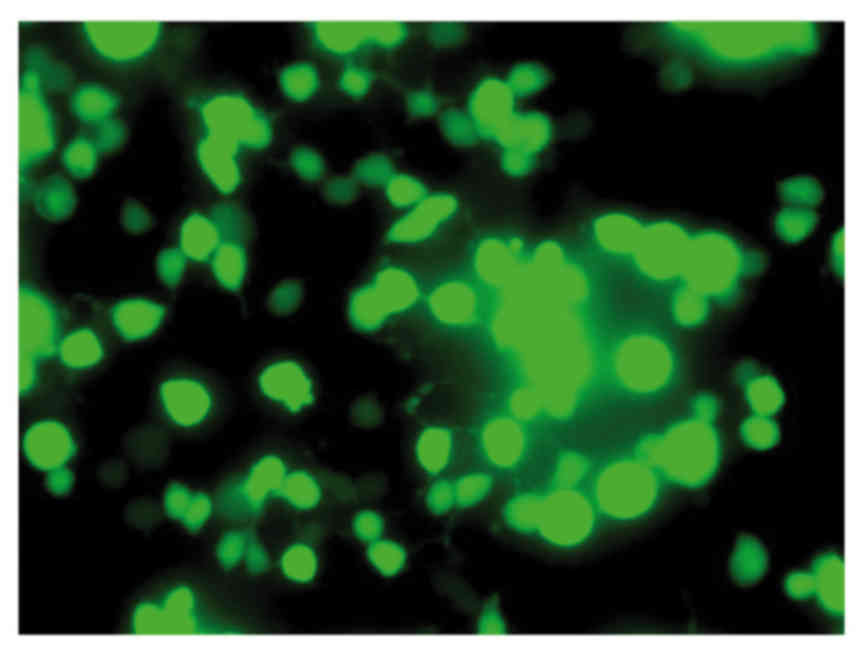
sequencing of the plasmid (data not shown). Green fluorescence was
observed in viable HT1080 cells following transfection with the
recombinant TERT plasmid (Fig. 1),
confirming expression of the plasmid.
Survivin and PKB expression decreases
but caspase-3 expression increases following TERT knockdown
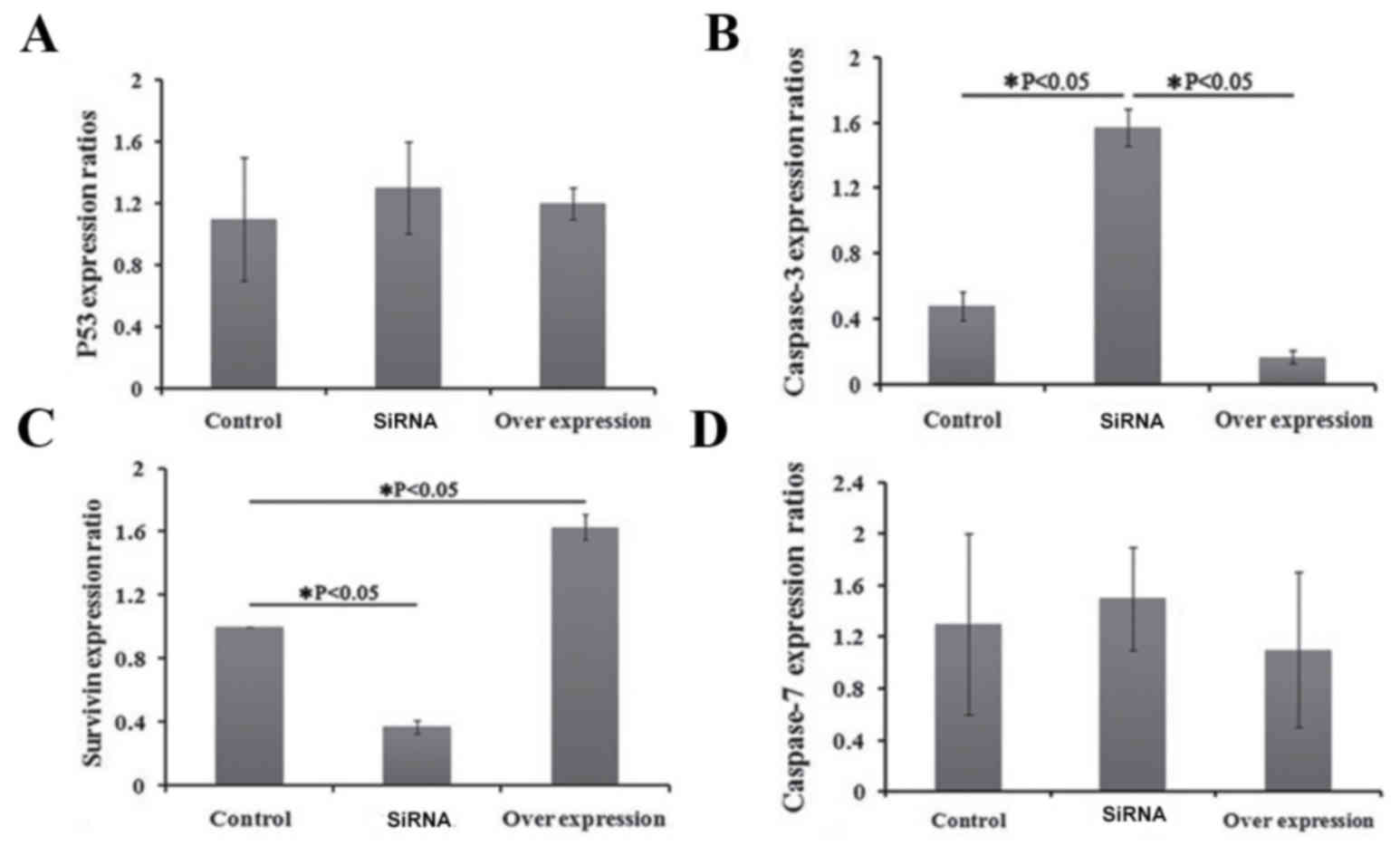
The effects of recombinant plasmid transfection on
the expression levels of p53, survivin, caspase-3 and caspase-7 in
HT1080 cells were detected by RT-qPCR. There was no significant
difference in the expression levels of p53 and caspase-7 genes
prior to and following TERT knockdown (P>0.05; Fig. 2). However, the expression levels of
survivin decreased significantly and the expression levels of
caspase-3 increased significantly following TERT knockdown
(P<0.05; Fig. 2). The expression
levels of survivin increased significantly and the expression
levels of caspase-3 decreased significantly following TERT
overexpression (Fig. 2).
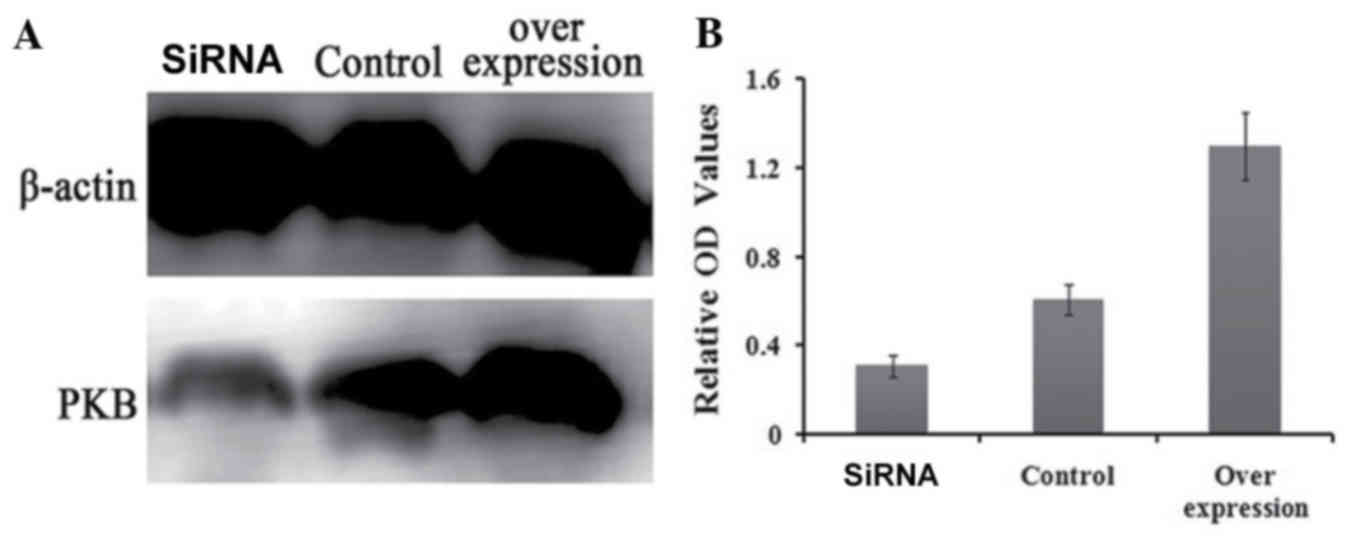
Western blotting analysis revealed that the
expression of PKB decreased significantly at the protein level
following TERT knockdown (P<0.05), while it increased
significantly when TERT was overexpressed (P<0.05; Fig. 3).
Discussion
The biological role of TERT is to add telomeric DNA
to the end of eukaryotic chromosomal DNA to ensure the activity of
cells (5). Telomeres serve an
important role in maintaining cellular chromosome stability and
activity in the cells of various species (6). In the present study, TERT overexpression
significantly promoted the growth of HT1080 cells, while TERT
knockdown significantly inhibited growth. TERT affects cell growth,
as it regulates the expression of telomerase, which affects
telomere length, and the length of telomeres can affect the cell
growth state (7–9). Previous studies have demonstrated that
TERT knockdown inhibits the growth of bladder cancer and prostate
cancer (2,10). These results suggest that the TERT
gene can control fibrosarcoma growth.
Previous studies have identified that TERT inhibits
cell apoptosis (11,12), which could account for the increased
tumor growth observed following TERT overexpression. In the current
study, the expression levels of caspase-3, caspase-7, survivin and
p53 were measured prior to and following TERT knockdown. TERT
knockdown did not significantly affect the expression of caspase-7
or p53; however, it significantly affected the expression of
caspase-3 and survivin. Survivin has been demonstrated to promote
cell survival (13), whereas
caspase-3 promotes apoptosis (14).
In the present study, TERT overexpression and knockdown
significantly decreased and increased caspase-3 expression,
respectively, indicating that TERT may serve a role in the growth
of fibrosarcoma through caspase-3 and survivin.
PKB is a signalling molecule that regulates cell
apoptosis and survival, and serves an important role in wound
repair (15,16). In the present study, TERT was
identified to regulate the expression of PKB. Previous studies have
demonstrated that the PKB signaling pathway is widely associated
with cell apoptosis, survival, growth and protein synthesis
(17–20). Signal transmission in the cytoplasm
through PKB promotes cell cycle progression by glycogen synthase
kinase-3 β (21), and accelerates
cell apoptosis through the inhibition of p21 or p53 (22). The results of the present study
suggest that TERT affects the growth of fibrosarcoma via cell
survival and apoptosis through the PKB signalling pathway.
The present study established a fibrosarcoma cell
model overexpressing TERT. In this cell line, the effects of TERT
on cell growth appeared to be mediated through the survivin,
caspase-3 and PKB signalling pathways. These results provide
important information for the application of hTERT-targeted
therapies for the treatment of fibrosarcoma and similar
malignancies, such as giant cell bone tumors.
References
|
1
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandellini P, Folini M, Bandiera R, De
Cesare M, Binda M, Veronese S, Daidone MG, Zunino F and Zaffaroni
N: Down-regulation of human telomerase reverse transcriptase
through specific activation of RNAi pathway quickly results in
cancer cell growth impairment. Biochem Pharmacol. 73:1703–1714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hastie ND, Dempster M, Dunlop MG, Thompson
AM, Green DK and Allshire RC: Telomere reduction in human
colorectal carcinoma and with ageing. Nature. 346:866–868. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McFadden N, Bailey D, Carrara G, Benson A,
Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P,
Macdonald A and Goodfellow I: Norovirus regulation of the innate
immune response and apoptosis occurs via the product of the
alternative open reading frame 4. PLoS Pathog. 7:e10024132011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folini M, Brambilla C, Villa R, Gandellini
P, Vignati S, Paduano F, Daidone MG and Zaffaroni N: Antisense
oligonucleotide-mediated inhibition of hTERT, but not hTERC,
induces rapid cell growth decline and apoptosis in the absence of
telomere shortening in human prostate cancer cells. Eur J Cancer.
41:624–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cong YS, Wright WE and Shay JW: Human
telomerase and its regulation. Microbiol Mol Biol Rev. 66:407–425.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blackburn EH, Greider CW and Szostak JW:
Telomeres and telomerase: The path from maize, Tetrahymena and
yeast to human cancer and aging. Nat Med. 12:1133–1138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou L, Zhang P, Luo C and Tu Z:
shRNA-targeted hTERT suppress cell proliferation of bladder cancer
by inhibiting telomerase activity. Cancer Chemother Pharmacol.
57:328–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorbunova V, Seluanov A and Pereira-Smith
OM: Expression of human telomerase (hTERT) does not prevent
stress-induced senescence in normal human fibroblasts but protects
the cells from stress-induced apoptosis and necrosis. J Biol Chem.
277:38540–38549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu K, Hodes RJ and Weng Np: Cutting edge:
Telomerase activation in human T lymphocytes does not require
increase in telomerase reverse transcriptase (hTERT) protein but is
associated with hTERT phosphorylation and nuclear translocation. J
Immunol. 166:4826–4830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connor DS, Schechner JS, Adida C, Mesri
M, Rothermel AL, Li F, Nath AK, Pober JS and Altieri DC: Control of
apoptosis during angiogenesis by survivin expression in endothelial
cells. Am J Pathol. 156:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dale-Nagle EA, Satriotomo I and Mitchell
GS: Spinal vascular endothelial growth factor induces phrenic motor
facilitation via extracellular signal-regulated kinase and Akt
signaling. J Neurosci. 31:7682–7690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paterniti I, Esposito E, Mazzon E,
Bramanti P and Cuzzocrea S: Evidence for the role of
PI(3)-kinase-AKT-eNOS signalling pathway in secondary inflammatory
process after spinal cord compression injury in mice. Eur J
Neurosci. 33:1411–1420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gheysarzadeh A and Yazdanparast R:
Inhibition of H2O2-induced cell death through FOXO1 modulation by
EUK-172 in SK-N-MC cells. Eur J Pharmacol. 697:47–52. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HS, Seong KM, Kim JY, Kim CS, Yang
KH, Jin YW and Nam SY: Chronic low-dose radiation inhibits the
cells death by cytotoxic high-dose radiation increasing the level
of AKT and acinus proteins via NF-κB activation. Int J Radiat Biol.
89:371–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang W, Ju JH, Lee KM, Nam K, Oh S and
Shin I: Protein kinase B/Akt1 inhibits autophagy by down-regulating
UVRAG expression. Exp Cell Res. 319:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng X, Huang Z, Mao X, Wang J, Wu G and
Qiao S: N-carbamylglutamate enhances pregnancy outcome in rats
through activation of the PI3K/PKB/mTOR signaling pathway. PLoS
One. 7:e411922012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siraskar B, Völkl J, Ahmed MS, Hierlmeier
M, Gu S, Schmid E, Leibrock C, Föller M, Lang UE and Lang F:
Enhanced catecholamine release in mice expressing PKB/SGK-resistant
GSK3. Pflugers Arch. 462:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vichalkovski A, Gresko E, Hess D,
Restuccia DF and Hemmings BA: PKB/AKT phosphorylation of the
transcription factor Twist-1 at Ser42 inhibits p53 activity in
response to DNA damage. Oncogene. 29:3554–3565. 2010. View Article : Google Scholar : PubMed/NCBI
|