Introduction
Ovarian cancer is a clinically significant health
problem globally. Estimates suggested that there would be
>20,000 new cases of ovarian cancer and ~15,000 mortalities due
to ovarian cancer in the United States in 2015 (1). Tumor cells spread by direct extension,
transcoelomic dissemination, lymphatic involvement and,
occasionally, via the hematogenous route (2–4). According
to FIGO staging, cancers involving the retroperitoneal lymph nodes
are classified as stage III cancers (5). However, systematic lymphadenectomy has
demonstrated that lymph node metastasis also occurs during the
early stages (stages I and II) in 14.2% of all patients (6). Systematic lymphadenectomy, which is
predominantly performed in the para-aortic and pelvic regions
(6,7),
is associated with multiple complications and is considered quite
invasive (8,9). The most frequent complications
associated with retroperitoneal lymphadenectomy include
postoperative mortality, vascular injury, lymphocyst formation,
deep venous thrombosis and pulmonary embolism (10–12).
Therefore, there is a need for the development of less invasive and
more sensitive methods for the detection of lymphatic metastasis in
ovarian cancer. Several models for ovarian cancer are commonly
used, including xenografts, genetically engineered mouse models,
laying hen models, ovarian cancer stem cells and three-dimensional
culture models (13–15). Among these models, xenografts are the
most common due to the high tumor rates, relatively low cost and
short experimental period. Intraperitoneal, subcutaneous, and
orthotopic implantation methods are conventional techniques for
establishing ovarian tumors and have been adapted to mimic
different clinical scenarios.
However, despite its importance for cancer diagnosis
and prognosis, preclinical evaluation of lymph node metastasis is
difficult in small animals (16). The
aim of the present study was to develop an appropriate model for
studying metastatic ovarian cancer localized in the retroperitoneal
lymph nodes using near-infrared (NIR) fluorescence imaging in a
xenograft mouse model. These data are expected to provide insights
into the further applications of intra-operative identification of
lymphatic metastasis and studies of the mechanisms of lymphatic
metastasis.
Materials and methods
Cell lines and animals
SKOV-3 cells, established from a human ovarian
adenocarcinoma, were obtained from the Shanghai Institute of Cell
Biology of the Chinese Academy of Sciences (Shanghai, China). The
SKOV-3-LN subline, which has the potential to induce higher rates
of lymphatic metastasis, peritoneal dissemination and bloody
ascites, was established previously in our laboratory (17). Cells were grown in McCoy's 5A medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and were
supplemented with 10% (v/v) fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, and 100
µg/ml streptomycin. Cells were maintained at 37°C in a humidified
incubator containing 5% CO2. Cultures were passaged
every 3 days.
Female BALB/c nude mice (7–8 weeks old) were
purchased from the Animal Center of the Chinese Academy of Sciences
(Shanghai, China) and maintained under specific pathogen-free
conditions at the Department of Laboratory Animal Science, Fudan
University (Shanghai, China). A total of 6 mice (weighing ~20 g)
were used in the present study, they were raised in an atmosphere
of 22±2°C with 65±5% humidity, with a 12 h light-dark cycle and ad
libitum access to water and food. The present study was performed
with the approval of the Animal Ethics Committee of the Obstetrics
and Gynecology Hospital, Fudan University and in accordance with
the Guide for the Ethical Treatment of Laboratory Animals from the
Ministry of Science and Technology of the People's Republic of
China (publication no. 2006-398).
Synthesis of NIR nanoparticles
Nanoparticles with a high lymph node labeling
efficiency were synthesized as follows. The poly
(phenylenevinylene) derivative poly
[2-methoxy-5-(2-ethylhexyloxy)-1, 4-phenylenevinylene; MEH-PPV;
molecular weight (MW): 150,000-250,000 Da] was purchased from
J&K, Inc. (Beijing, China). PS-PEG-COOH (MW: 21,700 Da for the
PS moiety; 1,200 Da for PEG-COOH; polydispersity, 1.25) was
purchased from Polymer Source, Inc. (Montreal, Canada). Silicon 2,
3-naphthalocyanine bis (trihexylsilyloxide) (NIR775) was purchased
from Sigma Aldrich; Merck KGaA (Darmstadt, Germany). NIR775-doped
NIR nanoparticles were prepared as previously described (18). In a typical procedure, a solution of
tetrahydrofluoride (THF) containing 125 µg/ml MEH-PPV, 125 µg/ml
PS-PEG-COOH, and 1.5 µg/ml NIR775 dye was prepared. An aliquot of
the mixture (2 ml) was then dispersed into 10 ml water under
vigorous sonication. Extra THF was evaporated at an elevated
temperature (below 90°C) under the protection of nitrogen. Sizes
and morphologies of NIR nanoparticles were determined using a
Tecnai G2 Spirit Bio-twin transmission electron microscope (TEM;
Thermo Fisher Scientific, Inc.) at 120 kV. TEM samples were
prepared by dripping the nanoparticle solution (5 µl, 500 µg/ml)
onto a carbon-supported copper grid and allowed to dry at room
temperature prior to observation. The grid can then be directly
observed using a TEM following evaporation of the water (18). The absorption spectra were recorded on
a Shimadzu UV-2550 ultraviolet (UV)-Vis spectrometer (Shimazdu
Corporation, Kyoto, Japan). Fluorescence emission spectra were
collected with an Edinburgh LFS-920 spectrophotometer (Edinburgh
Instruments, Ltd., Livingston, UK). Particles were concentrated (3
min/2,685.6 × g at room temperature) using Amicon Ultra 4 ml
centrifugal filters with a membrane nominal molecular weight limit
of 50 kDa (Merck KGaA) for in vivo injection and
imaging.
Generation and analysis of
fluorescently labeled epithelial ovarian cancer (EOC) cells using
lentivirus
SKOV-3-LN cells were infected with lentivirus
(LV-GIDL32812-RNAi (14974-1; Genechem Co., Ltd., Shanghai, China)
containing an 11.4 kb vector for the mCherry sequence (GV298
Genechem Co., Ltd.) and a puromycin resistance gene grown in
McCoy's 5A medium with extra 5 µg/ml polybrene (Genechem Co., Ltd.)
added for lentivirus infection enhancement during infection for 16
h. Infection continued by replacing the medium back to normal
McCoy's 5A medium for another 72 h. Clones infected with a blank
control lentivirus was used for a negative control. Clones were
selected in medium containing puromycin (1 µg/ml) for 48 h. During
infection, cells were maintained at 37°C in a humidified incubator
containing 5% CO2. Fluorescently-labeled EOC cells were
named SKOV3-M and SKOV3-LN-M cells. Cultured SKOV3-LN and
SKOV3-LN-M cells were trypsinized, washed in PBS, and observed by
fluorescence microscope (Nikon ECLIPSE Ti; NIS-Elements software v.
4.0; Nikon Corporation, Tokyo, Kanto, Japan). Analysis for mCherry
expression was performed by fluorescence-activated cell sorting
(FACS) at a cell density of 105 cells/ml using the FL3
channel on a FACS Caliber instrument (BD Biosciences, San Jose, CA,
USA) and FCSExpress V3.1 software (De Novo Software, Glendale, CA,
USA). Cells at different densities (2×105,
1×105, 5×104 and 2.5×104
cells/well) were imaged in 96-well plates using an IVIS Spectrum
Imaging System (PerkinElmer, Inc., Waltham, MA, USA).
Establishment of tumor xenografts and
in vivo imaging
Cultured SKOV3-LN-M cells were trypsinized, washed
in PBS and resuspended in Hanks' Balanced Salt Solution (Thermo
Fisher Scientific, Inc.). Next, 1×106 cells in a 30 µl
volume were injected into the left ovary of mice. A total of four
mice were injected and imaged. Mice were imaged using
excitation/emission 587/610 nm filters for detection of the mCherry
fluorescence signal and using excitation/emission 465/780 nm
filters for detection of the nanoparticle fluorescence signal in
situ using the IVIS Spectrum System (PerkinElmer, Inc.) 5 weeks
following injection. A total of ~50 µg nanoparticles were delivered
by tail veil injection 24 h prior to imaging, and the mice were
fasted to achieve the maximum decrease in autofluorescence. The
mice were then sacrificed via cervical dislocation, and images were
analyzed using Living Imaging software v. 4.4 (PerkinElmer,
Inc.).
Once the imaging was completed, retroperitoneal
lymph nodes were harvested, preserved in 4% paraformaldehyde,
sectioned (4 µm thick) for subsequent hematoxylin and eosin
(H&E) staining and immunohistochemistry (IHC) for the detection
of human cytokeratin (CK) 8 and 7. As the para-aortic lymph nodes
are the most common metastatic nodes, imaging and pathological
verification were limited to these lymph nodes. In IHC staining,
sections were de-waxed and rehydrated after being heated at 60°C
for 1 h. Antigen retrieval was performed by incubation of the
slides with EDTA (PH 9.0; Wuhan Boster Biological Technology Ltd.,
Wuhan, China) at 100°C for 30 min. Following cooling to room
temperature, endogenous peroxidase blocking was performed by
incubation with 3% hydrogen peroxidase for 25 min in the dark at
room temperature and 3% bovine serum albumin (Beijing Solarbio
Science & Technology, Co., Ltd., Beijing, China) was used for
background blocking at room temperature for 30 min. Incubation with
primary antibody anti-human CK7 (1:100; clone OV-TL 12/30; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA; cat. no. M7018)
or anti-human CK8 (1:100; clone TS-1; Thermo Fisher Scientific,
Inc.; cat. no. MA5-14428) was performed overnight at 4°C.
Subsequently, the slides were incubated with peroxidase-conjugated
anti-mouse IgG (1:1; Dako; Agilent Technologies, Inc.; cat. no.
K5007) for 50 min at room temperature followed by staining with
diaminobenzidine and nuclei counter-stain at room temperature for
10 sec each. The results of H&E staining and IHC were observed
and carefully checked under a Leica DM2500 Microscope by at least
two pathologists independently, using Leica LAS v. 4.2 software
(Leica Microsystems GmbH, Wetzlar, Hesse, Germany).
Results
In vitro fluorescence of cancer
cells
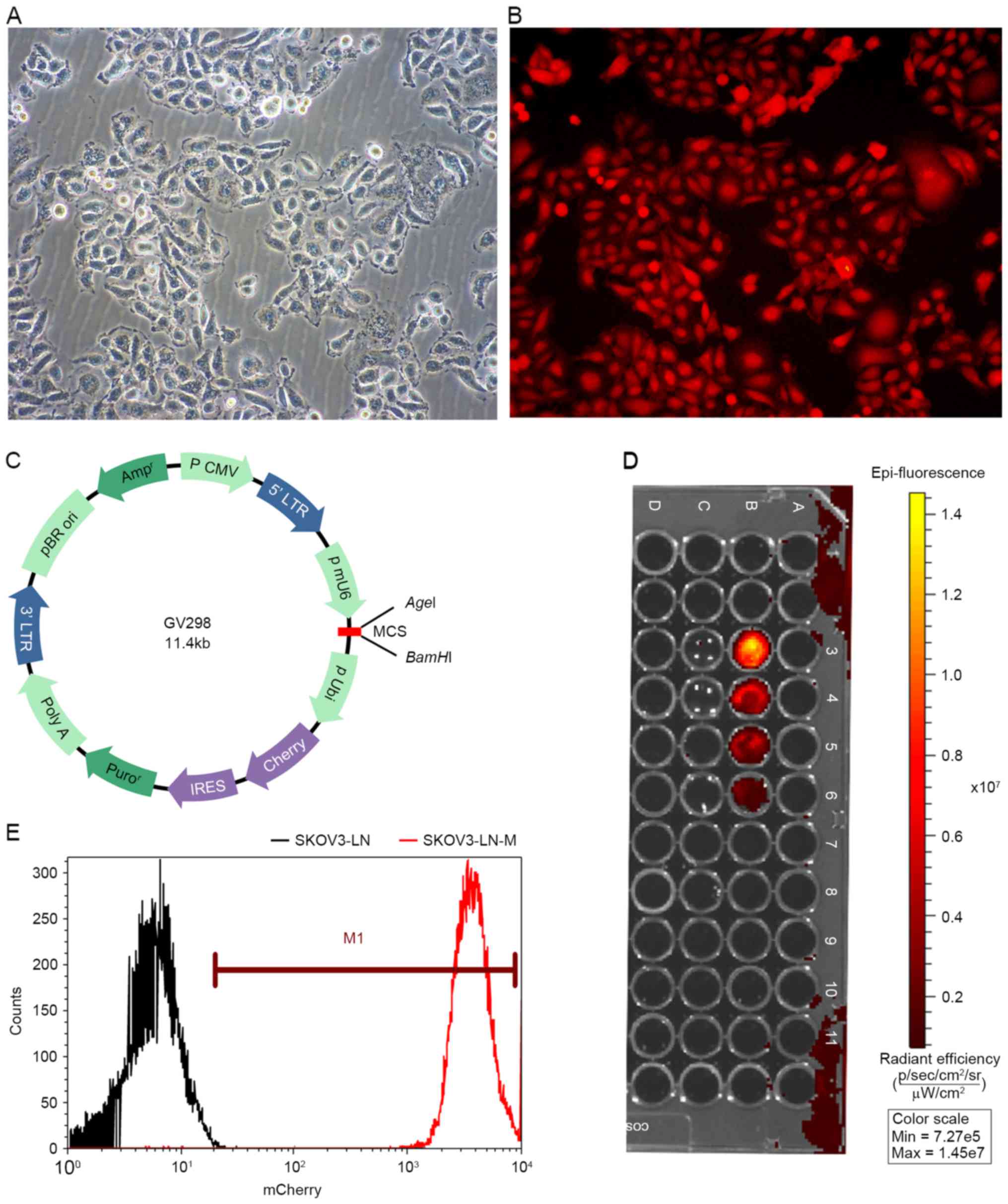
Fluorescently-labeled EOC cells were imaged to
observe mCherry fluorescence. SKOV3-LN cells grew in clusters, and
SKOV3-LN-M cells exhibited strong fluorescence. FACS analysis
revealed that 99.8% of SKOV3-LN cells were labeled with mCherry.
Furthermore, images of cells at different densities revealed that
the emitted fluorescence signals varied according to the cell
density in SKOV3-LN-M cells. At the least, 2.5×104 cells
were detected in vivo. In contrast, no fluorescence signal
was detected in SKOV3-LN cells (Fig.
1).
Characterization of NIR
nanoparticles
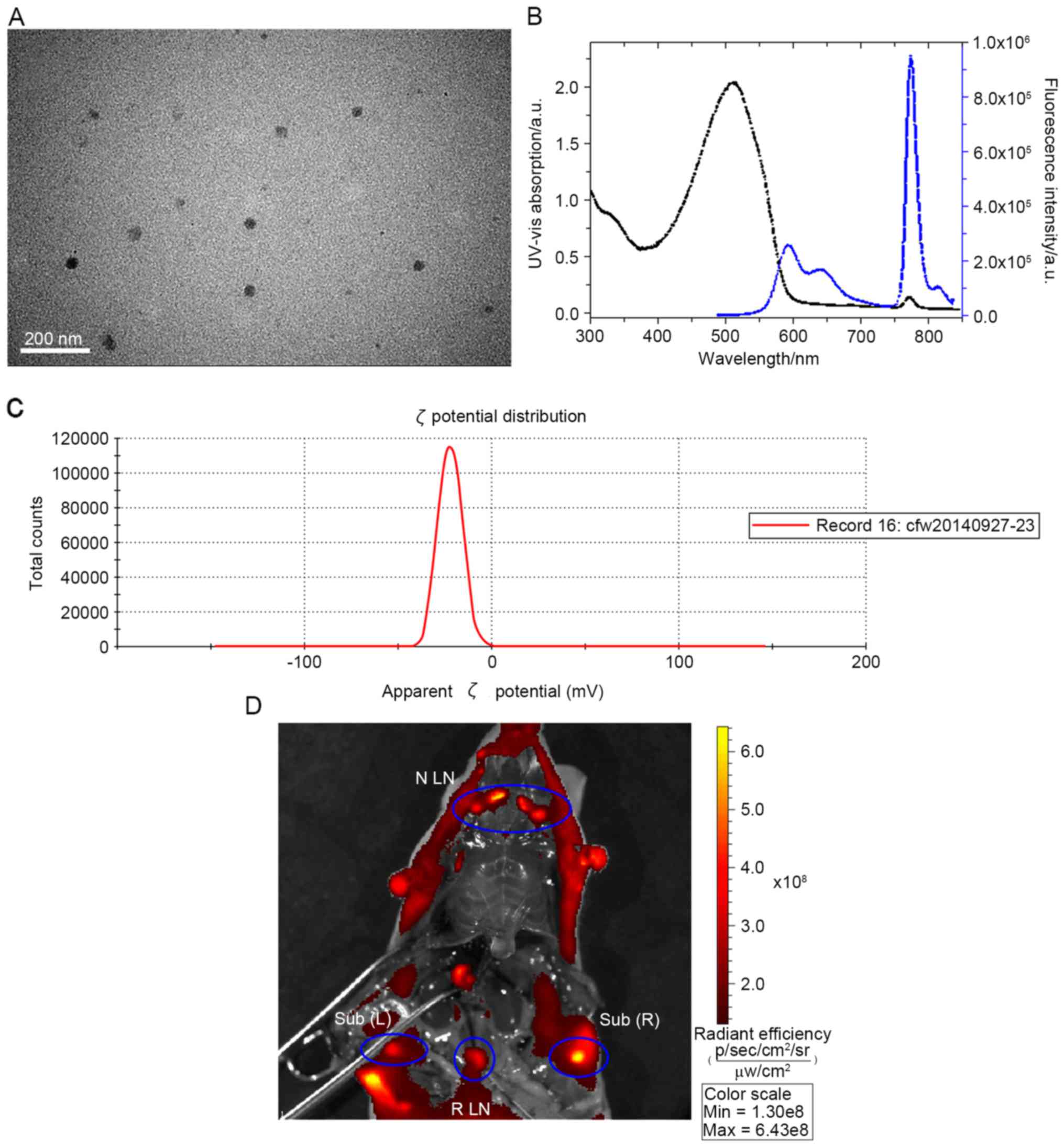
TEM images revealed that the nanoparticles were
dispersed with an average diameter of ~23 nm (Fig. 2A). The NIR nanoparticles exhibited a
broad UV–Vis band with a maximum at 510 nm (Fig. 2B). Under excitation at 480 nm, these
nanoparticles exhibited weak MEH-PPV emission at 595 nm, but a
strong NIR peak at 776 nm. ζ potential analysis predicted that the
NIR nanoparticles had a ζ potential >-30 mV (Fig. 2C). Identification of the lymph nodes
was evaluated using an in vivo imaging system. Major lymph
nodes, including lymph nodes in the neck region, subiliac lymph
nodes and the retroperitoneal lymph nodes, were visualized
(Fig. 2D).
In vivo imaging of tumor
xenografts
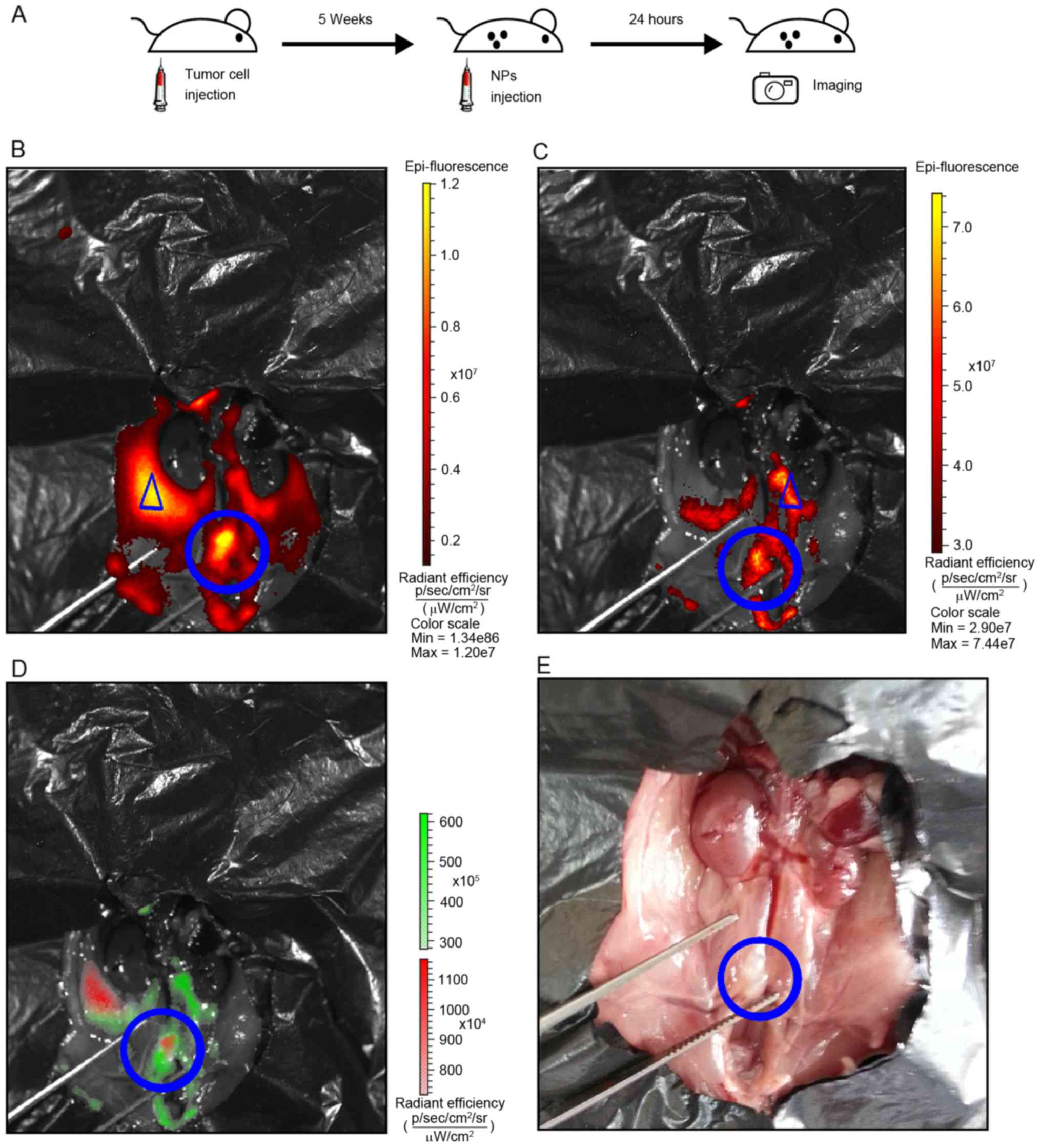
Tumor xenografts were established and imaged as
illustrated in the flowchart in Fig.
3A and described in the materials and methods section. Tumor
cells exhibited a strong and well-defined fluorescent signal for
mCherry (Fig. 3B). Notably, the
fluorescence signal of the nanoparticles was observed in the lymph
nodes and adjacent potential lymphatic vessels (Fig. 3C). Spectral unmixing analysis
demonstrated that the mCherry and nanoparticle signals overlapped,
suggesting that metastatic lymph nodes were detected (Fig. 3D). The corresponding gross anatomy of
the retroperitoneal lymph nodes is depicted in Fig. 3E.
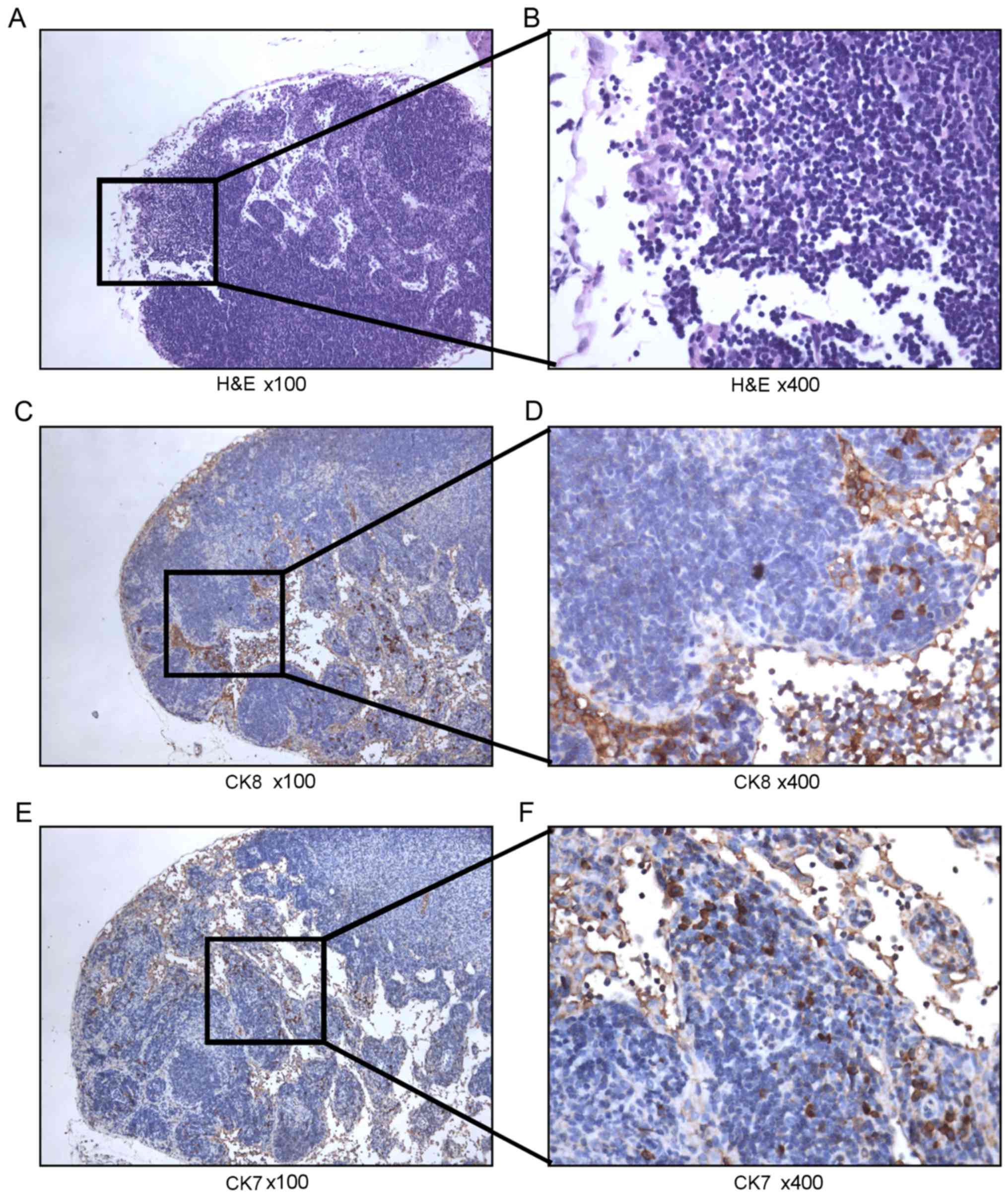
H&E staining was then performed on the lymph
nodes identified as potentially metastatic. However, the
imaging-positive lymph nodes tested negative following
histopathological examination (Fig. 4A
and B). Therefore, IHC was performed for the detection of human
CK7 and CK8 as the next analytical step. From this analysis,
human-derived epithelial tumor cells were successfully identified.
Analysis of human CK8 expression revealed the presence of
nesting-like positive cells (Fig. 4C and
D). In addition, analysis of human CK7, which is used to
characterize epithelial tumors (19),
revealed the presence of sporadic strongly positive cells (Fig. 4E and F), suggesting that these lymph
nodes contained metastatic ovarian tumor cells.
Discussion
The aim of the present study was to develop a
fluorescent label for the analysis of lymphatic metastasis in
vivo using fluorescently-labeled ovarian cancer cells. Imaging
results of the cells in vitro revealed the presence of
mCherry fluorescence. In addition, metastatic retroperitoneal lymph
nodes were successfully identified in vivo by
co-localization with lymph nodes labeled by NIR nanoparticles.
Of the metastatic lymph nodes, para-aortic and
pelvic lymph nodes are the most common in patients with ovarian
cancer. Furthermore, lymphogenous metastasis is associated with
disease progression and recurrence (7,20).
Cytoreductive surgery is the standard approach for the management
of ovarian cancer, and systematic lymphadenectomy is frequently
conducted despite the associated complications and complexity of
the surgical procedures (8,9). However, isolation of a specific single
node as the sentinel node is difficult prior to or during surgery
(21).
It may be possible to apply positron emission
tomography (PET)/computed tomography as a pre-operative imaging
step in order to address this clinical dilemma. In ovarian cancer,
few studies have examined the accuracy of these imaging modalities
for the detection of metastasis (22). Owing to the spatial resolution of
current PET scanners (4–6 mm) (23),
lymph nodes <5 mm in diameter may not be identified in
early-stage ovarian cancer (22). In
recurrent cases, a size threshold of 0.5 cm and 41% detection
sensitivity of retroperitoneal lymph node metastases have been
reported (24,25). As a result, the detection of
metastatic lymph nodes <5 mm in diameter is impracticable with
PET; thus, several imaging agents have been developed for the
visualization of early or recurrent ovarian tumors.
Folate receptor (FR) α and human epidermal growth
factor receptor (HER)-2 are widely used as targeting agents.
FRα-targeted fluorescent agents have been applied experimentally in
intra-operative imaging to facilitate staging and accurate radical
cytoreductive surgery (26). This
method has demonstrated promising results in histopathologically
confirmed tumor deposits measuring <1 mm for up to 8 h following
injection. However, this study only addressed the peritoneal tumor
deposits and did not report the capacity for detection of lymphatic
systems (26). HER-2-targeted
nanoparticles and low-molecular-weight proteins have also been
employed as imaging agents in tumor-bearing mice in optical or PET
imaging (27–29). Early-stage tumors with diameters of
1–2 mm were detectable in orthotopic ovarian tumor xenografts and
small metastatic lesions (~1 mm) in the peritoneal cavity and in
lung metastasis (27). However, this
detection was limited to HER-2-overexpressing SKOV-3 tumors, and
lymphatic involvement has not been observed or investigated.
Application of NIR fluorescence imaging, which
utilizes contrast agents with fluorescent characteristics in the
NIR spectrum (700–900 nm), has emerged to allow researchers to
overcome problems with anatomical guidance and tumor
identification, particularly for minimally invasive surgeries.
Identification of the vasculature, biliary tract in
cholecystectomy, adrenal glands in partial adrenalectomy and nerves
in thymectomy, as well as real-time visualization of ureters and
tissue perfusion assessment in colorectal anastomosis, have been
facilitated by navigation with NIR fluorescence imaging (30). For tumor delineation, NIR fluorescence
agents targeting FRα and αvβ3 integrin have been reported in
ovarian cancer (31). Targeted αvβ3
integrin imaging of intraperitoneal tumor xenografts has been
demonstrated to have 100% sensitivity, 88% specificity and a mean
target to background ratio of 2.2 (31). However, detection of lymphatic
metastasis using this method has not been reported. NIR
fluorescence agents have also been studied in sentinel lymph node
mapping; The Food and Drug Administration-approved indocyanine
green (ICG; 800 nm) is the most common agent used in this context,
and targeted contrast agents are not required when ICG is used
(30). The feasibility and accuracy
of sentinel lymph node mapping using ICG has been demonstrated in
melanoma, colorectal cancer, breast cancer, non-small cell lung
cancer, esophageal cancer, and gastric cancer (32–37).
Furthermore, NIR fluorescence-based sentinel lymph node mapping has
been studied in vulvar cancer, cervical cancer, and endometrial
cancer (38–40); but few studies concerning sentinel
lymph node mapping in ovarian cancer have been reported. Therefore,
based on the above-mentioned studies, there is a need for less
invasive and highly sensitive detection of lymphatic metastasis in
ovarian cancer.
In the present study, nanoparticles were used for
highly efficient lymph node labeling. Major lymph nodes, including
the mandibular lymph nodes, superficial parotid lymph nodes,
subiliac lymph nodes, and retroperitoneal medial iliac lymph nodes
were identified 24 h following injection (excitation/emission:
465/780 nm). Identification of the lymph nodes was matched with the
anatomy of murine lymph nodes (41).
In addition, metastatic tumor cells were labeled with mCherry
fluorescent protein; a general-purpose red fluorescent protein with
superior photostability (42). As
mCherry has a separate excitation/emission peak of 587/610 nm, it
was possible to co-localize the metastatic lymph nodes where the
two signals overlapped. Instead of using a targeting receptor, for
example FRα, HER-2 or αvβ3-integrin, the metastatic tumors were
successfully located based on the spatial overlapping of tumor
signals and lymph node signals, allowing tumor delineation
regardless of the expression of target receptors. Furthermore, with
the identification of spatial overlapping signals, it is also
possible to apply this method to track the metastatic tumors in the
lymphatic system in time-course studies, which provide additional
insights into the mechanisms of lymphatic metastasis. While routine
H&E staining of these imaging-positive lymph nodes yielded
histopathologically negative results, IHC analysis successfully
identified human-derived epithelial tumor cells, demonstrating the
high sensitivity of the imaging model of the present study for
metastatic ovarian cancer lymph nodes. Thus, this method may
contribute to the identification of metastatic lymph nodes when
positive signals are presented and a more proactive pathological
analysis is required. With further investigation, this model may be
used for intra-operative imaging due to its potential for the
sensitive identification of lymphatic metastasis, and it may also
facilitate sentinel lymph node sampling in further studies.
Furthermore, simple nanoparticles were successfully delivered to
the metastatic lymph nodes, providing the opportunity for packaging
of chemotherapy agents and delivery of these agents to metastatic
lymphatic lesions.
The limitations of the present study include the
potentially shallow tissue penetration depth of the imaging
modality and the interference of autofluorescence, which is
frequently observed in fluorescent signal analyses. Because of the
interference of autofluorescence, all of the images were collected
subsequent to the removal of the gastrointestinal and reproductive
system tissues, and with black sheets covering the surrounding
tissues.
In the present study, metastatic retroperitoneal
lymph nodes were identified by co-localization of lymph nodes and
tumor cells with NIR fluorescence nanoparticles in a xenograft
mouse model. The results suggested that this method may yield
important insights, and that it may be possible to apply these data
to intra-operative identification of lymphatic metastasis, analysis
of the mechanisms of lymphatic metastasis and evaluation of
lymphatic drug delivery.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant no. 81472423); The
Shanghai Natural Science Foundation of China (grant no.
13ZR1404300); The Experimental Animal Special Fund of Science and
Technology Commission of Shanghai (grant no. 13140901500); Chinese
Natural Science Foundation project (grant nos. 81301261 and
21374059); Key Project for Major Diseases of Health System of
Shanghai (grant no. 2013ZYJB0201) and National Key Research and
Development Program (grant nos. 2016YFC1303100, 2016YFC1303101,
2016YFC1303102 and 2016YFC1303103).
Glossary
Abbreviations
Abbreviations:
|
NIR
|
near-infrared
|
|
MEH-PPV
|
poly (2-methoxy
−5-[2-ethylhexyloxy]-1, 4-phenylenevinylene)
|
|
MW
|
molecular weight
|
|
THF
|
tetrahydrofluoride
|
|
UV
|
ultraviolet
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
CK
|
cytokeratin
|
|
PET
|
positron emission tomography
|
|
HER-2
|
human epidermal growth factor receptor
2
|
|
FR
|
folate receptor
|
|
ICG
|
indocyanine green
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan DS, Agarwal R and Kaye SB: Mechanisms
of transcoelomic metastasis in ovarian cancer. Lancet Oncol.
7:925–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadducci A, Cosio S, Zola P, Sostegni B,
Ferrero AM, Teti G, Cristofani R and Sartori E: The clinical
outcome of epithelial ovarian cancer patients with apparently
isolated lymph node recurrence: A multicenter retrospective Italian
study. Gynecol Oncol. 116:358–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutch DG and Prat J: 2014 FIGO staging for
ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol.
133:401–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kleppe M, Wang T, Van Gorp T, Slangen BF,
Kruse AJ and Kruitwagen RF: Lymph node metastasis in stages I and
II ovarian cancer: A review. Gynecol Oncol. 123:610–614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereira A, Magrina JF, Rey V, Cortes M and
Magtibay PM: Pelvic and aortic lymph node metastasis in epithelial
ovarian cancer. Gynecol Oncol. 105:604–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Camara O and Sehouli J: Controversies in
the management of ovarian cancer-pros and cons for lymph node
dissection in ovarian cancer. Anticancer Res. 29:2837–2843.
2009.PubMed/NCBI
|
|
9
|
Trimbos JB: Lymphadenectomy in ovarian
cancer: Standard of care or unnecessary risk. Curr Opin Oncol.
23:507–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
di Re F, Baiocchi G, Fontanelli R, Grosso
G, Cobellis L, Raspagliesi F and di Re E: Systematic pelvic and
paraaortic lymphadenectomy during cytoreductive surgery in advanced
ovarian cancer: Potential benefit on survival. Gynecol Oncol.
62:360–365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scarabelli C, Gallo A, Zarrelli A,
Visentin C and Campagnutta E: Systematic pelvic and para-aortic
lymphadenectomy during cytoreductive surgery in advanced ovarian
cancer: Potential benefit on survival. Gynecol Oncol. 56:328–337.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Re F and Baiocchi G: Value of lymph
node assessment in ovarian cancer: Status of the art at the end of
the second millennium. Int J Gynecol Cancer. 10:435–442. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaw TJ, Senterman MK, Dawson K, Crane CA
and Vanderhyden BC: Characterization of intraperitoneal, orthotopic
and metastatic xenograft models of human ovarian cancer. Mol Ther.
10:1032–1042. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricci F, Broggini M and Damia G:
Revisiting ovarian cancer preclinical models: Implications for a
better management of the disease. Cancer Treat Rev. 39:561–568.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lengyel E, Burdette JE, Kenny HA, Matei D,
Pilrose J, Haluska P, Nephew KP, Hales DB and Stack MS: Epithelial
ovarian cancer experimental models. Oncogene. 33:3619–3633. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Servais EL, Colovos C, Bograd AJ, White J,
Sadelain M and Adusumilli PS: Animal models and molecular imaging
tools to investigate lymph node metastases. J Mol Med (Berl).
89:753–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang Y, Pu T, Cai Q, Hong S, Zhang M, Li
G, Zhu Z and Xu C: Identification of lymphatic metastasis
associated genes in a metastatic ovarian cancer cell line. Mol Med
Rep. 12:2741–2748. 2015.PubMed/NCBI
|
|
18
|
Xiong L, Shuhendler AJ and Rao J:
Self-luminescing BRET-FRET near-infrared dots for in vivo
lymph-node mapping and tumour imaging. Nat Commun. 3:11932012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Painter JT, Clayton NP and Herbert RA:
Useful immunohistochemical markers of tumor differentiation.
Toxicol Pathol. 38:131–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pereira A, Pérez-Medina T, Magrina JF,
Magtibay PM, Rodríguez-Tapia A, de León J, Peregrin I and
Ortiz-Quintana L: Correlation between the extent of intraperitoneal
disease and nodal metastasis in node-positive ovarian cancer
patients. Eur J Surg Oncol. 40:917–924. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Classe JM, Cerato E, Boursier C, Dauplat
J, Pomel C, Villet R, Cuisenier J, Lorimier G, Rodier JF, Mathevet
P, et al: Retroperitoneal lymphadenectomy and survival of patients
treated for an advanced ovarian cancer: The CARACO trial. J Gynecol
Obstet Biol Reprod (Paris). 40:201–204. 2011.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Signorelli M, Guerra L, Pirovano C,
Crivellaro C, Fruscio R, Buda A, Cuzzucrea M, Elisei F, Ceppi L and
Messa C: Detection of nodal metastases by 18F-FDG PET/CT in
apparent early stage ovarian cancer: A prospective study. Gynecol
Oncol. 131:395–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitajima K, Murakami K, Yamasaki E, Kaji
Y, Fukasawa I, Inaba N and Sugimura K: Diagnostic accuracy of
integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer:
Comparison with enhanced CT. Eur J Nucl Med Mol Imaging.
35:1912–1920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bristow RE, Giuntoli RL II, Pannu HK,
Schulick RD, Fishman EK and Wahl RL: Combined PET/CT for detecting
recurrent ovarian cancer limited to retroperitoneal lymph nodes.
Gynecol Oncol. 99:294–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pannu HK, Cohade C, Bristow RE, Fishman EK
and Wahl RL: PET-CT detection of abdominal recurrence of ovarian
cancer: Radiologic-surgical correlation. Abdom Imaging. 29:398–403.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Dam GM, Themelis G, Crane LM, Harlaar
NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ,
van der Zee AG, et al: Intraoperative tumor-specific fluorescence
imaging in ovarian cancer by folate receptor-alpha targeting: First
in-human results. Nat Med. 17:1315–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Satpathy M, Wang L, Zielinski R, Qian W,
Lipowska M, Capala J, Lee GY, Xu H, Wang YA, Mao H and Yang L:
Active targeting using HER-2-affibody-conjugated nanoparticles
enabled sensitive and specific imaging of orthotopic HER-2 positive
ovarian tumors. Small. 10:544–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren G, Webster JM, Liu Z, Zhang R, Miao Z,
Liu H, Gambhir SS, Syud FA and Cheng Z: In vivo targeting of
HER2-positive tumor using 2-helix affibody molecules. Amino Acids.
43:405–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miao Z, Ren G, Jiang L, Liu H, Webster JM,
Zhang R, Namavari M, Gambhir SS, Syud F and Cheng Z: A novel
18F-labeled two-helix scaffold protein for PET imaging of
HER2-positive tumor. Eur J Nucl Med Mol Imaging. 38:1977–1984.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schols RM, Connell NJ and Stassen LP:
Near-infrared fluorescence imaging for real-time intraoperative
anatomical guidance in minimally invasive surgery: A systematic
review of the literature. World J Surg. 39:1069–1079. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harlaar NJ, Kelder W, Sarantopoulos A,
Bart J, Themelis G, van Dam GM and Ntziachristos V: Real-time near
infrared fluorescence (NIRF) intra-operative imaging in ovarian
cancer using an α(v)β(3-)integrin targeted agent. Gynecol Oncol.
128:590–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Vorst JR, Schaafsma BE, Verbeek
FP, Swijnenburg RJ, Hutteman M, Liefers GJ, van de Velde CJ,
Frangioni JV and Vahrmeijer AL: Dose optimization for near-infrared
fluorescence sentinel lymph node mapping in patients with melanoma.
Br J Dermatol. 168:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cahill RA, Anderson M, Wang LM, Lindsey I,
Cunningham C and Mortensen NJ: Near-infrared (NIR) laparoscopy for
intraoperative lymphatic road-mapping and sentinel node
identification during definitive surgical resection of early-stage
colorectal neoplasia. Surg Endosc. 26:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Troyan SL, Kianzad V, Gibbs-Strauss SL,
Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F and
Frangioni JV: The FLARE intraoperative near-infrared fluorescence
imaging system: A first-in-human clinical trial in breast cancer
sentinel lymph node mapping. Ann Surg Oncol. 16:2943–2952. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamashita S, Tokuishi K, Miyawaki M, Anami
K, Moroga T, Takeno S, Chujo M, Yamamoto S and Kawahara K: Sentinel
node navigation surgery by thoracoscopic fluorescence imaging
system and molecular examination in non-small cell lung cancer. Ann
Surg Oncol. 19:728–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kubota K, Yoshida M, Kuroda J, Okada A,
Ohta K and Kitajima M: Application of the hypereye medical system
for esophageal cancer surgery: A preliminary report. Surg Today.
43:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tajima Y, Murakami M, Yamazaki K, Masuda
Y, Kato M, Sato A, Goto S, Otsuka K, Kato T and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging
during laparoscopic surgery in gastric cancer. Ann Surg Oncol.
17:1787–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hutteman M, van der Vorst JR, Gaarenstroom
KN, Peters AA, Mieog JS, Schaafsma BE, Löwik CW, Frangioni JV, van
de Velde CJ and Vahrmeijer AL: Optimization of near-infrared
fluorescent sentinel lymph node mapping for vulvar cancer. Am J
Obstet Gynecol. 206:89. e1–e5. 2012. View Article : Google Scholar
|
|
39
|
van der Vorst JR, Hutteman M, Gaarenstroom
KN, Peters AA, Mieog JS, Schaafsma BE, Kuppen PJ, Frangioni JV, van
de Velde CJ and Vahrmeijer AL: Optimization of near-infrared
fluorescent sentinel lymph node mapping in cervical cancer
patients. Int J Gynecol Cancer. 21:1472–1478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Holloway RW, Bravo RA, Rakowski JA, James
JA, Jeppson CN, Ingersoll SB and Ahmad S: Detection of sentinel
lymph nodes in patients with endometrial cancer undergoing
robotic-assisted staging: A comparison of colorimetric and
fluorescence imaging. Gynecol Oncol. 126:25–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Van den Broeck W, Derore A and Simoens P:
Anatomy and nomenclature of murine lymph nodes: Descriptive study
and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol
Methods. 312:12–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shaner NC, Steinbach PA and Tsien RY: A
guide to choosing fluorescent proteins. Nat Methods. 2:905–909.
2005. View Article : Google Scholar : PubMed/NCBI
|