Introduction
Uterine cervical cancer is the third most commonly
diagnosed cancer and the fourth leading cause of cancer-associated
mortality in women globally (1). For
patients with early-stage cervical cancer, surgery remains the
first-choice therapy. Other adjuvant therapies, including
chemotherapy and radiotherapy, are also required for late-stage
patients and occasionally for postoperative early-stage patients.
The aim of radiation therapy is to deliver a high therapeutic dose
of ionizing radiation to the tumor within the tolerance of normal
tissue. Radiotherapy of uterine cervical cancer covers all cells
within its radiation field, affecting cancer cells and normal
tissue, particularly the pelvic organs. As traditional strategies
lack targeting specificity, the study of novel agents to enhance
therapeutic sensitivity is urgently required.
Gold-based nanomaterials have emerged as highly
effective platforms for theranostic agents (2). An in vitro study in bovine aortic
endothelial cells confirmed that it is possible to use gold
nanoparticles to enhance the radiation dose to the cells in the
kilovoltage range of X-ray beams in order to reduce the risk of
side effects from superficial X-ray treatments (3). A previous study also demonstrated that
gold nanoparticles increase the cytotoxicity of radiation in MCF-7
cells and decrease the local damage to normal tissue surrounding
the breast cancer tissue (4).
Previous studies have demonstrated that the degree
of radiosensitization was dependent on the average number of gold
nanoparticles internalized within the cells (5). A major challenge of the present study
was to identify suitable tumor-specific biomarkers to conjugate to
gold nanoparticles to achieve targeted delivery. MET
proto-oncogene, receptor tyrosine kinase (c-Met) is the receptor
for hepatocyte growth factor (HGF) (6). c-Met overexpression is associated with
the proliferation, invasion and metastasis of cancer cells
(7). In the study by Baykal et
al (8), overexpression of c-Met
was observed in 59.6% of invasive cervical carcinoma specimens.
According to Kaplan-Meier univariate survival analysis and
multivariate Cox regression analysis, the overexpression of c-Met
is an independent variable for disease-free survival (8).
In the present study, HGNs with a diameter of ~56 nm
were synthesized. Polyethylene glycol (PEG), which densely coats
the HGN surface in order to reduce non-specific binding, is a
neutral polymer (9); pegylation is a
modification that imparts functionality onto the HGN, decreasing
immunogenicity and the clearance rate (10). PEG complexes carrying different
functional groups (carboxyl and sulfhydryl) readily conjugate to
the gold surface through covalent bonds using thiol-terminated
compounds, and combine to targeting antibodies by forming amide
bonds. The present study investigated whether anti-c-Met/HGNs
enhanced cytotoxicity on cervical cancer cells undergoing X-ray
radiation therapy in vitro, and the underlying mechanisms
were studied.
Materials and methods
Synthesis of HGNs
A total of 180 mg silver nitrate (Aladdin Chemistry
Co., Ltd. Shanghai, China) was dissolved in 100 ml deionized water
under rigorous stirring. High-purity nitrogen was then passed into
the solution to create a nitrogen atmosphere which was maintained
during the whole reaction. Following rapid heating to boiling
point, 1 ml 1% sodium citrate (Aladdin Chemistry Co., Ltd.) was
added to the reaction solution and the solution was heated to boil
for another 30 min, following which the solution was cooled to
ambient temperature. Hollow gold nanospheres were prepared using
the galvanic replacement reaction between HAuCl4 and
colloidal silver in an aqueous solution under refluxing conditions,
as follows. A total of 10 ml silver colloid was added to a 50 ml
flask under magnetic stirring and then heated to 60°C. Meanwhile,
an aqueous solution of 5 ml HAuCl4 (1 mM, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was slowly added to the flask
through a syringe pump at a rate of 45 ml/h under magnetic
stirring. The solution was heated for 30 min and concentrated at
5,000 × g at room temperature for 5 min following this. The HGNs
was characterized by ultraviolet-visible-near infrared (UV-Vis-NIR)
spectrophotometry and transmission electron microscopy (TEM;
JEM-1011; JEOL, Ltd., Tokyo, Japan).
Synthesis of anti-c-Met/HGNs
A total of four sub-steps were involved in
anti-c-Met/HGN synthesis. First, to synthesize PEG Mixtures, 0.017
g methoxy polyethylene glycol carried with sulfhydryl (Shanghai
Yare Biotech, Inc., Shanghai, China) and 0.003 g
carboxymethyl-PEG-thiol (Shanghai Seebio Biotech, Inc., Shanghai,
China) were dissolved in 2 ml double-distilled water. Second, to
synthesize PEG-HGNs, 200 µl 10 nM HGN solution was added to 200 µl
of the previously prepared PEG mixture, and the solution was mixed
at 4°C for 8 h. Third, for functionalization of the carboxyl group,
30 min following the addition of 5 µl
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (40 mg/ml) and
N-hydroxysuccinimide (60 mg/ml) into the aforementioned PEG-HGN
dispersion at room temperature, the solution was concentrated at
9,000 × g at room temperature for 5 min. Next, the supernatant was
discarded and double-distilled water added to a final volume of 200
µl. Fourth, to synthesize anti-c-Met/HGNs, 3 µl anti-c-Met antibody
(cat. no. EP1415Y, Abcam, Cambridge, MA, USA) was added to the
solutions at room temperature for 2 h, then incubated at 4°C for 8
h. The absorbance of anti-c-Met/HGNs was examined by UV-Vis-NIR
spectrophotometry at 300–900 nm wavelength.
Cell line and culture conditions
The human cervical cancer CaSki cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% mycoplasma-free fetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) plus 1%
streptomycin/penicillin (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China). Cells were incubated in a
CO2 incubator (HF240; Shanghai Lishen Scientific
Equipment Co., Ltd., Shanghai, China) under standardized conditions
(37°C, 5% CO2, 100% humidity). The medium was changed
2–3 times a week.
Immunofluorescent staining
CaSki cells were seeded on 15-mm microscope cover
glass slides. Following an overnight incubation under standardized
conditions (37°C, 5% CO2, 100% humidity), cells were
fixed with 4% paraformaldehyde for 15 min, washed three times with
PBS, and cells were blocked with normal goat serum (Bosterbio Co.,
Ltd., Wuhan, China) for 30 min at room temperature. Following this,
the cells were incubated with anti-c-Met antibody (cat. no.
EP1454Y; 1:500 dilution; Abcam) in a wet box overnight at 4°C.
Following three PBS washes, cells were stained with Dylight 488
conjugated goat anti-rabbit IgG (A23220; 1:200 dilution; Abbkine
Scientific Co., Ltd., Redlands, CA, USA) for 1 h in the dark at
room temperature. The nuclei was stained with 100 µl DAPI
(4′,6-diamidino-2-phenylindole) staining solution (1.0 µg/ml
Bosterbio Co., Ltd.) for 3 min at room temperature in the dark. The
fluorescently labeled c-Met was observed and images were captured
with a fluorescence inverted microscope (IX81; Olympus Corporation,
Tokyo, Japan).
Cell uptake of HGNs and
anti-c-Met/HGNs
CaSki cells (200,000) were cultured in 25
cm2 cell culture flasks. The complete RPMI-1640 medium
was replaced with FBS-free RPMI-1640 medium when cells reached 70%
confluence. HGNs alone and anti-c-Met/HGNs were then added at
different intervals (4, 8, 12, 24, 48 and 72 h). The cells were
thoroughly washed with PBS, collected and resuspended into
double-distilled water to a final volume of 1 ml. Cell counting was
performed for each sample using a hemocytometer. A total of 1 ml
aqua regia was dropped into each sample overnight to lyse the
cells. Following this, double-distilled water was added to each
sample to a final volume of 10 ml, the acid was neutralized, the
particles filtered out and the gold mass of each sample was
measured by inductively coupled plasma atomic emission
spectroscopy.
Cytotoxicity of HGNs and
anti-c-Met/HGNs
Cells were seeded in a 96-well culture plate at
~7.5×103 cells per well and incubated at 37°C and 5%
CO2 overnight. The original complete RPMI-1640 medium
was then replaced with FBS-free RPMI-1640 medium containing
different concentrations (ranged from 0.0–3.3 nM) of HGNs and
anti-c-Met/HGNs for 24 h. Following three washes with PBS, fresh
medium containing 10% FBS was added to each well. Cell viability
was measured using a Cell Counting Kit-8 (CCK-8; Nanjing Enogene
Biotech Co., Ltd., Nanjing, China) assay based on the optical
density value of the cells in each well at a 450 nm wavelength.
Next, 10 µl of CCK-8 reagent was added into each well and cells
were incubated for an additional 2 h at 37°C. The results were
determined using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) measuring the absorbance at 450 nm. The cell
viability of each group was calculated using GraphPad Prism version
7.0 (GraphPad Software, Inc., La Jolla, CA, USA). The experiment
was performed in triplicate.
Cell cycle assay
Subsequent to culturing with anti-c-Met/HGNs for 24
h, cells were fixed using 70% cold ethanol overnight at 4°C.
Following two washes with cold PBS, cells were resuspended and
treated with 10 µg/ml RNase for 30 min at 37°C, cells were stained
with 200 µl propidium iodide (PI; 50 µg/ml) for 30 min at 4°C.
Analysis was performed using a FACS Calibur flow cytometer (BD
Biosciences, San Jose, CA, USA). Experiments were performed in
triplicate. For each sample, 5,000 cells were measured. The data on
cellular DNA content and cell cycle were analyzed using FlowJo
v.10.0 software (Tree Star, Inc., Ashland, OR, USA).
Radiation cytotoxicity assay
Cells were seeded and incubated as aforementioned.
Cells were incubated with HGNs and anti-c-Met/HGNs at laddered
concentrations. An equal volume of RPMI 1640 medium was added to
the control group. After 24 h, cells were washed twice with PBS,
and fresh medium with 10% FBS was added. The cells were then
divided into three groups: Control, HGNs and anti-c-Met/HGNs. Cells
were irradiated with 6 MeV X-rays from a medical electron linear
accelerator (Primus; Siemens AG, Munich, Germany) at various
radiation doses (0, 2.5, 5, 7.5 and 10 Gy). The distance between
the source and the cells was 25 cm. Following irradiation, cells
were incubated at 37°C until they were analyzed. Cell viability was
measured 8 h following irradiation using the aforementioned CCK-8
assay. All experiments were performed in triplicate.
Cell apoptosis assay
Cell apoptosis analysis was measured by flow
cytometry using a Fluorescein Isothiocyanate (FITC) Annexin V
Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA)
according to the manufacturer's protocol. Cells were seeded in a
6-well plate and treated with 5 Gy radiation using a 6 MeV X-ray.
After 3 h, cells were harvested using EDTA-free trypsin and
resuspended in 100 µl 1x binding buffer (diluted 10x binding buffer
from the kit with ddH2O to 1x binding buffer). Following
the adjustment of cell numbers in each sample to an appropriate
density, 5 µl FITC Annexin V and 5 µl PI were added. Another 200 µl
binding buffer was added following incubation for 15 min at room
temperature in the dark. Flow cytometry was conducted on a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA)
immediately following this. Data analysis was performed using
FlowJo v.10.0 software (Tree Star, Inc.). Experiments were
conducted in triplicate.
Western blot analysis
Cells were cultured for 48 h following irradiation
and the cell proteins of each sample were then extracted using a
Total Protein Extraction kit (Beyotime Institute of Biotechnology,
Haimen, China) in accordance with the manufacturer's protocol. An
equivalent quality of total protein (20 µg) in each sample was
loaded and electrophoresed by 15% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. Following blocking with 5% w/v
skimmed milk powder for 1 h at room temperature, blots were
incubated with antibodies against caspase-3 (cat. no. 9665), B-cell
lymphoma 2 (Bcl-2; cat. no. 4223) and Bcl-associated X, apoptosis
regulator (Bax; cat. no. 5023; Cell Signaling Technology, Inc.,
Danvers, MA, USA; 1:1,000 dilution) overnight at 4°C. The internal
control was GAPDH (cat. no. 2118; Cell Signaling Technology, Inc.,
Danvers, MA, USA; 1:1,000 dilution). Protein-bound membranes were
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (cat. no. 7074; Cell Signaling
Technology, Beverly, MA, USA; 1:2,000 dilution) for 1 h at room
temperature. Bands were visualized by enhanced chemiluminescence
(EMD Millipore, Billerica, MA, USA) using an ImageQuant LAS 4000
mini (General Electric Company, Boston, MA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Quantitative data were expressed as
the mean ± standard deviation. One-way analysis of variance and
Student-Newman-Keuls tests were used. P<0.05 was considered to
indicate statistical significance.
Results
Characterization of HGNs and
anti-c-Met/HGNs
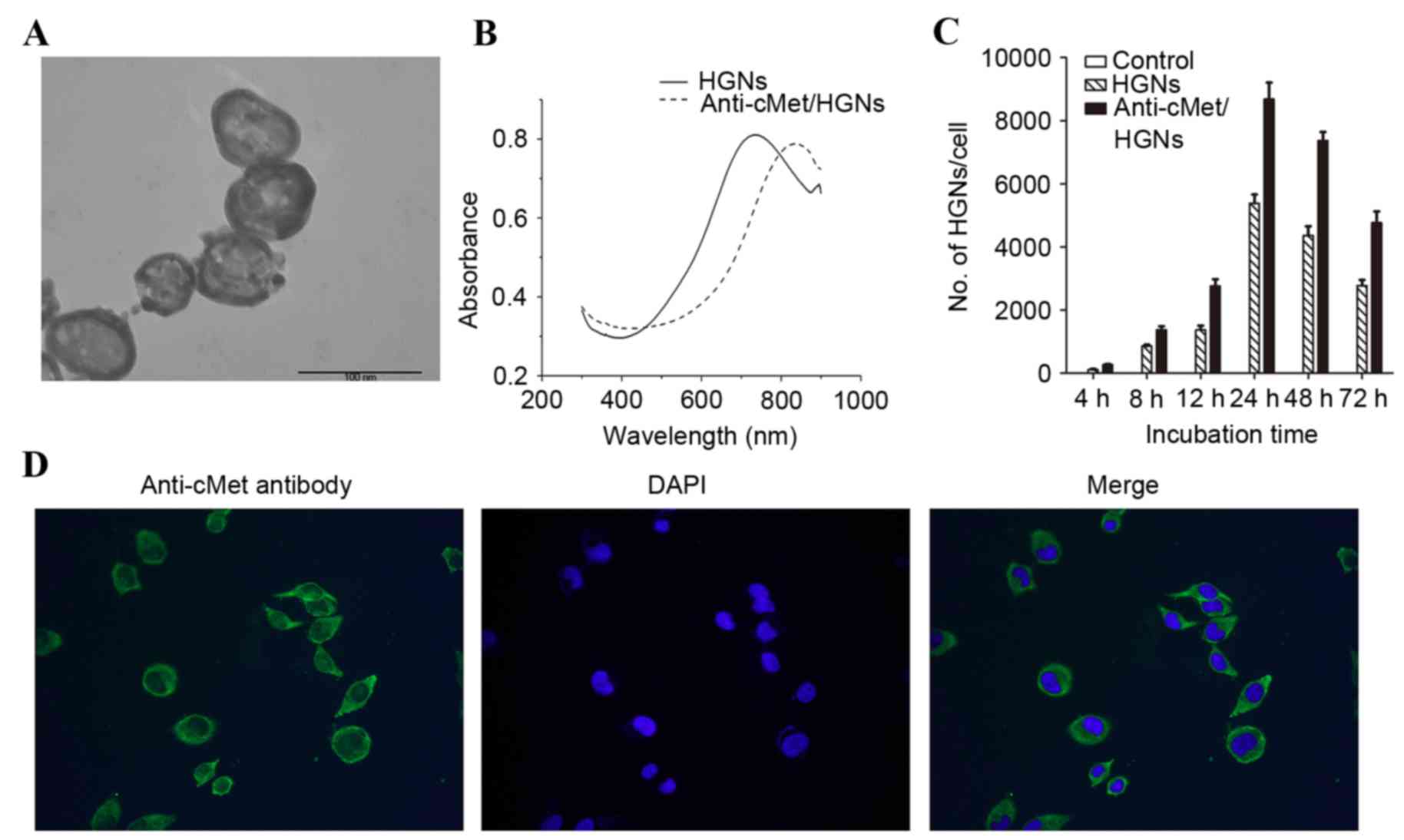
The HGNs synthesized possessed a homogeneous
morphology (Fig. 1A), a mean diameter
of 56.25±6.13 nm and an average wall thickness of 6.56±1.33 nm, as
measured using TEM. A redshift of the plasma resonance peak
occurred following the modification of naked HGNs (Fig. 1B).
Uptakes of HGNs and
anti-c-Met/HGNs
HGNs are known to be taken into cancer cells by
endocytosis (11). CaSki cells
internalized more anti-c-Met/HGNs than naked HGNs at each time
interval. Peak uptake concentration for naked HGNs and
anti-c-Met/HGNs was observed at 24 h. Following incubation with the
nanoparticles for 24 h, the average numbers of the nanoparticles
internalized by each cell was 5,378±401 for naked HGNs and
8,681±742 for anti-c-Met/HGNs (Fig.
1C).
Fluorescence measurements
c-Met is a receptor tyrosine kinase that is a
functional receptor for HGF. c-Met is a member of one of the
membrane protein superfamilies, the tyrosine kinase family of
growth-factor receptors, whose expression is associated with
proliferation, morphogenesis and suppression of apoptosis (12). Overexpression of c-Met has been
observed in different pathological types of cervical cancer
(8). Immunofluorescence analysis was
used to verify the presence of a layer of green fluorescent
staining on the cell membrane of CaSki cells, indicating the
overexpression of c-Met (Fig.
1D).
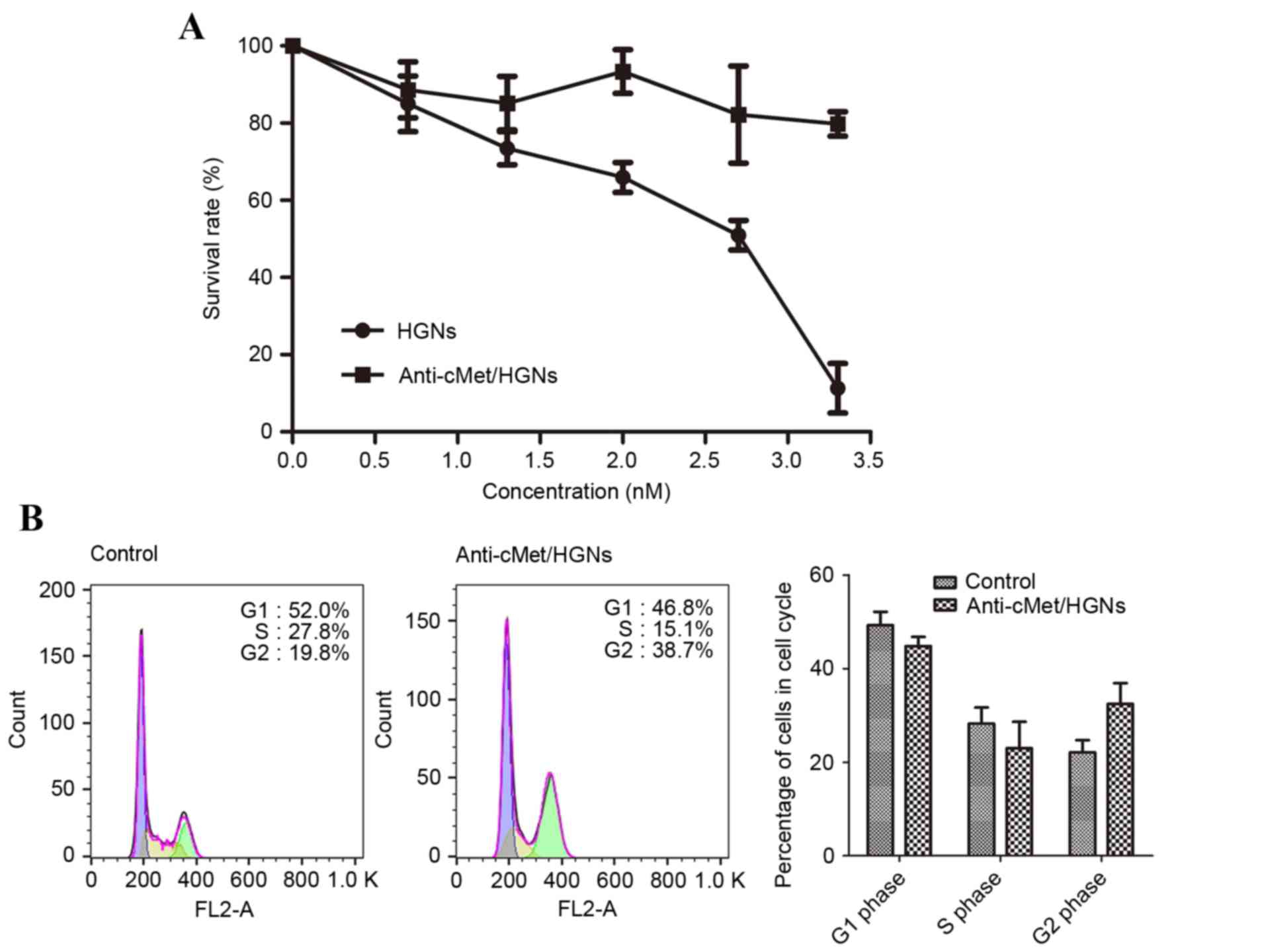
Cytotoxicity of HGNs and
anti-c-Met/HGNs
Cells were incubated with either naked HGNs or
anti-c-Met/HGNs at increasing concentrations, and the cytotoxicity
of the nanospheres was measured by a CCK-8 test. Every condition
was conducted in triplicate. The half maximal inhibitory
concentration (IC50) for naked HGNs in CaSki cells was
2.7 nM (Fig. 2A). PEG passivation of
HGN surfaces was performed to minimize cytotoxicity. Following the
incubation of CaSki cells with 2 nM HGN or anti-c-Met/HGN the cell
survival rate was 65.92±3.19% for those treated with HGNs alone and
93.39±4.92% for those treated with anti-c-Met/HGNs (Fig. 2A).
Cell cycle assay
Cells were incubated with 3 nM HGNs or
anti-c-Met/HGNs for 24 h. Compared with the control group, the
group co-cultured with anti-c-Met/HGNs exhibited an increase in the
number of cells in the G2/M phase and fewer cells in the G0/G1
phase (Fig. 2B). In the control
group, the amount of cells in the G2/M phase was 19.8%, which
increased to 38.7% in cells treated with anti-c-Met/HGN, a
significant difference (P<0.05). Correspondingly, the ratio of
cells in the G0/G1 phase in these groups was 52.0 and 46.8%,
respectively. HGNs arrested cancer cells at the G2/M phase, which
is the most radiosensitive phase in the cell cycle (13), and thus increased the radiation
sensitivity of cancer cells.
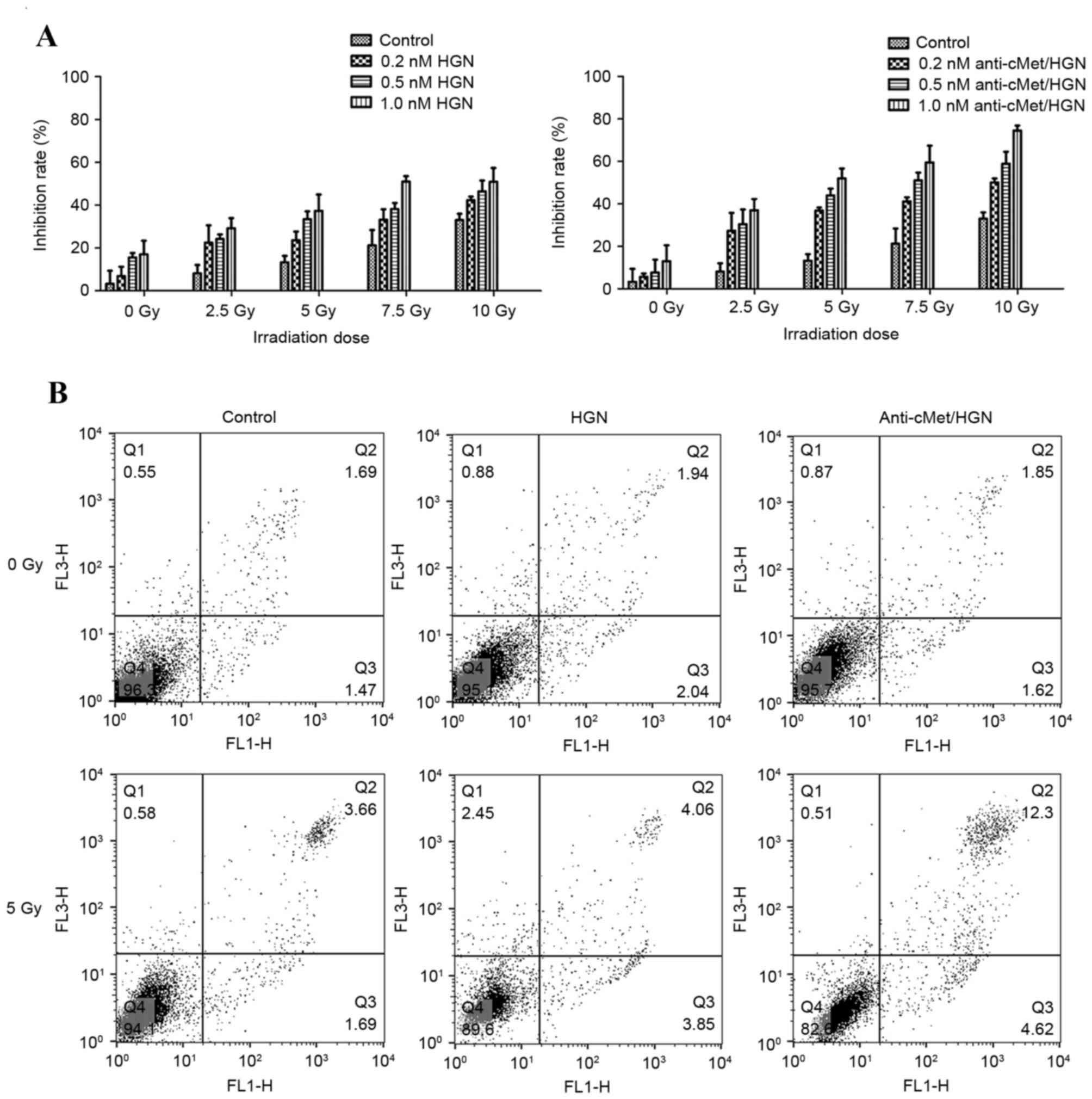
Radiation cytotoxicity assay
Treatment with anti-c-Met/HGNs enhanced radiation
sensitivity of CaSki (Fig. 3A).
Compared with groups treated with X-ray radiation alone, those
treated with anti-c-Met/HGNs exhibited a markedly higher cellular
proliferation inhibition rate. X-ray irradiation alone (5 Gy)
induced an inhibition rate of 13.2±2.5%, whereas incubation with
naked HGNs (1.0 nM) with X-ray irradiation increased the inhibition
rate to 37.3±6.3% and incubation with 1.0 nM anti-c-Met/HGNs with
irradiation increased it further to 52.0±3.8%. A significant
enhancement in inhibition rate was observed, averaging 38.7%
(P<0.05) compared with controls.
Cell apoptosis assay
Flow cytometry was used to determine cell apoptosis
3 h following radiation. The flow cytometry graphs were sectioned
into four quadrants. Dual staining with FITC Annexin V and PI was
used to identify early apoptotic cells (Annexin V-positive,
PI-negative; lower-right quadrant), viable cells (Annexin
V-negative, PI-negative; lower-left quadrant) and late apoptotic
together with dead cells (Annexin V-positive, PI-positive;
upper-right quadrant). The results revealed that the total cell
apoptotic rates of the anti-c-Met/HGNs with X-ray group was
significantly higher than that of the negative control group in
CaSki cells (P<0.05; Fig. 3B).
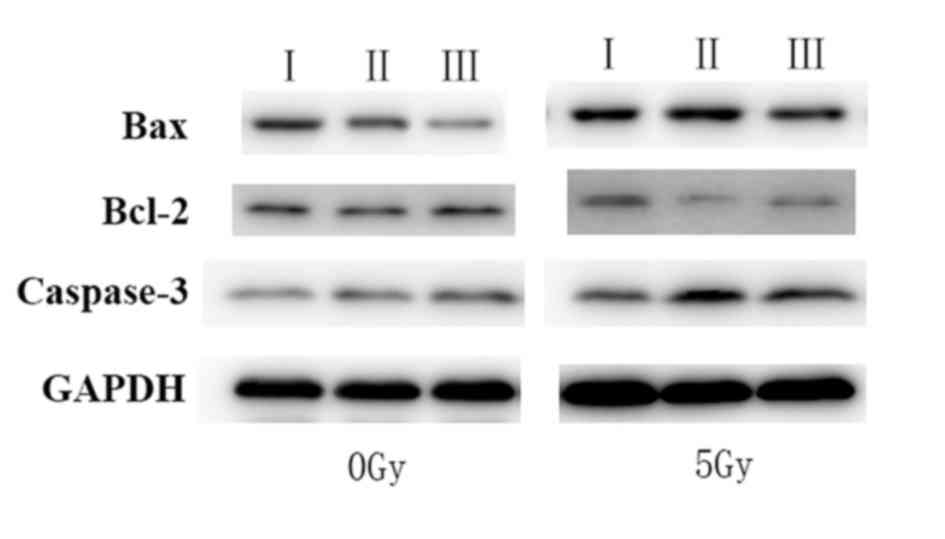
Western blot analysis
The expression of Bcl-2, caspase-3 and Bax was
assessed by western blot analysis to verify the overexpression of
apoptosis-related proteins (Fig. 4).
The results demonstrated that the expression of proteins associated
with apoptosis, including Bax and caspase-3, were increased,
whereas the expression of Bcl-2 was markedly decreased in cells
incubated with anti-c-Met/HGNs and treated with radiation, compared
with other groups. The results indicated that X-ray irradiation may
induce apoptosis in CaSki cells by disturbing the balance between
Bax and Bcl-2, as well as intervening in the Fas ligand
pathway.
Discussion
As it is the most common gynecologic malignancy,
patients with early-stage cervical cancer ordinarily have good
prognosis when they undergo radical surgery. Nevertheless, patients
exhibiting high-risk features identified on pathologic examination
who undergo surgery, patients with larger early-stage tumors (IB2
and IIA, diameter of >4 cm) and patients with advanced-stage
tumors (IIB-IVA), which were classified according to International
Federation of Obstetrics and Gynecology stage (1995) are
administered radiation therapy to eliminate the tumor foci or to
reduce the risk of relapse (14). The
emergence of radioresistance reduces the effectiveness of radiation
therapy, meaning that a novel agent is required to enhance
sensitivity and to improve targeting specificity.
Nanotechnology is an emerging technique that is used
to improve cellular targeting and sensitivity to radiation therapy
(2). HGNs are novel therapeutic
agents which possess biocompatibility, ease of synthesis and
modification, and a high Z-coefficient, indicating a strong
therapeutic potential (15). HGNs
have been demonstrated to enhance sensitivity to radiotherapy by
altering the cell-cycle distribution, and a great deal of progress
has been made in designing HGN-conjugating agents for targeted
radiotherapy against malignant tumors (16,17). HGNs
have a high atomic number, which leads to a greater absorption of
X-rays than standard agents. HGNs are also able to bind multiple
proteins that are targeted to cell-surface receptors, which are
overexpressed in cancer cells (18).
The size and shape of gold nanoparticles alters their cellular
uptake and biomedical applications. Chithrani et al
(19) investigated the features of
gold nanoparticles synthesized at a range of sizes (1–100 nm
diameter) and shapes (1:1 to 1:5 aspect ratio). The authors
revealed that the cellular uptake of nanoparticles was dependent on
the size. The maximum uptake occurred at a size of 50 nm. A study
by Osaki et al (20) suggested
that endocytosis is highly size-dependent, as 50-nm nanoparticles
entered cells via endocytosis more efficiently than smaller ones.
These results suggested the optimal size of nanoparticles is ~50
nm.
In the present study, HGNs of 56.25±6.13 nm in
diameter and 6.56±1.33 nm in wall thickness were synthesized
through a galvanic replacement reaction using colloidal silver as
the sacrificial template and HAuCl4 as the precursor to
gold under refluxing conditions. OPSS-PEG-NHS was used to modify
HGNs to reduce their cytotoxicity, impart biocompatibility and
extend blood circulation time. The PEG-coated HGNs were conjugated
to anti-c-Met monoclonal antibodies, as c-Met has been confirmed to
be overexpressed in different pathological categories of cervical
cancer by histopathology (8). Using
immunofluorescent staining, c-Met was revealed to be overexpressed
on the surface of CaSki cells. The bioconjugation of the anti-c-Met
antibody to HGN led to a slight red shift in the infrared
absorption peak of nanospheres.
One major challenge in the field of nanoparticle
therapy is the identification of an appropriate concentration of
nanoparticles to minimize toxicity and maximize therapeutic
efficacy. Owing to the toxicity and low biological compatibility, a
high dose of naked gold nanospheres may cause severe cytotoxic
responses. In the present study, uptake concentrations of
nanoparticles reached peak levels at 24 h and then diminished. The
cytotoxicity of HGNs was therefore assessed using different HGN
concentrations following incubation for 24 h. The IC50
of naked HGNs was 2.7 nM in CaSki cells. As the naked HGN and
antibody-conjugated HGN groups were being used, the experimental
concentration used for the following experiments with radiation
therapy was 1.0 nM.
Owing to their ability to reduce the effective dose
of radiation, radiation sensitizers are of high clinical value.
Metallic materials have been reported to arrest the cell cycle at
the most radiosensitive phase (G2/M phase) to enhance the
sensitivity of cancer cells towards radiation (13,21). The
present study confirmed that the HGNs synthesized caused CaSki
cells to accumulate at the G2/M phase. A previous study on
nanotechnology also revealed the potential for enhanced tumor
destruction by radiation therapy (3).
Garnica-Garza et al (22)
examined the feasibility of using X-ray beams to treat prostate
cancer loaded with gold nanoparticles and the effect of the
nanoparticle concentration. The present study was performed with 6
MeV X-ray energies at various radiation doses (0, 2.5, 5, 7.5 and
10 Gy). The distance of the cells from the radiation source was 25
cm and the effect of radiation was tested by the CCK-8 assay. The
enhancement of radiation sensitivity for anti-c-Met/HGNs is
presented in Fig. 3A.
HGNs were confirmed to mediate the radiation effect
on CaSki cells using double-labeled Annexin V and PI. Annexin V
staining precedes the loss of membrane integrity, which accompanies
the late stages of cell death that results from either apoptotic or
necrotic processes. Treatment with X-rays alone or naked HGNs with
X-rays had no significant effect on cancer apoptosis rate; however,
X-ray radiation in the presence of the antibody-conjugated HGNs
significantly increased the apoptotic rate compared with that of
the control group (P<0.05). Furthermore, the expression of
apoptosis-associated proteins 48 h following X-ray radiation was
also probed. It has been suggested that an interaction between the
Fas pathway and Bcl-2 family members may define the rate of
apoptosis (23). Once Fas has been
activated, other downstream effector caspases, such as caspase-3,
are motivated to initiate the execution phase of apoptosis
(24). In the present study, the
expression of Bax and caspase-3 were significantly elevated and
that of Bcl-2 was significantly decreased in cells treated with
anti-c-Met-conjugated HGNs and X-ray radiation, compared with
radiation alone and with naked HGNs and radiation (Fig. 4). Therefore, X-ray radiation is likely
to have induced apoptosis in CaSki cells by disrupting the balance
between Bcl-2 and Bax and activating the Fas signaling pathway.
The present study indicated that, compared with
conventional radiotherapy strategies, low-dose X-ray radiation
combined with anti-c-Met/HGN treatment is a promising therapeutic
approach for the targeted elimination of cervical cancer cells.
Further work will also be required to assess the functionality of
HGNs in animal models and make them suitable for clinical use.
The HGNs described in the present study exhibited
potential in radiation therapy for tumor ablation. The
anti-c-Met/HGNs synthesized in the present study form part of a
novel therapeutic approach for the targeted elimination of cervical
cancer cells. Future work will also be required to ensure
functionality of HGNs in animal models to make them suitable for
clinical employment.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81372809),
the National Clinical Research Center for Gynecological Oncology
(grant no. 2015BAI13B05) and the Natural Science Foundation of
Shandong Province (grant no. ZR2016HB03).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daniel MC and Astruc D: Gold
Nanoparticles: Assembly, supramolecular chemistry,
quantum-size-related properties, and applications toward biology,
catalysis, and nanotechnology. Chem Rev. 104:293–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahman WN, Bishara N, Ackerly T, He CF,
Jackson P, Wong C, Davidson R and Geso M: Enhancement of radiation
effects by gold nanoparticles for superficial radiation therapy.
Nanomedicine. 5:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kong T, Zeng J, Wang X, Yang X, Yang J,
McQuarrie S, McEwan A, Roa W, Chen J and Xing JZ: Enhancement of
radiation cytotoxicity in breast-cancer cells by localized
attachment of gold nanoparticles. Small. 4:1537–1543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chithrani DB, Jelveh S, Jalali F, van
Prooijen M, Allen C, Bristow RG, Hill RP and Jaffray DA: Gold
nanoparticles as radiation sensitizers in cancer therapy. Radiat
Res. 173:719–728. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bottaro DP, Rubin JS, Faletto DL, Chan AM,
Kmiecik TE, Woude GF Vande and Aaronson SA: Identification of the
hepatocyte growth factor receptor as the c-met proto-oncogene
product. Science. 251:802–804. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birchmeier C, Birchmeier W, Gherardi E and
Woude GF Vande: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baykal C and Ayhan A, Al A, Yüce K and
Ayhan A: Overexpression of the c-Met/HGF receptor and its
prognostic significance in uterine cervix carcinomas. Gynecol
Oncol. 88:123–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Lofton C, Popp M and Tan W: Using
luminescent nanoparticles as staining probes for Affymetrix
GeneChips. Bioconjug Chem. 18:610–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zhao W and Tan W: Bioconjugated
silica nanoparticles: Development and applications. Nano Res.
1:992008. View Article : Google Scholar
|
|
11
|
Tsai SW, Chen YY and Liaw JW: Compound
cellular imaging of laser scanning confocal microscopy by using
gold nanoparticles and dyes. Sensors (Basel). 8:2306–2316. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizuno S and Nakamura T: HGF-MET cascade,
a key target for inhibiting cancer metastasis: The impact of NK4
discovery on cancer biology and therapeutics. Int J Mol Sci.
14:888–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner J, Koumenis C, Kute TE, Planalp RP,
Brechbiel MW, Beardsley D, Cody B, Brown KD, Torti FM and Torti SV:
Tachpyridine, a metal chelator, induces G2 cell-cycle arrest,
activates checkpoint kinases, and sensitizes cells to ionizing
radiation. Blood. 106:3191–3199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petignat P and Roy M: Diagnosis and
management of cervical cancer. BMJ. 335:765–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dorsey JF, Sun L, Joh DY, Witztum A, Kao
GD, Alonso-Basanta M, Avery S, Hahn SM, Al Zaki A and Tsourkas A:
Gold nanoparticles in radiation research: Potential applications
for imaging and radiosensitization. Transl Cancer Res. 2:280–291.
2013.PubMed/NCBI
|
|
16
|
Geng F, Song K, Xing JZ, Yuan C, Yan S,
Yang Q, Chen J and Kong B: Thio-glucose bound gold nanoparticles
enhance radio-cytotoxic targeting of ovarian cancer.
Nanotechnology. 22:2851012011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roa W, Zhang X, Guo L, Shaw A, Hu X, Xiong
Y, Gulavita S, Patel S, Sun X, Chen J, et al: Gold nanoparticle
sensitize radiotherapy of prostate cancer cells by regulation of
the cell cycle. Nanotechnology. 20:3751012009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain S, Hirst DG and O'Sullivan JM: Gold
nanoparticles as novel agents for cancer therapy. Br J Radiol.
85:101–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chithrani BD, Ghazani AA and Chan WC:
Determining the size and shape dependence of gold nanoparticle
uptake into mammalian cells. Nano Lett. 6:662–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osaki F, Kanamori T, Sando S, Sera T and
Aoyama Y: A quantum dot conjugated sugar ball and its cellular
uptake. On the size effects of endocytosis in the subviral region.
J Am Chem Soc. 126:6520–6521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson GD: Radiation and the cell cycle,
revisited. Cancer Metastasis Rev. 23:209–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garnica-Garza HM: Contrast-enhanced
radiotherapy: Feasibility and characteristics of the physical
absorbed dose distribution for deep-seated tumors. Phys Med Biol.
54:5411–5425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krammer PH: CD95(APO-1/Fas)-mediated
apoptosis: Live and let die. Adv Immunol. 71:163–210. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slot KA, Voorendt M, de Boer-Brouwer M,
van Vugt HH and Teerds KJ: Estrous cycle dependent changes in
expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and
pro- and active caspase-3 in the rat ovary. J Endocrinol.
188:179–192. 2006. View Article : Google Scholar : PubMed/NCBI
|