Introduction
Glioma is the most common intracranial primary
tumor, with invasive growth and high postoperative recurrence. It
is a serious threat to the life and health of patients. At present,
the pathogenesis of glioma is not clear (1). Previous studies have demonstrated that
the occurrence and development of glioma is associated with
apoptosis (2–4). The traditional treatment principle is
postoperative radiotherapy prior to chemotherapy, or either
chemotherapy or radiotherapy alone, but it is difficult to improve
the clinical efficacy (5); the cure
rate is low. In previous years, through considerable investigation
(6–8),
researchers have been seeking new treatments for cerebral
glioma.
At present, numerous anti-tumor drugs have been
developed in domestic and foreign fields, but the majority of drugs
are expensive and have untoward effects. Drug resistance is also a
problem; therefore, the clinical application of these drugs is
restricted (9–11). In previous years, numerous new drugs
for the treatment of glioma have been developed. Carmustine, as a
cell-cycle phase nonspecific alkylating antineoplastic agent
commonly used in the treatment of brain glioma (12), inhibits DNA synthesis and RNA
production by alkylating DNA and RNA, as well as blocking the
activity of DNA polymerases. It has the most notable effect on cell
cycle transition between the G1 phase and S phase, blocks S phase
progression and even has an impact on cells at the G0 phase.
However, long-term use of carmustine may lead to delayed
myelosuppression. In the present study, carmustine was used as a
positive control. New drug treatments with high efficiency, low
toxicity and low price have become the focus of research in recent
years. Tumstatin, which is used in the present study, is a novel
tumor angiostatin from active fragments of the angiogenesis
inhibition region in the thrombospondin-1 protein and angiostatin
linked via a lysine. It consists of 24 amino acids, with a
molecular weight of 2,707 kDa. It was developed and synthesized by
GL Biochem Ltd. (Shanghai, China). The present study focuses on
researching a novel tumstatin that affects C6 glioma cells, and
investigating the mechanism of its inhibition effect on glioma by
detection of apoptosis and mitochondrial membrane potential changes
in glioma cells (13). The present
study aims to provide a theoretical basis for additional research
on the novel tumstatin treatment of gliomas.
Materials and methods
Cells
The C6 brain glioma cell line was purchased from the
Shanghai Institute of Cellular Biology of Chinese Academy of
Sciences (Shanghai, China). Cells were maintained in Dulbecco's
modified Eagle's medium (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) with 10% fetal calf serum (Tianjin Haoyang Biological
Manufacture Co., Ltd., Tianjin, China) at 37°C in an incubator with
5% CO2.
Reagents
The mitochondrial membrane potential assay kit with
JC-1 (catalog no. C2006), and the cell cycle and apoptosis
detection kit (catalog no. C1052) were purchased from Beyotime
Institute of Biotechnology (Haimen, China). Ethidium bromide
(catalog no. E8751), dimethyl sulfoxide (DMSO) and MTT were
purchased from Sigma-Aldrich (EMD Millipore, Billerica, MA, USA).
Acridine orange (catalog no. 1AB10220) was purchased from Ding Guo
Changsheng Biotechnology Co., Ltd. (Beijing, China). The novel
tumstatin was designed by Laboratory of Chemistry and Molecular
Biology Institute of Frontier Medical Sciences, Jilin University
(Changchun, China) and synthesized by GL Biochem Ltd. (Shanghai,
China), with a purity of 98%. Carmustine (catalog no. 1407011) was
purchased from Jinyao Pharmaceutical Co., Ltd. (Tianjin, China;
serial number).
Main instruments
The Nikon ECLIPSE 80i fluorescence microscope (Nikon
Corporation, Tokyo, Japan), a CO2 incubator (model,
NCO-15AC; Sanyo Manufacturing Co., Osaka, Japan), the AN YANG super
clean bench (model: BSC-BOO II B2; Anyang Technology Development
Co., Ltd., Suzhou, China), a flow cytometer (BD Biosciences,
Franklin Lakes, CA, USA) and a microplate reader (model: 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) were all used in the
present study.
Cellular viability by MTT
At the logarithmic growth phase, C6 brain glioma
cells were digested with trypsin (Ameresco, Inc., Framingham, MA,
USA), cells were counted, and 100 µl of cell suspension was added
to each well of a 96-well plate at 37°C in a 5% CO2
atmosphere and incubated for 4 h. When cells attached, plates were
randomly divided for the Mock group, carmustine group and novel
tumstatin of different concentrations (1,000, 1,500 and 2,000
µg/ml), with a total treatment volume of 20 µl/well in 96-well
plate. The Mock group was treated with culture medium at an equal
volume, and each group had 8 wells. At 37°C and in a 5%
CO2 atmosphere for 24, 48 and 72 h, each well was
incubated for 4 h with MTT (20 µl). The reaction was then
terminated with DMSO (150 µl/well), and cells were shocked for 10
min on the plate oscillator. An enzyme immunoassay instrument
detected the absorbance, and absorption wavelength was set at 490
nm.
Acridine orange/ethidium bromide
(AO/EB) double staining to detect cells apoptosis
C6 brain glioma cells in the logarithmic growth
phase were digested with 0.25% trypsin. The cell suspension was
adjusted to 3×105 cells/ml and seeded onto 6-well plates
at 37°C in a 5% CO2 atmosphere for 4 h. This was then
randomly divided for the Mock group, the carmustine group and novel
tumstatin of different concentrations (1,000, 1,500 and 2,000
µg/ml), and 2 wells were allocated to each group (50 µl/well in a
total volume of 2 ml/well in 6-well plate). Coverslips were
removed, covered with cells and then placed on a slide. This was
followed by the dropwise addition of 5 µl of AO/EB dye to the
coverslips. Slides were observed immediately by fluorescence
microscopy, and images were captured (×20). The number of apoptotic
cells per field was counted, and the apoptosis rate was calculated
(% apoptotic cells = number of apoptotic cells/total number of
cells ×100%).
Detection of mitochondrial membrane
potential (JC-1)
C6 brain glioma cells in the logarithmic growth
phase were digested with 0.25% trypsin and inoculated in 6-well
plates with 3.0×105 cells/ml, at 37°C in a 5%
CO2 atmosphere for 4 h. This was randomly divided for
the Mock group, the carmustine group and novel tumstatin of
different concentrations (1,000, 1,500 and 2,000 µg/ml), and two
wells were allocated to each group (50 µl/well with 2 ml). Carbonyl
cyanide m-chlorophenylhydrazone (CCCP; according to the ratio of
1:1,000) was added to the culture medium of each cell as a positive
control group. In the carmustine group and novel tumstatin of
different concentrations, 0.5 ml JC-1 staining liquid (Beyotime
Institute of Biotechnology, Haimen, China) was added to each well.
Following incubation for 20 min at 37°C, the supernatant was
discarded, and cells were rinsed twice with JC-1 (1X) buffer in an
ice bath, and 1 ml of culture medium was added to each well. This
was observed under the fluorescence microscope, at the excitation
wavelength of 490 nm and emission wavelength of 530 nm, and images
were captured (×20).
Cell cycle detection by flow
cytometry
Subsequent to treatment (method 2), cells were
collected with cold PBS, percussed into a single cell, centrifuged,
washed twice with PBS and resuspended with 500 µl PBS. The
following components were then added: 1 mg/ml propidium iodide
(PI); 10 mg/ml RNase A; and 0.01% Triton-X100, protected from light
for 30 min at 37°C. The cell cycle was detected by flow cytometry,
and the percentage of each cell cycle accounted for the
results.
Analysis of apoptosis by Annexin
V-fluorescein isothiocyanate (FITC)/PI
Following treatment (method 2), cells were collected
with cold PBS, resuspended with 195 µl Annexin V-FITC and incubated
for 10 min away from light. PI (10 µl) was then added, and cells
were mixed gently in an ice bath (protected from light) and
detected by flow cytometry. The results were expressed as apoptosis
rate.
Statistical analysis
Statistical analysis was performed by SPSS 13
statistical software (SPSS, Inc., Chicago, IL, USA). The data were
expressed as the mean ± standard deviation. Statistical
significance was compared between the treatment and the control
groups by one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cellular viability by MTT
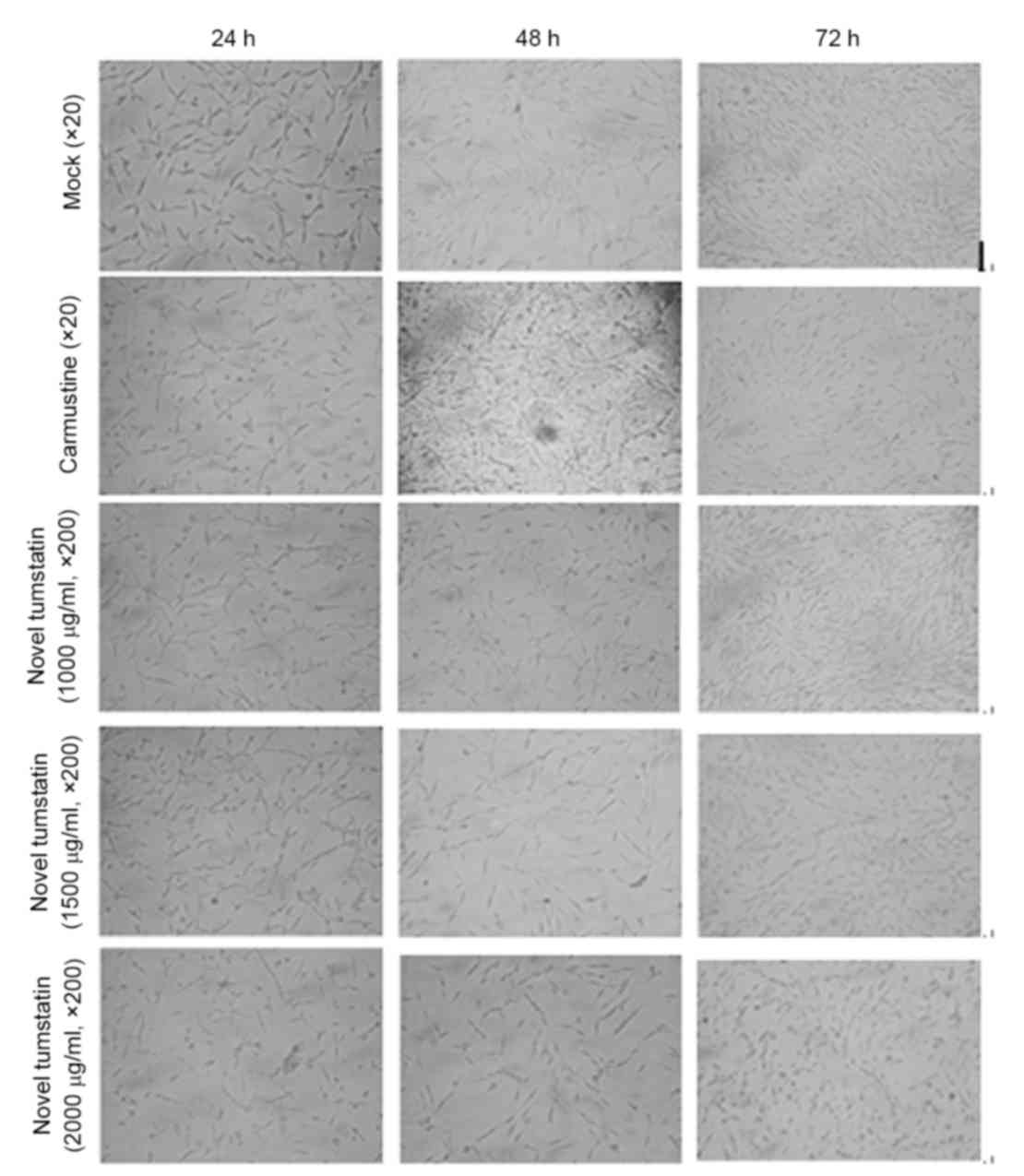
The results demonstrated the effect of different
dose models of tumstatin on C6 glioma cells. At 24 h, cells were
spindle-shaped, which was observed in each experimental group under
a fluorescence microscope, and no significant difference was
observed between each group (P>0.05). At 48 h, novel tumstatin
significantly inhibited C6 glioma cell growth (P<0.05), whereas
growth of the Mock group markedly increased. Significant
differences were visible in the novel tumstatin (2,000 µg/ml) group
compared with the Mock group and novel tumstatin (1,000 µg/ml;
P<0.05). No significant difference was observed in the novel
tumstatin (2,000 µg/ml) group compared with the carmustine group
(P>0.05). At 72 h, each experimental group showed accelerated
and spiral cell growth. Among these groups, cells in the carmustine
group and the novel tumstatin group grew more slowly compared with
the Mock group. At 72 h, the novel tumstatin group had no
inhibitory effects on C6 cell growth (Table I and Fig.
1).
 | Table I.Effect of novel tumstatin on the
activity of C6 cells. |
Table I.
Effect of novel tumstatin on the
activity of C6 cells.
|
| OD value |
|---|
|
|
|
|---|
| Group | 24 h | 48 h | 72 h |
|---|
| Mock group | 0.201±0.034 | 0.247±0.037 | 0.250±0.043 |
| Carmustine group (100
µg/ml) | 0.194±0.030 |
0.193±0.034a | 0.221±0.039 |
| Novel tumstatin group
(1,000 µg/ml) | 0.201±0.025 | 0.231±0.063 | 0.218±0.032 |
| Novel tumstatin group
(1,500 µg/ml) | 0.199±0.037 |
0.194±0.036a | 0.212±0.025 |
| Novel tumstatin group
(2,000 µg/ml) | 0.200±0.028 |
0.183±0.035a,b | 0.215±0.023 |
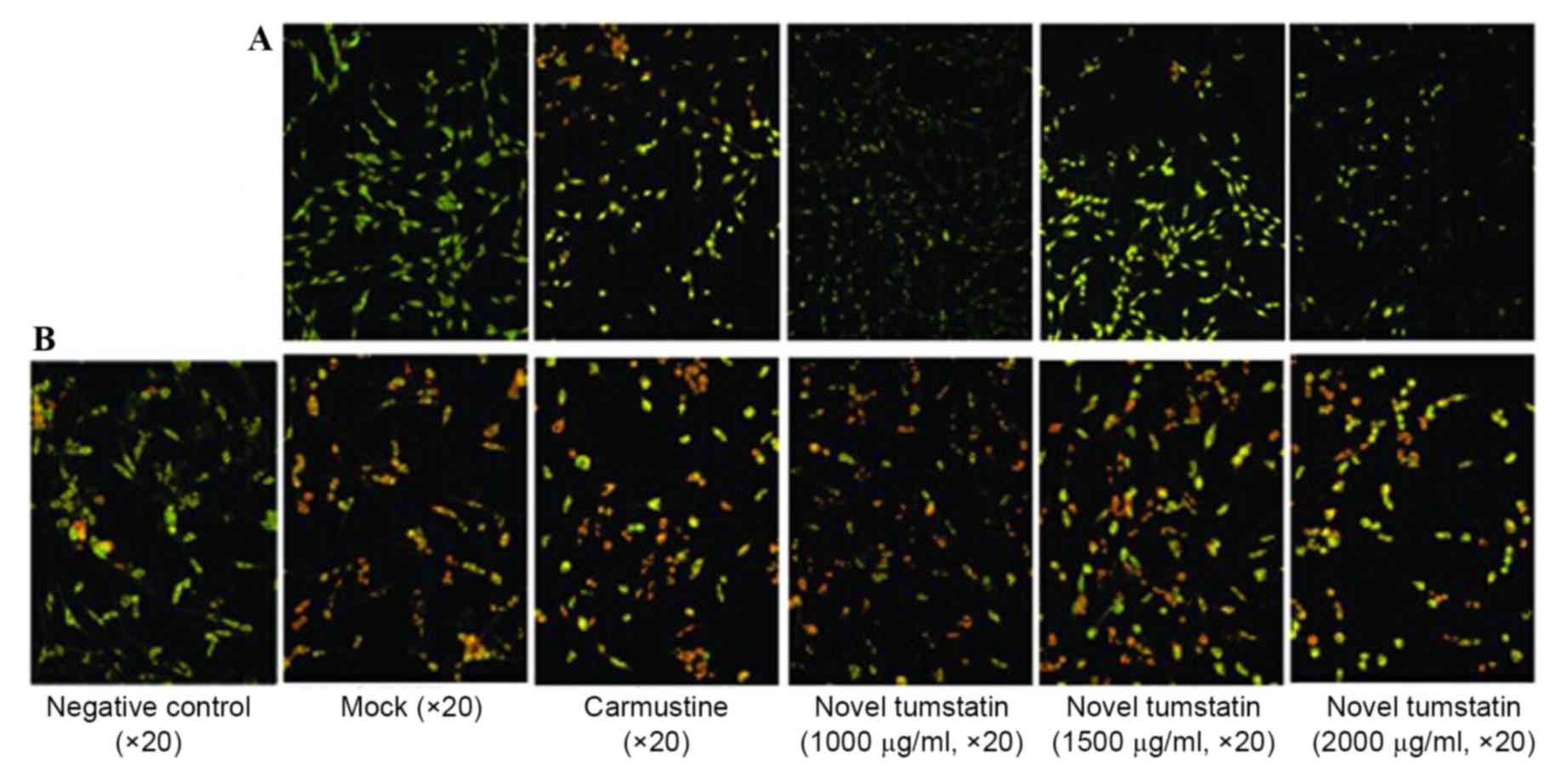
AO/EB double staining
immunofluorescence and morphological observation of cell
apoptosis
C6 glioma cells were treated with novel tumstatin
for 48 h, and using AO/EB staining, apoptosis was observed under a
fluorescence microscope. The results showed a large number of cells
in the Mock group were stained green, and the cells were
spindle-shaped with a whole nucleus and clear demarcation. With the
increase in drug concentration, the number of normal cells was
gradually reduced, while the number of apoptotic cells, which
emitted red fluorescence in the cytoplasm and nucleus, increased.
Significant differences were visible in the novel tumstatin (2,000
µg/ml) group compared with the Mock group and novel tumstatin
(1,000 µg/ml; P<0.05). No significant difference was observed in
the novel tumstatin (2,000 µg/ml) group compared with the
carmustine group (P>0.05; Table
II and Fig. 2A).
 | Table II.Apoptotic effect of novel tumstatin on
C6 glioma cells after 48 h. |
Table II.
Apoptotic effect of novel tumstatin on
C6 glioma cells after 48 h.
|
| 48 h |
|---|
|
|
|
|---|
| Group | Mitochondrial
membrane potential | AO/ED double
staining |
|---|
| CCCP positive control
group |
74.80±17.52 |
|
| Mock group | 20.00±6.20 | 10.00±2.00 |
| Carmustine group (100
µg/ml) |
39.85±4.38a |
44.20±11.43a |
| Novel tumstatin group
(1,000 µg/ml) | 18.60±5.02 | 18.40±4.92 |
| Novel tumstatin group
(1,500 µg/ml) | 29.80±3.42 |
35.80±8.28a |
| Novel tumstatin group
(2,000 µg/ml) |
40.20±6.83a,b |
43.80±5.01a,b |
Effects of the novel tumstatin on C6
brain glioma cell mitochondrial membrane potential
Under the fluorescence microscope, in the CCCP
positive control group, mitochondrial membrane potential of C6
glioma cells was lost completely following treatment with novel
tumstatin (10 µm) for 20 min, and JC-1 staining showed green
fluorescence. In the Mock group, the majority of cells were stained
red, and cells had slender protuberance. Following treatment with
the novel tumstatin (2,000 µg/ml) group on C6 glioma cells for 48
h, the majority of cells showed green fluorescence, and no cell had
protrusions or appeared rounded, suggesting that cells were
apoptotic; red fluorescent cells were spindle-shaped. However, the
protuberance was short compared with the normal control group, the
novel tumstatin (1,000 µg/ml) group and the novel tumstatin (1,500
µg/ml) group. Significant differences were visible in the novel
tumstatin (2,000 µg/ml) group compared with the Mock group and the
novel tumstatin (1,000 µg/ml) group (P<0.05). No significant
difference was observed in the novel tumstatin (2,000 µg/ml) group
compared with the carmustine group (P>0.05; Table II and Fig.
2B).
Effects of the novel tumstatin on
brain glioma cell cycle and apoptosis
The present study showed that at 48 h, novel
tumstatin altered the cell cycle. In the novel tumstatin (2,000
µg/ml) group, the G0/G1 phase rate increased significantly, while
the number of cells in the S phase decreased significantly
(P<0.05). Significant differences were visible in the novel
tumstatin (2,000 µg/ml) group compared with the Mock group and the
novel tumstatin (1,000 µg/ml) group (P<0.05). No significant
difference was observed in the novel tumstatin (2,000 µg/ml) group
compared with the carmustine group (Table III and Fig. 3). Results showed that the novel
tumstatin (2,000 µg/ml) group evidently promoted apoptosis of C6
glioma cells.
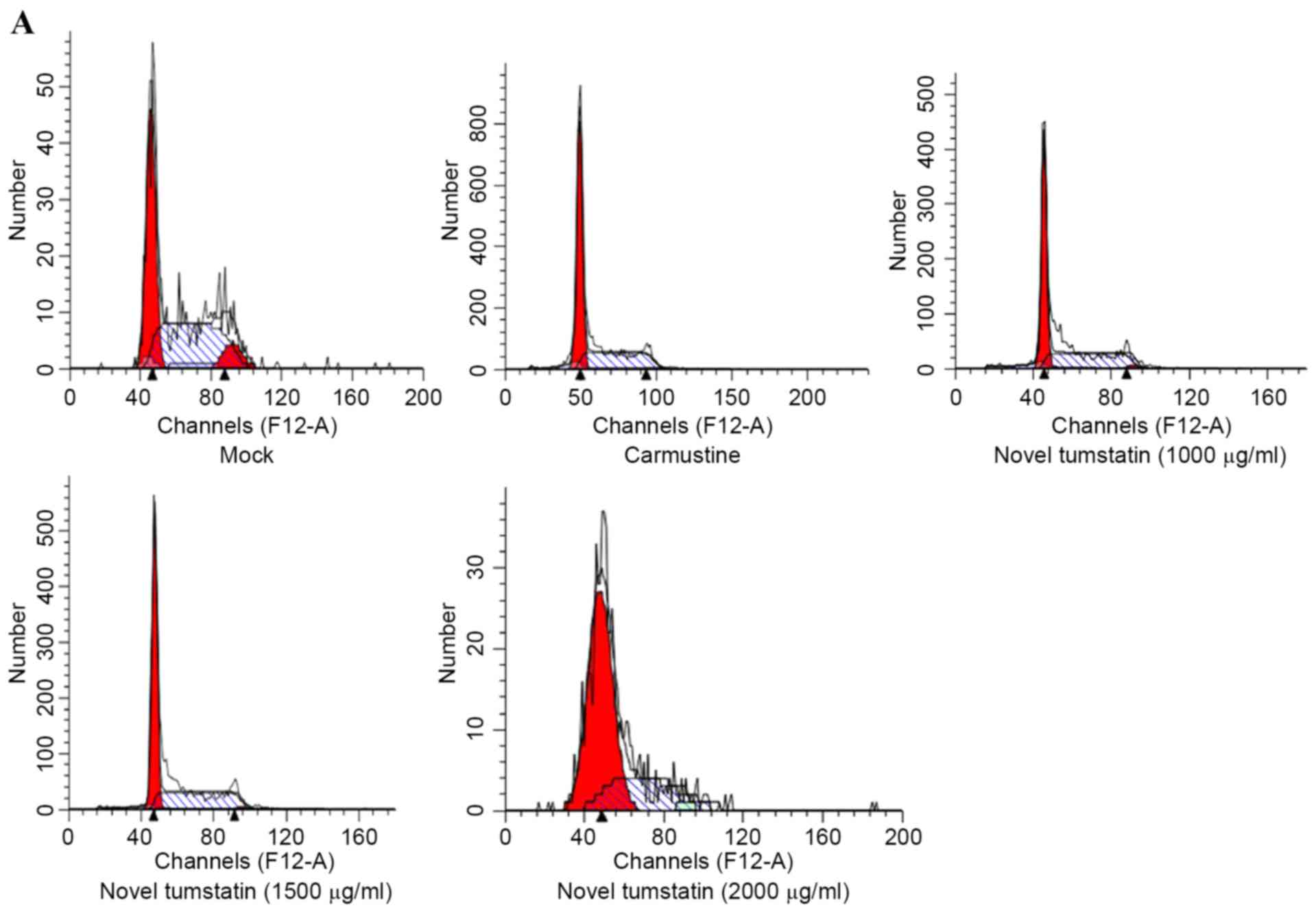 | Figure 3.(A) Effects of different doses of
novel tumstatin on the cell cycle of C6 glioma cells after 48 h.
Cells were treated with different doses (0, 1,000, 1,500 and 2,000
µg/ml) of novel tumstatin and carmustine for 48 h, then analyzed by
flow cytometry. (B) Effects of different doses of novel tumstatin
on the cell apoptosis of C6 glioma cells after 48 h. Cells were
treated with different doses (0, 1,000, 1,500 and 2,000 µg/ml) of
novel tumstatin and carmustine for 48 h, and were then double
labeled with Annexin V-fluorescein isothiocyanate/propidium iodide
and analyzed by flow cytometry. |
 | Table III.Novel tumstatin impact on the cell
cycle and apoptosis of C6 glioma cells. |
Table III.
Novel tumstatin impact on the cell
cycle and apoptosis of C6 glioma cells.
| Group | G0/G1-phase | S-phase | G2/M-phase | Apoptosis rate,
% |
|---|
| Mock group | 46.41±8.57 | 49.67±9.94 | 3.92±4.00 | 4.88±0.
81 |
| Carmustine group (100
µg/ml) | 62.68±2.98 | 36.23±3.49 | 1.08±0.55 | 36.78±8.33 |
| Novel tumstatin group
(1,000 µg/ml) | 52.88±2.71 | 44.36±0.34 | 2.78±2.63 | 10.90±0.97 |
| Novel tumstatin group
(1,500 µl/ml) | 58.82±1.70 | 40.09±2.32 | 1.10±0.65 | 30.96±4.16 |
| Novel tumstatin group
(2,000 µl/ml) |
67.75±3.27a,b |
28.88±2.32a | 2.77±2.09 | 45.96±1.71 |
Discussion
In previous years, the incidence rate of malignant
tumors has been gradually rising. In brain tumors, the incidence of
malignant gliomas is the most evident (12), which accounts for ~35–60% of
intracranial tumors (14). The
pathogenesis of the disease remains unclear, and there has been no
major breakthrough in treatment of the disease. At present, surgery
combined with chemotherapy and radiotherapy reduces the recurrence
and metastasis of tumors; however, the cure rate is low and there
has been no treatment breakthrough. It is the focus of clinical
research on effective glioma treatment.
According to studies in the literature, numerous
antitumor drugs have been investigated, but the majority of the
drugs are expensive and have adverse reactions. There are also
problems of drug resistance; therefore, the clinical application of
these drugs is restricted (9–11). Therefore, current studies are focused
on searching for efficiency, low toxicity, efficacy and stability
of antitumor drugs, and an ideal peptide drug will possess these
advantages precisely. In the present study, the effects of
tumstatin were observed on neurogliocytoma through an in
vitro experiment using C6 glioma cells.
The development of tumors is a complex process and
is associated with cell apoptosis. The imbalance between cell
proliferation and apoptosis was associated with tumorigenesis and
development. Therefore, inhibition of tumor cell proliferation and
inducing the apoptosis of tumor cells have become important methods
for the treatment of cancer (15–18). The
present study used the MTT method and light microscopy to assess
the inhibition of proliferation and induction of apoptosis. The
results revealed that novel tumstatin has an inhibitory effect on
the proliferation of C6 glioma cells, and the most notable function
appears at 48 h; the novel tumstatin (2,000 µg/ml) group showed
significant inhibition of cell proliferation compared with the Mock
group and the novel tumstatin (1,000 µg/ml) group (P<0.05).
Under microscopy, the Mock group cells were in good condition, the
novel tumstatin (2,000 µg/ml) group had marked effects on
inhibition on cell growth, and the number of cells with decreased
growth state was low.
Research demonstrates that there are numerous
factors inducing cells to be apoptotic; the internal factors are
associated with the regulation of certain genes and mitochondrial
membrane potential, while the external factors consist of γ-ray,
hypoxia and anti-tumor drugs (19).
The level of apoptosis examined by flow cytometry was intuitive and
accurate (20–22). In the present study, the apoptosis
rate of cells in response to the novel tumstatin was investigated.
Tumstatin (2,000 µg/ml) significantly increased the apoptosis rate
of C6 glioma cells at 48 h.
The results showed that novel tumstatin and
carmustine induce C6 glioma cell apoptosis, thereby increasing the
percentage of G0/G1 phase cells; however, no significant difference
was observed between the tumstatin (2,000 µg/ml) and carmustine
groups (P>0.05). Following treatment with 2,000 and 1,000 µg/ml
tumstatin, a significant difference was observed in the percentage
of G0/G1 phase cells (P<0.05). Additionally, a more significant
decrease was observed in the percentage of S phase cells, DNA
synthesis and cell proliferation in the tumstatin (2,000 µg/ml)
group compared with the carmustine groups (P<0.05). Therefore,
it was speculated that novel tumstatin arrests the growth of C6
tumor cells through the G0/G1 phase and induces cell apoptosis, and
this effect is time and dose-dependent.
JC-1 is an ideal probe, which is widely used for the
detection of mitochondrial membrane potential. When mitochondrial
membrane potential is high, JC-1 gathers in the mitochondrial
matrix, which forms a polymer with red fluorescence. However, when
the mitochondrial membrane potential is low, JC-1 cannot gather in
the mitochondrial matrix and becomes a single state, emitting green
fluorescence. Detection of the mitochondrial membrane potential by
light color may be convenient. The decrease of mitochondrial
membrane potential is a hallmark of early apoptotic events
(23,24). This research adopts the JC-1 detection
model to observe the effect of novel tumstatin on mitochondrial
transmembrane potential of C6 brain glioma cells. The results show
that at 48 h, the mitochondrial membrane potential of the novel
tumstatin (2,000 µg/ml) group was decreased. Significant
differences were visible in the novel tumstatin (2,000 µg/ml) group
compared with the Mock group and the novel tumstatin (1,000 µg/ml;
P<0.05). No significant difference was observed in the novel
tumstatin (2,000 µg/ml) group compared with the carmustine group
(P>0.05). This indicated that novel tumstatin may act directly
on the mitochondria of C6 cells, and the mitochondrial membrane
permeability increased, causing a decrease in mitochondrial
transmembrane potential and thereby inducing cell apoptosis.
AO/EB staining was used to detect the apoptosis of
C6 cells. The results revealed that novel tumstatin acted on C6
glioma cells after 48 h. A large number of cells were dyed green in
the Mock group, and the cells were spindle-shaped with a visible
nucleus and clear demarcation. With an increase in drug
concentration, the number of normal cells gradually reduced, while
the number of apoptotic cells increased, which emitted red
fluorescence in the cytoplasm or nucleus. Significant differences
in the apoptosis rate were visible in the novel tumstatin (2,000
µg/ml) group compared with the Mock group and the novel tumstatin
(1,000 µg/ml) group (P<0.05). The proportion of apoptotic cells
increased gradually with the increase in drug concentration. The
results were consistent with the cell cycle and mitochondrial
membrane potential. Furthermore, a previous study demonstrated that
tumstatin elicits an evident change in the cell cycle by
down-regulating cyclinD1 expression (not shown) (25); it causes a significant increase in the
number of cells in the G0/G1 phase, but decreases the number of
cells in the S phase. Additionally, tumstatin can notably inhibit
proliferation, but triggers apoptosis in C6 glioma cells, which is
possibly associated with a decline in mitochondrial membrane
potential. These results may provide pharmacological evidence for
tumstatin as a new anti-tumor drug in the treatment of brain
glioma.
In summary, novel tumstatin significantly inhibits
the proliferation of C6 glioma cells, inducing cell apoptosis. The
possible molecular mechanism of apoptosis is associated with the
decrease in mitochondrial membrane potential. On this basis,
additional studies will explore the impact of novel tumstatin on
the protein expression and cell signal transduction.
References
|
1
|
Auffinger B, Spencer D, Pytel P, Ahmed AU
and Lesniak MS: The role of glioma stem cells in chemotherapy
resistance and glioblastoma multiforme recurrence. Expert Rev
Neurother. 15:741–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sancho-Martinez I and Martin-Villalba A:
Tyrosine phosphorylation and CD95: A FAScinating switch. Cell
Cycle. 8:838–842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eisele G, Roth P, Hasenbach K, Aulwurm S,
Wolpert F, Tabatabai G, Wick W and Weller M: APO010, a synthetic
hexameric CD95 ligand, induces human glioma cell death in vitro and
in vivo. Neuro Oncol. 13:155–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konkankit VV, Kim W, Koya RC, Eskin A, Dam
MA, Nelson S, Ribas A, Liau LM and Prins RM: Decitabine
immunosensitizes human gliomas to NY-ESO-1 specific T lymphocyte
targeting through the Fas/Fas ligand pathway. J Transl Med.
9:1922011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao JX, Liu XQ and Dong BJ: Primary study
of bevacizumab combined with temozolomide for recurrent glioma.
Chin Clin Oncol. 39:920–922. 2011.
|
|
6
|
Do N, Weindl G, Grohmann L, Salwiczek M,
Koksch B, Korting HC and Schäfer-Korting M: Cationic
membrane-active peptides-anticancer and antifungal activity as well
as penetration into human skin. Exp Dermatol. 23:326–331. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loud JT, Peters JA, Fraser M and Jenkins
J: Applications of advances in molecular biology and genomics to
clinical cancer care. Cancer Nurs. 25:110–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hopkins AL and Groom CR: The druggable
genome. Nat Rev Drug Discov. 1:727–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okada M, Sato A, Shibuya K, Watanabe E,
Seino S, Suzuki S, Seino M, Narita Y, Shibui S, Kayama T and
Kitanaka C: JNK contributes to temozolomide resistance of stem-like
glioblastoma cells via regulation of MGMT expression. Int J Oncol.
44:591–599. 2014.PubMed/NCBI
|
|
10
|
Sze CI, Su WP, Chiang MF, Lu CY, Chen YA
and Chang NS: Assessing current therapeutic approaches to decode
potential resistance mechanisms in glioblastomas. Front Oncol.
3:592013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Silber JR, Bobola MS, Blank A and
Chamberlain MC: O(6)-methylguanine-DNA methyltransferase in glioma
therapy: Promise and problems. Biochim Biophys Acta. 1826:71–82.
2012.PubMed/NCBI
|
|
12
|
Xu JJ, Hong UQ, Zhao W, Hong J, Wen N,
Ling QI and Liu XJ: Induction of broccoli polypeptide on apoptosis
of C6 glioma cells. Jilin Daxue Xuebao. 39:8–11. 2013.
|
|
13
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferguson SD: Malignant gliomas: Diagnosis
and treatment. Dis Mon. 57:558–569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cobbs CS: Cytomegalovirus and brain tumor:
Epidemiology, biology and therapeutic aspects. Curr Opin Oncol.
25:682–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubrez L, Berthelet J and Glorian V: IAP
proteins as targets for drug development in oncology. Onco Targets
Ther. 9:1285–1304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartuzi P, Hofker MH and van de Sluis B:
Tuning NF-κB activity: A touch of COMMD proteins. Biochim Biophys
Acta. 1832:2315–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang YQQY, Bai X, Lu XD and Tan Y: Effects
of Survivin antisense oligonucleotides on proliferation of hepatic
cell line SMMC-7721. Jilin Daxue Xuebao. 39:278–281. 2013.
|
|
19
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stuckey DW and Shah K: TRAIL on trial:
Preclinical advances in cancer therapy. Trends Mol Med. 19:685–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fink MY and Chipuk JE: Survival of
HER2-positive breast cancer cells: Receptor signaling to apoptotic
control centers. Genes Cancer. 4:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dabbagh A and Rajaei S: The role of
anesthetic drugs in liver apoptosis. Hepat Mon. 13:e131622013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adrain C and Martin SJ: The mitochondrial
apoptosome: A killer unleashed by the cytochrome seas. Trends
Biochem Sci. 26:390–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Velde C Vande, Cizeau J, Dubik D, Alimonti
J, Brown T, Israels S, Hakem R and Greenberg AH: BNIP3 and genetic
control of necrosis-like cell death through the mitochondrial
permeability transition pore. Mol Cell Biol. 20:5454–5468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bo Sun, Jiajing Liu, Jiajun Chen, et al:
Experiment studies of novel tumstatin on C6 glioma cells in vitro.
Chin J Lab Diag. 20:707–709. 2016.
|