Introduction
Lung cancer is the primary cause of
cancer-associated mortalities and is ranked the highest in males
and second highest in females for mortality and morbidity rates
globally (1). Non-small cell lung
cancer (NSCLC), which constitutes 80–85% of lung cancer cases,
remains an aggressive type of lung cancer that is associated with
poor patient survival (2). Currently,
chemotherapy has reached a plateau of effectiveness in improving
patient survival, and it is the mainstay of treatment regimens and
new drugs, including erlotinib and gefitinib, that they will
eventually fail due to drug resistance (3,4);
therefore, novel approaches and drugs are urgently required in
order to improve the prognosis of patients with lung cancer.
Natural compounds remain a predominant source for
anticancer drug development. A total of 47% of the 155 anticancer
drugs approved from 1950–2006 were natural products or directly
derived from them (5). Matrine
(C15H24N2O) is the major alkaloid
component identified in Sophora alopecuroides roots, which
are widely utilized in traditional Chinese medicine and have a wide
range of pharmacological effects, including anti-cardiac,
anti-arrhythmic, anti-bacterial, anti-asthma, anti-diuretic, immune
suppressive and anti-inflammatory activities (6–9). It has
also been reported that matrine possesses antitumor activities
in vitro and in vivo (10–13). The
mechanisms underlying matrine's antitumor activities include the
inhibition of cell proliferation and the induction of apoptosis
(14–16). However, the low water solubility,
bioavailability and bioactivity of matrine, coupled with its
considerable side effects, which include general toxicity to the
central nervous system, have limited its utility as a therapeutic
drug (17,18). Therefore, studies have been focusing
on developing derivatives and analogues of matrine by total
synthesis or structural modifications in order to improve its
activity and bioavailability (19–21).
A previous study demonstrated that the amide bond
may be required for the antitumor activities of matrine as
following opening of the D-ring and breaking of the amide bond, the
anti-proliferative activities of matrine are lost (22). Based on the potential positions for
modifying matrine, the present study designed, synthesized and
characterized five matrine derivatives for their anti-proliferation
potential.
Materials and methods
Reagents and cell culture
Matrine (purity, 98%) was purchased from Shaanxi
Undersun Biomedtech Co., Ltd., Xian, China. Matrine was dissolved
in PBS to dilute to various concentrations (50, 100, 200, 400, 600,
800 µM) at the time of adding to cells. The derivatives were
dissolved in dimethyl sulphoxide (DMSO) to make a stock solution at
100 mM. MTT was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The apoptosis detection kit (Flowcellect™
Annexin Red kit) was purchased from EMD Millipore (Billerica, MA,
USA). The A549 human lung cancer cells, BT-20 human breast cancer
cells, MCF-7 human breast cancer cells and U20S human osteosarcoma
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in Dulbecco's modified
Eagle's medium high-glucose (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin at 37°C in an humidified atmosphere
containing 95% air and 5% CO2.
General procedure for the preparation
of matrine derivatives
A total of 6 mmol (3.0 ml) lithium diisopropylamide
[LDA; 2.0 mol/l in tetrahydrofuran (THF)] was added to 20 ml
anhydrous THF in dried three-necked bottle under a dry nitrogen
atmosphere and the solution was cooled to 0°C. Subsequently, 5 mmol
(1.24 g) matrine dissolved in anhydrous THF was added dropwise and
the solution was stirred for 30 min at 0°C. A total of 10 mmol of
the corresponding reagent (3-methylbenzyl bromide,
2-naphthaldehyde, 6-methoxy-2-naphthdehyde and methyl
6-bromo-2-naphthoate (all from Ameresco, Inc., Framingham, MA, USA)
was then added into the solution and stirred for another 3 h at
room temperature. Subsequently, the reaction was inhibited by
adding 10 ml saturated NH4Cl. Finally the solution was
extracted using dichloromethane (15 ml, 3X) at room temperature for
30 min, washed with saturated brine, dried over
Na2SO4 and concentrated (under −0.1 MPa
pressure) at room temperature, providing the corresponding final
products. For YF3-3, 50 mg (0.2 mmol) matrine was dissolved in 10
ml of ether and 1 ml (16 mmol) of CH3I was added. The
mixture was allowed to stand overnight at room temperature and no
precipitate of methiodide was observed. The mixture was refluxed
and a slow precipitate of methiodide was observed after ~3 h.
Refluxing was continued for a further 7 h, the reaction mixture was
evaporated and the residue was recrystallizated from
C2H5OH to provide 36 mg of needles.
Cell proliferation, apoptosis and cell
cycle assay
The experiments of cell proliferation, apoptosis and
cell cycle assay were performed as previously described (23).
An MTT assay was performed to measure proliferation.
Cells (BT-20, A549, MCF-7 and U20S) were seeded in 96-well plates
at a density of 4,000 cells/well and incubated for 24 h at 37°C
prior to the assay.
For cell cycle analysis, A549 cells were seeded in
12-well plates at a density of 1×105 cells per well and
incubated for 24 h at 37°C. Then YF3-5 (12.5, 25 or 50 µM) was
added. Following a further 24 h at 37°C, cells were harvested and
fixed with 75% ethanol for 2 h, stained with propidium iodide and
analyzed with flow cytometry.
For the apoptosis assay, A549 cells were seeded in
6-well plates at the density of 3×105 cells per well and
incubated for 24 h at 37°C. Then YF3-5 (25, 50 or 100 µM) was
added. Following a further 24 h at 37°C, cells were stained with
Annexin V-fluorescein isothiocyanate (FITC)/7-AAD and analyzed with
flow cytometry.
Oxidative stress assay
The effect of YF3-5 on the reactive oxygen species
(ROS) formation in A549 cells was determined by an oxidative stress
assay. Briefly, A549 cells at 80% confluency were treated with
differing concentration of YF3-5 (100, 50 and 25 µM) for 23.5 h at
room temperature. Subsequently, 50 µl of pre-warmed staining
solution containing dihydroethidium (DHE) and Hoechst dye were
added to the wells and incubated at 37°C in 5% CO2 for
30 min. The cells were then fixed with formaldehyde at room
temperature for 2 h and the nuclei intensity of ethidium, a product
of DHE oxidation, was determined using the Array Scan HCS reader
(Thermo Fisher Scientific, Inc.). In each well, ≥400 cells were
analyzed.
Molecular docking assay and ClogP
calculation
The computational docking test was performed using
Molecular Operating Environment 2008.10 (Chemical Computing Group
Inc., Montreal, Canada) as previously described (24). Briefly, an X-ray crystallography
structures of cyclin-dependent kinase (CDK)2 [Research
Collaboration for Structural Bioinformatics Protein Data Bank (PDB)
identity (ID): 2VV9], CDK4 (PDB ID: 2V9F), CDK6 (PDB ID: 1JOW),
cyclin D1 (PDB ID: 2W99) and cyclin E (PDB ID: 1W98) and the
corresponding ligand were downloaded from PDB (http://www.rcsb.org). The protein and compound YF3-5
were optimized to retain a low energy state prior to docking.
Docking score was refined by forcefield and evaluated using the
London dG scoring method. For the calculation of ClogP, ChemDraw
software version 14.0 was used, with default settings (PerkinElmer,
Inc., Waltham, MA, USA).
Western blot assay
A549cells (3×105 per well) were seeded in
6-well culture plates, treated with 25 or 50 µM YF3-5 for 12 and 24
h at 37°C and lysed in radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) supplemented
with a protease inhibitor cocktail and phenylmethane sulfonyl
fluoride. Whole-cell lysates were collected by centrifugation at
12,000 × g for 10 min at 37°C. The protein concentration was
measured with the BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Lysates containing 30 µg of protein were
separated by SDS-PAGE (8–15%) and transferred to nitrocellulose
membranes (EMD Millipore). Subsequently, blocking was performed
using 5% dried skimmed milk for 1 h at room temperature. The
membrane was then probed with antibodies against Rb (cat. no. 9309;
1:1,000; CST Biological Reagents Company Ltd., Shangai, China),
phosphorylated Rb (cat. no. 8516; 1:1,000; CST Biological Reagents
Company Ltd.) and β-actin (cat. no. 3700; 1:1,000; CST Biological
Reagents Company Ltd.) for 2 h at room temperature following
incubation with relative secondary antibodies (rabbit anti-mouse
mAb-HRP conjugate; cat. no. 58802 and mouse anti-rabbit mAb-HRP
conjugate; cat. no. 5127; 1:10,000; CST Biological Reagents Company
Ltd.) for 2 h at room temperature and detected using a
chemiluminescence substrate (Cell Signaling Technology, Inc.,
Danvers, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between two groups were compared using the Student's
t-tests. All experiments were repeated ≥3 times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Synthesis of five matrine
derivatives
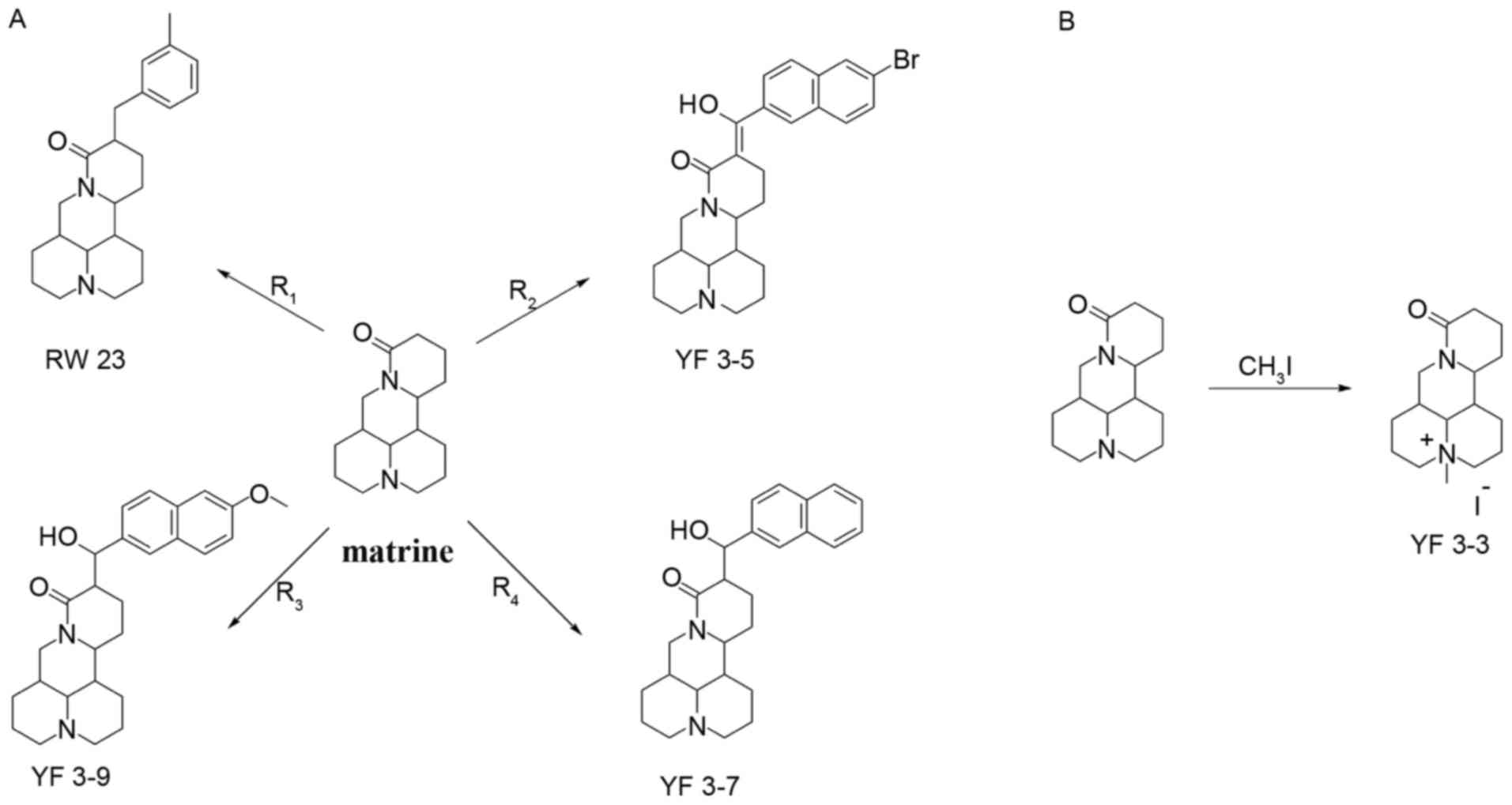
Four matrine derivatives were synthesized, as
presented in Fig. 1A and
characterized in Table I. Derivatives
of matrine were prepared by allowing matrine to react with
3-methylbenzyl bromide, 2-naphthaldehyde, 6-methoxy-2-naphthdehyde
and methyl 6-bromo-2-naphthoate, respectively, in the presence of
LDA and nitrogen atmosphere at 0°C for 30 min, then at room
temperature for 3 h. For compound YF3-3, matrine was dissolved in
diethyl ether and reacted with CH3I (Fig. 1B).
 | Table I.Characterization of synthesized
compounds. |
Table I.
Characterization of synthesized
compounds.
| Compound | 1H | 13C | Infrared
radiation | MS | High resolution
MS |
|---|
| YF3-3 | 4.35 (dd, 1H), 3.87
(m, 1H), | 169.86, 66.76, 66.08,
65.78, | 2,919, 2,724, 2,489,
1,651 | 263 (M-I), 190
(38), | M+-I,
found 263.2122 |
|
| 3.72 (t, 1H), 3.29
(s, 3H), 2.97 (t, 1H), | 53.29, 46.30, 40.78,
40.50, |
| 106 (18), 101
(10) |
|
|
| 2.28 (m, 1H),
2.23–2.12 (m, 7H), | 32.76, 28.93,
24.12, 22.39, |
|
|
|
|
| 2.05–1.99 (m, 1H),
1.97–1.91 (m, 1H), | 18.85, 17.43,
17.24 |
|
|
|
|
| 1.91–1.86 (m, 1H),
1.86–1.80 (m, 1H), |
|
|
|
|
|
| 1.78–1.54 (m, 7H),
1.28–1.21 (m, 1H) |
|
|
|
|
| YF3-5 | 15.57 (m, 1H), 8.31
(d, J=6.0Hz, 1H), | 197.93, 166.62,
136.39, 135.11, | 3,369, 3,042,
2,950, 2,868, 2,776, | 482 (M+1,100), | MH+,
found 481.1494 |
|
| 8.24 (s, 1H),
8.09–7.95 (m, 3H), | 131.27, 130.97,
130.76, 130.10, | 1,600, 1,497,
1,426, 1,344, 1,293, | 478 (3), 332
(4), |
|
|
| 7.76–7.73 (m,
1H), | 129.86, 127.30,
125.84, | 1,231, 1,180,
1,099, 812, 771 | 248 (8), 190
(37), |
|
|
| 4.19–4.15 (m, 1H),
3.84 (m, 1H), | 122.77, 63.53,
57.23, 57.18, |
| 168 (6), 106
(20) |
|
|
| 3.00–2.96 (m, 1H),
2.76–2.71 | 53.41, 50.39,
43.66, 41.68, |
|
|
|
|
| (m, 3H), 2.21–2.14
(m, 1H), | 35.38, 27.75,
26.36, 25.73, |
|
|
|
|
| 1.79–1.27 (m,
15H) | 22.49, 21.14,
20.78 |
|
|
|
| YF3-7 | 7.86–7.83 (m, 3H),
7.81 (s, 1H), | 173.05, 139.16,
133.25, 133.20, | 3,343, 3,049,
2,961, 2,821, 1,643, | 404.90 (M+), 336
(1), | MH+,
found 405.2543 |
|
| 7.56 (dd, J=1.8Hz,
J=8.4 Hz, 1H), | 128.17, 128.02,
127.69, 126.48, | 1,465, 1,349,
1,109, 792 | 274 (7), 190 (54),
168 (6), |
|
|
| 7.50–7.45 (m, 2H),
4.89 (d, J=9.6 Hz, | 126.02, 125.83,
124.94, 76.81, |
| 106 (16), 101
(11) |
|
|
| 1H), 4.48 (dd,
J=4.2 Hz, J=12.6 Hz, 1H), | 63.22, 57.15,
57.12, 53.43, 47.61, |
|
|
|
|
|
3.82–3.77 (m, 1H), 3.14 (t,
J=12.6 Hz, | 44.16, 41.34,
35.21, 27.77, |
|
|
|
|
| 1H), 2.85–2.77 (m,
2H), 2.47–2.42 | 27.18, 26.01,
22.72, 21.15, 20.72 |
|
|
|
|
| (m, 1H), 2.07 (s,
1H), 2.04–1.93 |
|
|
|
|
|
| (m, 4H), 1.81–1.69
(m, 4H), 1.61–1.53 |
|
|
|
|
|
| (m, 2H), 1.50–1.45
(m, 1H), 1.44–1.34 |
|
|
|
|
|
| (m, 3H), 1.30–1.25
(m, 3H) |
|
|
|
|
| YF3-9 | 7.77 (m, 3H), 7.41
(m, 1H), 7.27 | 168.34, 167.32,
152.91, 132.08, | 3,331, 3,076,
2,950, 2,823, 1,621, | 434 (M+1,100), 404
(1), | MH+,
found 435.2648 |
|
| (m, 1H), 7.13 (m,
1H), 4.35 (m, 2H), | 129.62, 124.69,
123.85, 122.32, | 1,421, 1,356,
1,180, 1,099, 841 | 336 (1), 274 (4),
190 (36), |
|
|
| 3.86 (s, 3H), 3.44
(m, 4H), 2.98 (m, 1H), | 121.54, 120.64,
114.07, 100.95, |
| 185 (7), 106 (17),
101 (9) |
|
|
| 2.73 (m, 2H), 2.03
(m, 2H), 1.91–1.70 | 59.22, 58.48,
55.64, 52.59, 50.54, |
|
|
|
|
| (m, 4H), 1.68–1.54
(m, 5H), 1.53–1.42 | 48.58, 42.64,
39.34, 38.10, 36.43, |
|
|
|
|
| (m, 2H), 1.40–1.27
(m, 3H) | 31.01, 22.69,
21.49, 17.78, 16.97 |
|
|
|
| RW23 | 10.47 (m, 1H),
7.15–6.90 (m, 4H), | 166.77, 135.79,
133.02, 125.29, | 1,419.12, 1,460.19,
1,625.67, | 352 (M+1,100), | MH+,
found 353.2677 |
|
| 4.36 (dd, J=13.8
Hz, 4.8 Hz, 1H), | 123.37, 121.92,
121.51, 58.87, | 2,942.82 | 309 (2), 247
(4), |
|
|
| 4.00 (m, 1H), 3.22
(m, 4H), | 53.54, 52.47,
48.66, 39.03, |
| 219 (4), 190
(35), |
|
|
| 2.89 (m, 2H),
2.50–1.05 (20H) | 36.90, 32.80,
30.51, 23.06, |
| 177 (28), 150
(2), |
|
|
|
| 22.12, 21.43,
19.35, 16.66, |
| 105 (7), 41
(16) |
|
|
|
| 16.44, 16.02 |
|
|
|
Characterization of
YF3-3:4-methyl-10-oxotetradecahydro-1H, 5H-dipyrido
[2,1-f.3′,2′-ij][1,6] naphthyridin-4-ium iodide
The crude mixture obtained was purified by
recrystallation with ethanol and acetone; the appearance of the
compound was yellow crystal. The melting point (mp) was
268.1–270.2°C and the yield was 46%.
Characterization of
YF3-5:(Z)-11-((6-bromonaphthalen-2-yl)(hydroxy) methylene)
dodecahydro-1H,5H, 10H-dipyrido [2,1-f:3′,2′,1′-ij][1,6]
naphthyridin-10-one
The crude mixture obtained was purified by column
chromatography eluting with petroleum ether/ethyl acetate (1:1),
the appearance of the compound was white powder. The mp was
165.1–166.8°C and the yield was 56%.
Characterization of YF3-7:11-(hydroxy
(naphthalen-2-yl)methyl) dodecahydro-1H, 5H,
10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6] naphthyridin-10-one
The crude mixture obtained was purified by column
chromatography eluting with petroleum ether/ethyl acetate (100:80),
the appearance of the compound was white powder. The mp was
179.6–189.5°C and the yield was 51%.
Characterization of YF3-9:11-(hydroxy
(6-methoxynaphthalen-2-yl)methyl)dodecahydro-1H, 5H,10H-dipyrido
[2,1-f:3′,2′,1′-ij][1,6]naphthyridin-10-one
The crude mixture obtained was purified by column
chromatography eluting with ethyl acetate/methanol (10:1), the
appearance of the compound was white powder. The mp was
174.0–174.3°C and the yield was 63%.
Characterization of
RW-23:11-(3-methylbenzyl)
dodecahydro-1H,5H,10H-dipyrido[2,1-f:3′,2′,1′-ij][1,6]
naphthyridin-10-one
The crude mixture obtained was purified by column
chromatography eluting with petroleum ether/ethyl acetate (1:10),
the compound was a yellow, thick liquid; the hydrochloride form was
an orange solid. The mp was 60.5–62.3°C and the yield was 57%.
Anti-proliferation activities of five
matrine derivatives
The anti-proliferation effects of the matrine
derivatives were analyzed in A549 lung, BT-20 breast, MCF-7 breast
and U20S osteosarcoma cancer cells using an MTT assay for 48 h. As
presented in Table II, all compounds
exhibited anti-proliferation effects on the cancer cells. The
growth inhibition activity of matrine was moderate in the four
cancer cell lines, whereas YF3-5, YF3-7 and YF3-9 significantly
inhibited growth in comparison with matrine. YF3-5 exhibited the
most potent growth inhibition of all the cancer cell lines used in
this model. The anti-proliferation activity of YF3-5 [half-maximal
inhibitory concentration (IC50) ranging from 15.49–16.67
µM] was 9–29 times more potent compared with that of matrine
(IC50 ranging from 152.00–452.30 µM). It was suggested
that the improved anticancer activities of compounds were
consistent with their relative CLogP values determined by ChemDraw
software. Compound YF3-5 had the highest CLogP value, which
indicated that the structure modification of YF3-5 may increase its
lipophilicity and increase cell solubility. As there is a
requirement for novel treatment options for NSCLC, and as compound
YF3-5 was the most potent growth inhibitor against cancer cells
amongst the synthesized compounds that were analyzed, the present
study further investigated the efficacy and cytotoxic mechanisms
underlying YF3-5 using the A549 cell line.
 | Table II.Anti-proliferative activity of
Matrine and its derivatives. |
Table II.
Anti-proliferative activity of
Matrine and its derivatives.
|
| IC50
(µM) |
|
|---|
|
|
|
|
|---|
| Compounds | BT-20 | A549 | MCF-7 | U20S | CLogP |
|---|
| Matrine | 301.60±23.45 | 425.30±24.67 | 216.50±16.89 | 152.00±11.36 | 1.36 |
| YF3-3 | 495.50±34.38 | 353.90±17.67 | 204.50±17.82 | 189.10±12.86 | 3.25 |
| YF3-5 | 16.67±1.34 | 15.49±1.04 | 15.88±1.27 | 16.39±1.31 | 5.36 |
| YF3-7 | 136.60±10.45 | 117.90±6.89 | 126.10±8.96 | 85.09±5.223 | 3.76 |
| YF3-9 | 147.00±15.57 | 183.40±9.32 | 112.30±8.23 | 85.20±4.36 | 3.68 |
| RW-23 | 260.50±10.21 | 247.60±10.78 | 231.60±15.37 | 189.00±18.32 | 3.95 |
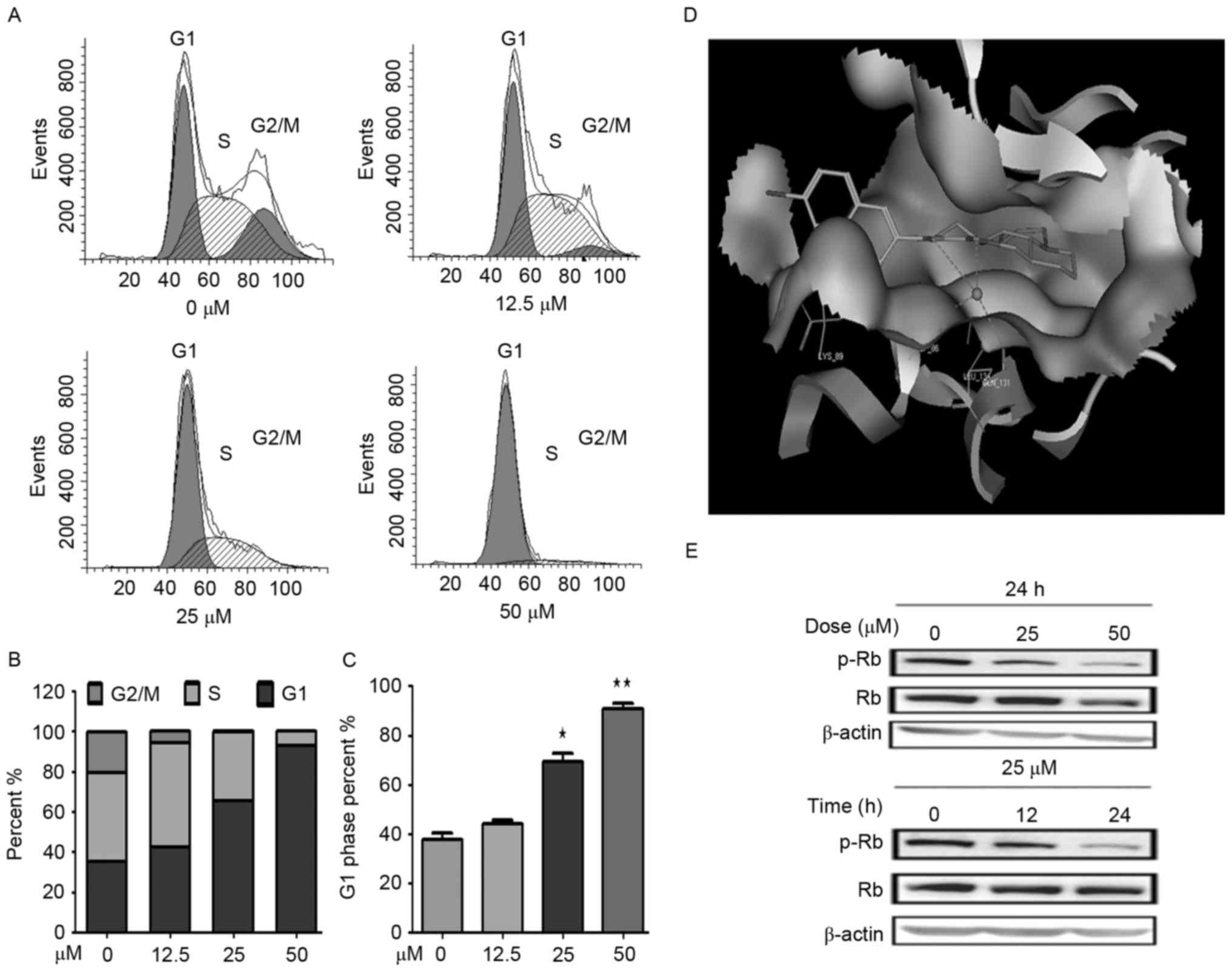
Derivative YF3-5 arrests cell cycle in
the G1 phase in A549 lung cancer cells
To determine whether the cytotoxicity of YF3-5
against A549cells involved cell cycle arrest, the present study
examined the cell cycle distribution of A549 cells treated with
YF3-5 using propidium iodide staining and flow cytometry analysis.
As presented in Fig. 2, after a 24-h
treatment with 12.5, 25 or 50 µM YF3-5, YF3-5 arrested the cell
cycle of A549 cells at the G1 phase in a dose-dependent
manner. YF3-5 increased the percentage of cells at the
G1 phase from 38% (vehicle control) to 69% (P<0.05)
and 91% (P<0.01) at concentrations of 25 and 50 µM, respectively
(Fig. 2A-C). To further investigate
the underlying mechanism of YF3-5-induced G1 cell cycle
arrest, the present study performed molecular docking of YF3-5 with
G1 regulators (25).
Molecular docking analysis of the YF3-5 complex with CDKs, cyclin E
and cyclin D1 structures (26) was
performed and the present study revealed that YF3-5 interacted with
CDK2, which has a vital role in G1 to S cell cycle phase
transition (Table III). The
three-dimensional structure demonstrated that compound YF3-5 may be
embedded into the long and narrow hydrophobic pocket formed by Lys
89, Gln 85, Asp 86, Ile 10 and Leu 134 of CDK2 (Fig. 2D). The naphthalene ring residues of
YF3-5 have hydrophobic interactions with the side chains of Lys 89
of the CDK2 and the carbonyl group and the hydroxyl group of YF3-5
may interact with the side chains of Gln 131 and Asp 86 of CDK2
(Fig. 2D). Retinoblastoma protein
(Rb) is a tumor suppressor which negatively regulated cell cycle at
the G1 phase (27). When
cells transit from G1 to S phase, CDK2 phosphorylates
Rb, which results in the transcription factor E2F transcription
factor 1 dissociating from Rb and entering the nucleus. Based on
the docking results, the present study speculated that compound
YF3-5 may attenuate the phosphorylation of Rb. To further elucidate
the effects of YF3-5 against Rb phosphorylation, western blotting
was performed to detect the expression level of phosphorylated Rb.
The results demonstrated that YF3-5 attenuated Rb phosphorylation
(Fig. 2E).
 | Table III.Molecular docking results of YF3-5
with proteins involved in G1 cell cycle phase regulation. |
Table III.
Molecular docking results of YF3-5
with proteins involved in G1 cell cycle phase regulation.
| Target | Docking score |
|---|
|
|
|---|
| Protein | PDB ID | Matrine | YF3-5 |
|---|
| CDK2 | 2VV9 | −10.398 | −15.864 |
| CDK4 | 2V9F |
−9.585 | −12.746 |
| CDK6 | 1JOW |
−7.312 |
−9.41 |
| Cyclin D1 | 2W99 |
−9.389 | −13.767 |
| Cyclin E | 1W98 |
−7.369 |
−7.563 |
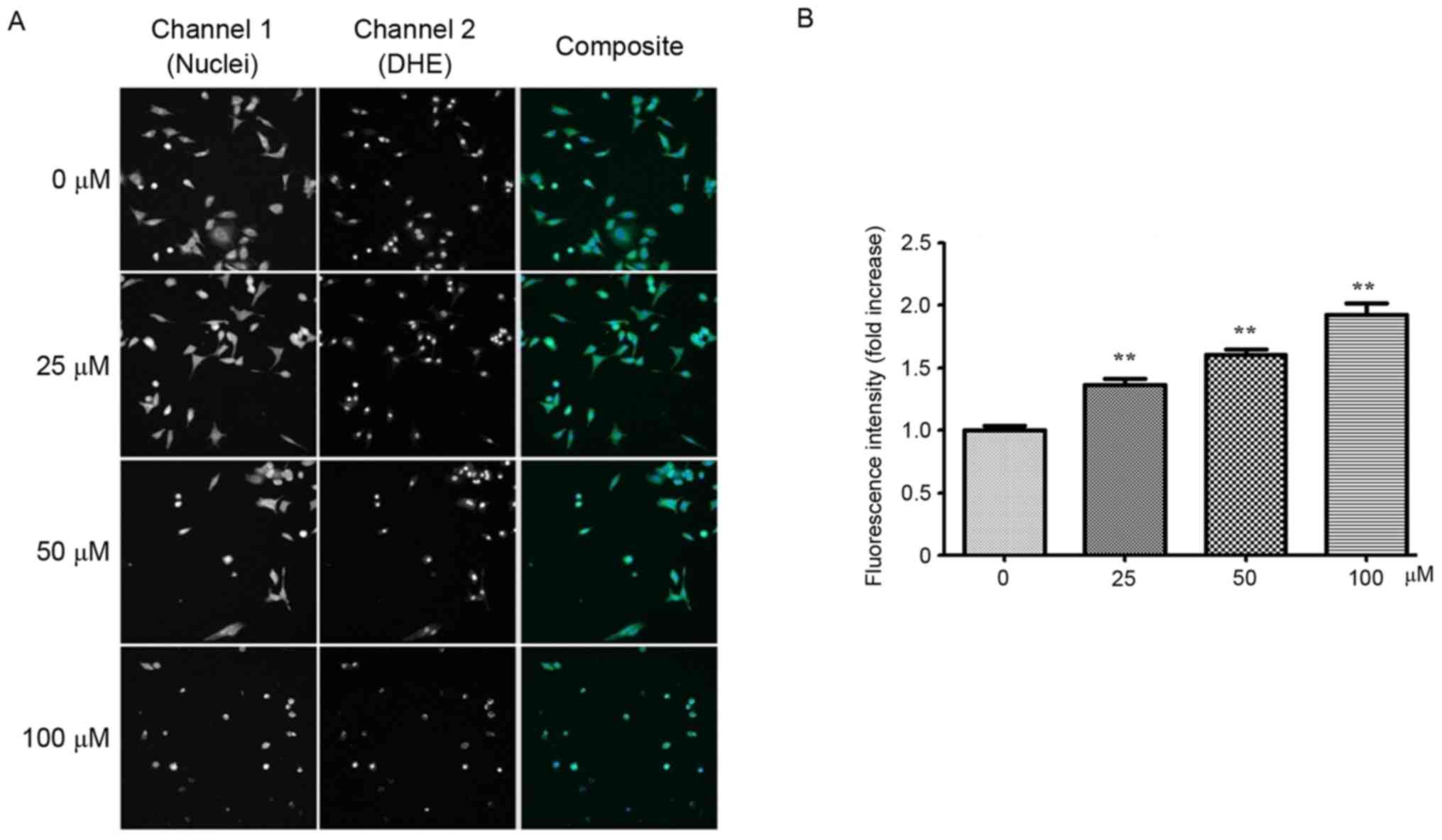
Derivative YF3-5 induces ROS
generation
ROS are chemically reactive molecules that serve an
important role in cancer development (28,29). It is
well established that certain chemotherapeutic agents generate ROS
in patients during cancer therapy (30). Therefore, it was of interest to
investigate whether YF3-5 also induced ROS generation in A549
cells. YF3-5 was demonstrated to significantly induce intracellular
ROS generation in A549 cells in a dose-dependent manner
(P<0.001). At concentrations of 25, 50 and 100 µM, YF3-5
increased intracellular ROS levels by 36.5, 60.5 and 92.2%,
respectively, compared with the vehicle control (Fig. 3).
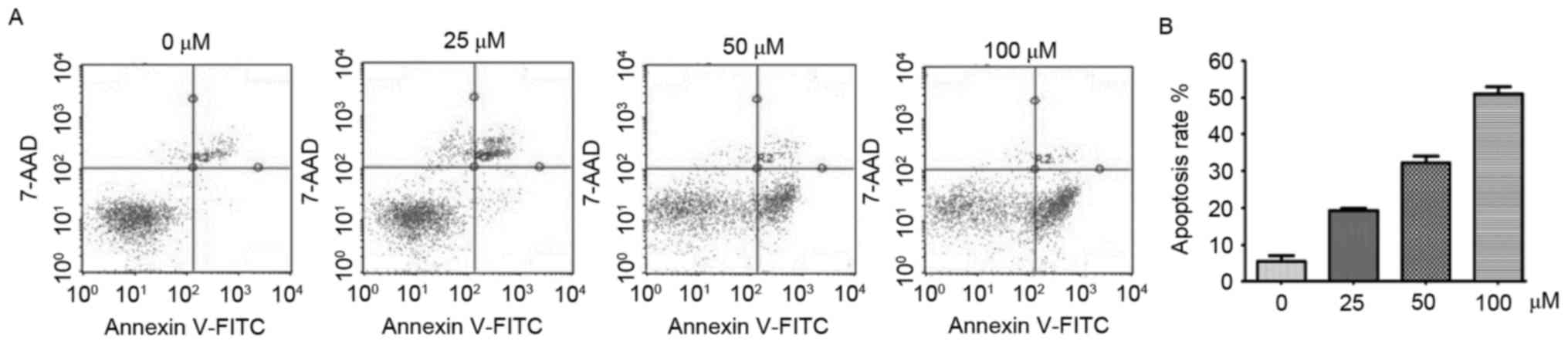
Derivative YF3-5 induces apoptosis via
generation of ROS in A549 lung cancer cells
Evidence has demonstrated that excessive ROS may
induce apoptosis via the extrinsic and intrinsic pathways (29,31).
Subsequently, the present study detected whether YF3-5 induced
apoptosis. Annexin V-FITC/7-AAD double staining assay was performed
to investigate the effect of YF3-5 on apoptosis of A549 cells. As
presented in Fig. 4A, following a
24-h treatment, YF3-5 significantly induced apoptosis in A549 cells
in a dose-dependent manner (P<0.01). At concentrations of 25, 50
and 100 µM, YF3-5 induced apoptosis of A549 cells by 18.58, 34.03
and 49.05%, respectively. To further elucidate the role of ROS in
YF3-5 induced cell apoptosis, cells were co-treated with ROS
scavenger N-acetylcysteine (NAC) and YF3-5 and cell apoptosis
assays were performed. The results demonstrated that exposure to
NAC prevented YF3-5 induced apoptosis (Fig. 4B), suggesting a role of ROS in YF3-5
induced apoptosis.
Discussion
The mortality rate of lung cancer has declined with
the emergence of tyrosine kinase inhibitors; however, problems
remain, including drug resistance. There is an urgent requirement
to develop new chemicals to improve lung cancer therapy. Matrine is
a Chinese traditional liver protective drug that displays antitumor
activity with moderate side effects. The present study used matrine
as a starting point and synthesized five matrine derivatives. MTT
results indicated that all the target compounds displayed improved
anticancer effects compared with matrine. By analyzing the
structure for relative activity, the present study revealed that
the carbon-carbon double bond at 14′ of matrine skeleton is
critical for improving the anticancer effects. In order to
investigate a more in-depth structure activity association, a
series of matrine derivatives should be investigated in future
studies.
Further investigation revealed that YF3-5 exhibited
the most significant growth inhibition against human lung cancer
cells by inducing apoptosis and G1 cell cycle arrest.
Molecular docking results indicated that YF3-5 interacted with
CDK2. Western blotting results revealed that treatment with YF3-5
induced phosphorylated Rb downregulation. These results suggested
that YF3-5 may arrest the cell cycle at the G1 phase by
interacting with CDK2 and inhibiting the phosphorylation of Rb.
Excessive ROS is also able to induce apoptosis (29,31). The
increased intracellular ROS expression levels induced by YF3-5 in
A549 cells suggested that there may be an association between ROS
generation and apoptosis induction mediated by YF3-5. The results
of the present study demonstrated that YF3-5 induces cell apoptosis
via the generation of ROS.
In conclusion, five matrine derivatives were
synthesized and their anticancer effects were evaluated in four
human cancer cell lines. YF3-5 displayed the strongest anticancer
effects and induced G1 phase cell cycle arrest and ROS
generation in A549 cells. Further results indicated that YF3-5
induced cell apoptosis via the generation of ROS. The present study
provided further information for predicting the structural
modifications of matrine derivatives to enable the synthesis of
novel potent antitumor agents.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 21262005), the Natural
Science Foundation of Guangxi (grant no. 2012GXNSFAA053158), the
Scientific Research Foundation of Guangxi University (grant no.
XJZ160924 and the Tennessee Center for Botanical Medicine
Research.
References
|
1
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group, :
Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 25 Suppl 3:iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ai J, Gao HH, He SZ, Wang L, Luo DL and
Yang BF: Effects of matrine, artemisinin, tetrandrine on cytosolic
[Ca2+]i in guinea pig ventricular myocytes. Acta Pharmacol Sin.
22:512–515. 2001.PubMed/NCBI
|
|
7
|
Cheng H, Xia B, Zhang L, Zhou F, Zhang YX,
Ye M, Hu ZG, Li J, Li J, Wang ZL, et al: Matrine improves
2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice.
Pharmacol Res. 53:202–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Chu W, Liu J, Xue X, Lu Y, Shan H
and Yang B: Antiarrhythmic properties of long-term treatment with
matrine in arrhythmic rat induced by coronary ligation. Biol Pharm
Bull. 32:1521–1526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
10
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Wang Z, Chong T and Ji Z: Matrine
inhibits proliferation and induces apoptosis of the
androgen-independent prostate cancer cell line PC-3. Mol Med Rep.
5:783–787. 2012.PubMed/NCBI
|
|
12
|
Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan
H, Luan G, Yagasaki K and Zhang G: Effects of matrine against the
growth of human lung cancer and hepatoma cells as well as lung
cancer cell migration. Cytotechnology. 59:191–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao H, Yang B, Hu R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanism related to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013.PubMed/NCBI
|
|
15
|
Tan C, Qian X, Jia R, Wu M and Liang Z:
Matrine induction of reactive oxygen species activates p38 leading
to caspase-dependent cell apoptosis in non-small cell lung cancer
cells. Oncol Rep. 30:2529–2535. 2013.PubMed/NCBI
|
|
16
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|
|
17
|
Xiang J and Jiang Y: Antiepileptic
potential of matrine via regulation the levels of
gamma-aminobutyric acid and glutamic acid in the brain. Int J Mol
Sci. 14:23751–23761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu ZG, Li MH, Wang JS, Wei DD, Liu QW and
Kong LY: Developmental toxicity and neurotoxicity of two
matrine-type alkaloids, matrine and sophocarpine, in zebrafish
(Danio rerio) embryos/larvae. Reprod toxicol. 47:33–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao F, Wang DE, Liu R, Tu Q, Liu JJ and
Wang J: Synthesis, characterization and activity evaluation of
matrinic acid derivatives as potential antiproliferative agents.
Molecules. 18:5420–5433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du NN, Li X, Wang YP, Liu F, Liu YX, Li
CX, Peng ZG, Gao LM, Jiang JD and Song DQ: Synthesis,
structure-activity relationship and biological evaluation of novel
N-substituted matrinic acid derivatives as host heat-stress cognate
70 (Hsc70) down-regulators. Bioorg Med Chem Lett. 21:4732–4735.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yong J, Wu X and Lu C: Anticancer advances
of matrine and its derivatives. Curr Pharm Des. 21:3673–3680. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, You Y, Wang S, Liu X, Liu B, Wang
J, Lin X, Chen M, Liang G and Yang H: Synthesis, characterization
and in vitro anti-tumor activities of matrine derivatives. Bioorg
Med Chem Lett. 22:4100–4102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu LC, Wen ZS, Qiu YT, Chen XQ, Chen HB,
Wei MM, Liu Z, Jiang S and Zhou GB: Largazole arrests cell cycle at
G1 phase and triggers proteasomal degradation of E2F1 in lung
cancer cells. Acs Med Chem Lett. 4:921–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L, Guo L, Liang Y, Liu X, Jiang L and
Wang L: Curcumin suppresses stem-like traits of lung cancer cells
via inhibiting the JAK2/STAT3 signaling pathway. Oncol Rep.
34:3311–3317. 2015.PubMed/NCBI
|
|
25
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Padilla F, Bhagirath N, Chen S, Chiao E,
Goldstein DM, Hermann JC, Hsu J, Kennedy-Smith JJ, Kuglstatter A,
Liao C, et al: Pyrrolopyrazines as selective spleen tyrosine kinase
inhibitors. J Med Chem. 56:1677–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng F, Qian J, Yue H, Li X and Xue K:
SUMOylation of Rb enhances its binding with CDK2 and
phosphorylation at early G1 phase. Cell Cycle. 15:1724–1732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: The roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Conklin KA: Chemotherapy-associated
oxidative stress: Impact on chemotherapeutic effectiveness. Integr
Cancer Ther. 3:294–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems and apoptosis. Free Radic Biol Med.
48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|