Introduction
The immune system of patients with cancer is often
in an inhibitory state, and is unable to fulfill responsibilities
of immunological surveillance (1).
These patients often suffer from decreased T cell counts, which
lowers their ability to cope with different pathogens (2). A large number of immunosuppressive cells
accumulate in tumor microenvironments, including exhausted
cytotoxic T lymphocytes, Tregs and myeloid derived suppression
cells, which limits the normal function of effector immune cells
(3).
Generation of adaptive immune responses mainly takes
place in secondary lymphoid organs, particularly lymph node (LN)
draining of pathological sites. Antigen-specific lymphocytes are
predominantly activated and expanded in draining LNs (4). However, the microenvironment in tumor
draining lymph nodes (TDLNs) of patients with cancer appears to be
in favor of immune tolerance (5).
Cell components of LNs consist of immune cells and stromal cells.
According to their different surface markers [CD31 and podoplanin
(gp38)] stromal cells can be separated into several subtypes
(6). Fibroblastic reticular cells
(FRCs; gp38+CD31−) are the main constructer
in LNs, which orchestrate a complex network of extracellular matrix
and collagens (4). Lymphocytes then
roam through this network to search for cognate antigens expressed
on the surfaces of antigen-presenting cells (7). This interaction between stromal cells
and immune cells in LNs is critical for the normal function of the
immune system (7–9). Simultaneously, FRCs are the main source
of interleukin-7 (IL-7) in the periphery, a survival signal to
lymphocytes immigrated into LNs (10). Competition for this limited signal may
control the size of the T lymphocyte pool (11). Lymphatic endothelial cells
(gp38+CD31+) circle around the lymphatic
vessel in LNs and are another producer of IL-7 in mice and human
fetal mesenteric LNs (12). These
stromal cells are candidate compartments for the maintenance of
peripheral immune tolerance (6,13–15).
Accordingly, the present study aimed to elucidate
whether tumor cells have an impact on LN stromal cells, to
deteriorate their immunosuppressive status in patients with cancer.
The present study focused on IL-7 produced by stromal cells, a
cytokine indispensable for the adaptive immune system (16). T cell homeostasis and
lymphopenia-induced proliferation are highly dependent on
peripheral IL-7 (17,18), and are also crucial for T
lymphopoiesis in the thymus (19) and
LN organogenesis (20–22). The present study identified that IL-7
produced by FRCs in TDLNs of tumor-bearing mice was significantly
decreased. Inadequate supply of IL-7 in TDLNs was insufficient to
support the survival of T cells in LNs, which will aggravate the
immunosuppressive status of cancer.
Materials and methods
Mice
Approval for animal experiments was obtained from
the Institutional Ethics Committee of the Third Military Medical
University (TMMU; Chongqing, China). Female 6-week-old C57BL/6
mice, weighting 16–18 g, came from the Center of Experimental
Animals of TMMU. They were housed in SPF-class laboratory animal
room at a temperature of 22–26°C and humidity of 50–60%. They were
maintained under a 12 h dark/12 h light cycle, supplied with
sterile food and water ad libitum. All animals were assigned to
control and tumor-bearing groups randomly. A total of
1×106 Lewis lung carcinoma cells, suspended in 100 µl
PBS buffer, were inoculated in the right inguinal region of
mice.
Patient cohorts
A total of 40 patients diagnosed with colon cancer
at different Dukes stages (23) who
had received surgical excision in Xinqiao Hospital were included in
the present study. Ethical approval for the project was obtained
from the Institutional Ethics Committee of the TMMU. Written
informed consent was obtained from each patient included in the
present study.
LN biopsy specimens
A total of 40 patients with colon cancer at
different Dukes stages were enrolled into the present study. LN
biopsies, from patients undergoing surgical resection or
colonoscopic biopsy were obtained from the Department of Pathology,
Xinqiao Hospital (Chongqing, China) randomly. All LN biopsies came
from mesenteric LNs which were defined as TDLNs. According to the
criteria of Dukes stages (23), each
stage (A-D) contained 10 patients, and there were 1 to 4 LNs for
each patient. The study mainly focused on the changes of IL-7,
number of T cells and FRCs in TDLNs. As a result, TDLNs from Dukes
stage A were used as control groups in the patient experiment. Mice
were sacrificed 28 days following tumor inoculation, and
subsequently the LNs of the mice were removed from the inguinal
region. The right LNs of the inguinal region were defined as TDLNs
while the left sides were defined as non-TDLNs (nTDLNs). LNs from
control group mice were defined as control specimens (Con). Each LN
biopsy was immediately immersed in 4% neutral buffered
paraformaldehyde and paraffin embedded.
Flow cytometry
Flow cytometry analysis was performed as previously
described (24). LNs were dissected
and digested thoroughly with enzyme mix comprised of RMPI-1640,
containing 1 mg/ml collagenase IV (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 2% fetal bovine serum (Tianjin TBD standard,
Tianjin, China) and 50 µg/ml DNase I (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The following antibodies were
used: Anti-CD45 (clone 30-F11); anti-podoplanin (clone8.1.1);
anti-CD31 (cloneMEC13.3); anti-CD4 (clone GK1.5); and anti-CD8
(clone 53–6.7; all from BioLegend, Inc., San Diego, CA, USA). All
the dilutions were 1:100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RNA was converted to complemenatry (c)DNA
using PrimeScript Reverse Transcription reagent kit with gDNA
eraser (Takara Bio Inc., Otsu, Japan). The cDNA from was used for
RT-qPCR analysis of IL-7. PCR primer pairs were as follows: IL-7
forward, 5′-CTGCAGTCCCAGTCATCAGTA-3′ and reverse,
5′-GTGGCACTCAGATGATGTGACA-3′ and β-actin forward,
5′-CCTGAGGCTCTTTTCCAGCC-3′ and reverse,
5′-AGAGGTCTTTACGGATGTCAACGT-3′ (25).
Total IL-7 mRNA was measured using SYBR-Green reagents (Takara Bio
Inc.) and run in triplicate on the Applied Biosystems 7500 fast
real-time PCR Detection system (Thermo Fisher Scientific, Inc.
Carlsbad, CA, USA), normalized to β-actin by employing the
2−∆∆Cq method (26).
Thermocycling conditions were as follows: 95°C for 30 sec, followed
by 40 cycles of 95°C for 5 sec, 59°C for 34 sec, 95°C for 15 sec,
60°C for 60 sec and 95°C for 15 sec.
Immunofluorescence microscopy
All staining was performed as previously described
using 10 µm tissue sections mounted on glass slides (8,9). Primary
antibodies were as follows: Anti-mouse ER-TR7 (dilution, 1:100;
cat. no. sc-73355; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA); anti-mouse IL-7 (dilution, 1:50; cat. no. sc-7921; Santa Cruz
Biotechnology Inc.); and rabbit IgG isotype (cat. no. A7016;
Beyotime Institute of Biotechnology, Beijing, China). Secondary
antibodies were fluorescein isothiocyanate-conjugated anti-rat
(dilution, 1:200; cat. no. A0557; Beyotime Institute of
Biotechnology) and Cy3-conjugated anti-rabbit IgG (dilution, 1:100;
cat. no. A0516; Beyotime Institute of Biotechnology).
Western blot analysis
Portions of each LN sample were applied to 12%
SDS-PAGE and detected using a specific goat gp38 antibody
(dilution, 1:1,000; cat. no. AF3244; R&D Systems, Inc.,
Minneapolis, MN, USA). A horseradish peroxidase-conjugated
anti-goat IgG (dilution, 1:5,000; cat. no. A0181; Beyotime
Institute of Biotechnology) was used to visualize the signal by ECL
prime western blotting (Beyotime Institute of Biotechnology). The
blots were quantified by measuring the relative band intensity
normalized to changes in β-actin intensity.
Immunohistochemistry
All staining procedures were performed as previously
described (27). Subsequent to
tissues being deparaffinized and rehydrated using dimethylbenzene
and gradient concentration of ethanol, heat-induced epitope
retrieval was performed with water bath heating in EDTA antigen
retrieval buffers (pH 8.0; Zhongshan Company, Beijing, China) for
10 min, cooling to room temperature. The sections were then blocked
with 5% bovine serum albumin (BSA) blocking reagent (Boster
Systems, Inc., Pleasanton, CA, USA) and 3%
H2O2. Primary antibodies were diluted in 5%
BSA and incubated overnight at 4°C. The next day, slides were
washed using PBS and stained with secondary antibody, horseradish
peroxidase-conjugated anti-rabbit (dilution, 1:1,000; cat. no.
A0208; Beyotime Institute of Biotechnology). Light micrographs were
captured using an Olympus BX60 upright microscope (magnification,
×40; 0.75 numerical aperture).
Primary antibodies were as follows: Anti-mouse CD3
(dilution, 1:100; cat. no. ab16669; Abcam, Cambridge, MA, USA);
anti-mouse CD8 (dilution, 1:100; cat. no. sc-7188; Santa Cruz
Biotechnology Inc.); anti-human IL-7 (dilution, 1:50; cat. no.
sc-7921; Santa Cruz Biotechnology Inc.); rabbit IgG isotype (cat.
no. A7016; Beyotime Institute of Biotechnology); anti-human CD8
(cat. no. ZA-0508; Zhongshan Company, Beijing, China); and
anti-human desmin (cat. no. ZA-0610; Zhongshan Company).
Quantitative image analysis was performed using 5–10 randomly
acquired, high-powered images (magnification, ×400). The number of
cells in each image was manually counted and the percentage of IL-7
and desmin area was calculated with an automated action program in
Image-Pro Plus (version 6.0; Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
Statistical analysis was performed with one-way
analysis of variance followed by a Newman-Keuls test using GraphPad
Prism software (version 6.0; GraphPad Software Inc., La Jolla, CA,
USA). The Spearman correlation test was conducted to analyze the
existing associations. P<0.05 was considered to indicate a
statistically significant difference.
Results
Loss of FRCs in TDLNs
Lewis lung carcinoma cells were inoculated in the
right inguinal region of 6-week-old C57BL/6 mice. Following tumor
inoculation for 28 days, mice were sacrificed and LNs from the two
sides of the inguinal region were collected. LNs were then digested
thoroughly with enzyme mix comprised of RPMI-1640 containing
collagenase IV and DNase I (24).
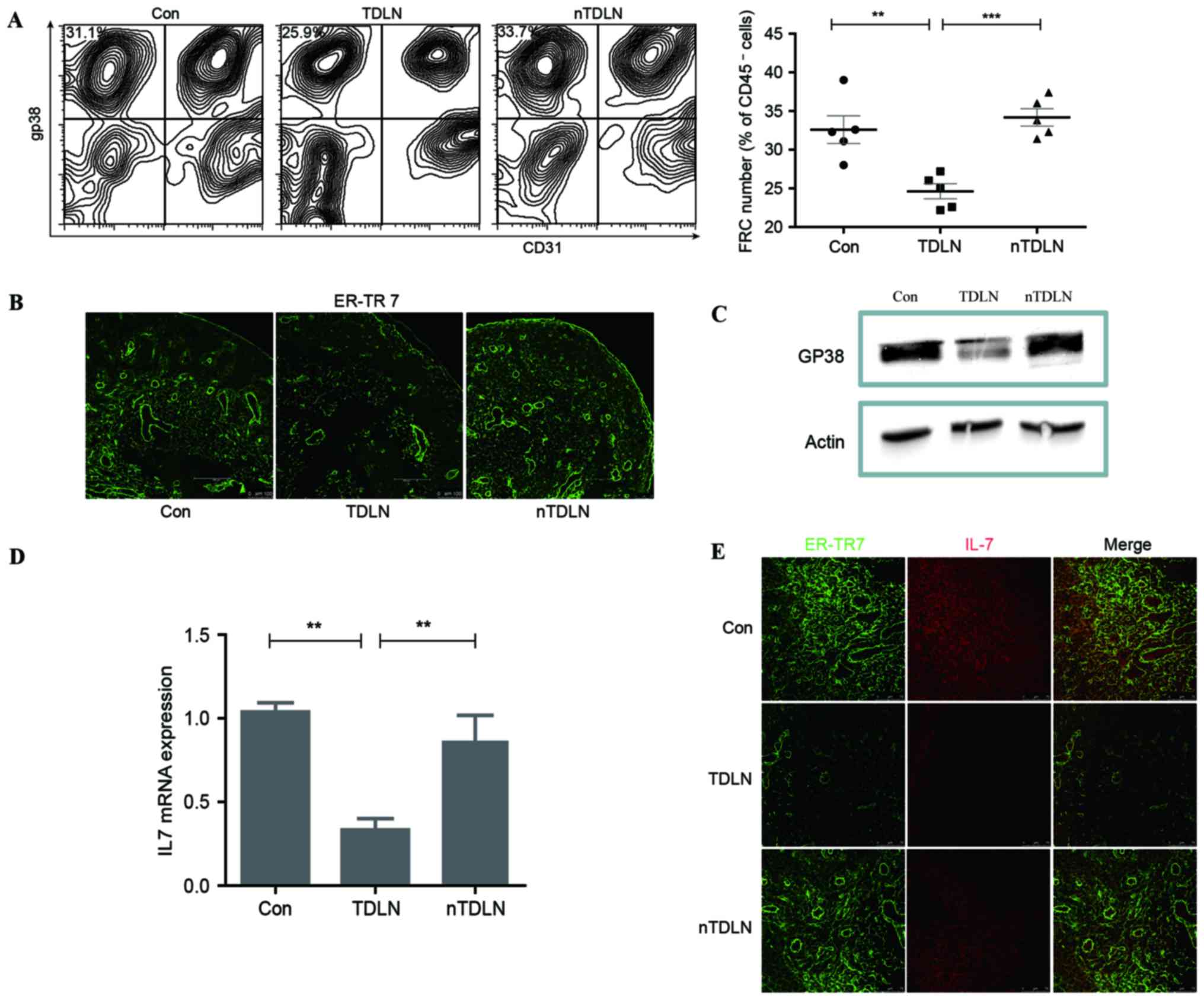
Using flow cytometry, a significant decrease (P<0.01) was
observed in the number of FRCs (gp38+CD31−)
in CD45− cells in TDLNs compared with the control group
(Fig. 1A). ER-TR7 and gp38 were
reported as markers of FRCs and required for proper formation and
organization of LNs (11,28,29). To
validate the effect of tumors on stromal cells in LNs,
immunofluorescent staining of ER-TR7 was performed, which
demonstrated that the density of ER-TR7+ FRCs was
markedly decreased in TDLNs compared with control LNs (Fig. 1B). Western blot analysis of gp38
showed a similar change in TDLN (Fig.
1C). These results indicated that the population of FRCs in
TDLNs was affected in tumor-bearing mice.
IL-7 is significantly decreased in
TDLNs
FRCs are resident in LNs and generate the majority
of IL-7 in the periphery, which is essential for the homeostasis of
lymphocytes (10). It was examined
whether loss of FRCs impacts IL-7 expression. IL-7 transcript
measured by RT-qPCR was reproducibly decreased by more than one
half in the TDLNs compared with contralateral non-TDLNs and control
groups (Fig. 1D). Immunofluorescent
staining of IL-7 confirmed the descent of IL-7 expression in TDLNs
(Fig. 1E), as was gp38 detection in
TDLNs compared with those of the control. These results indicated
that loss of FRCs in TDLNs was responsible for the decreased IL-7
secretion.
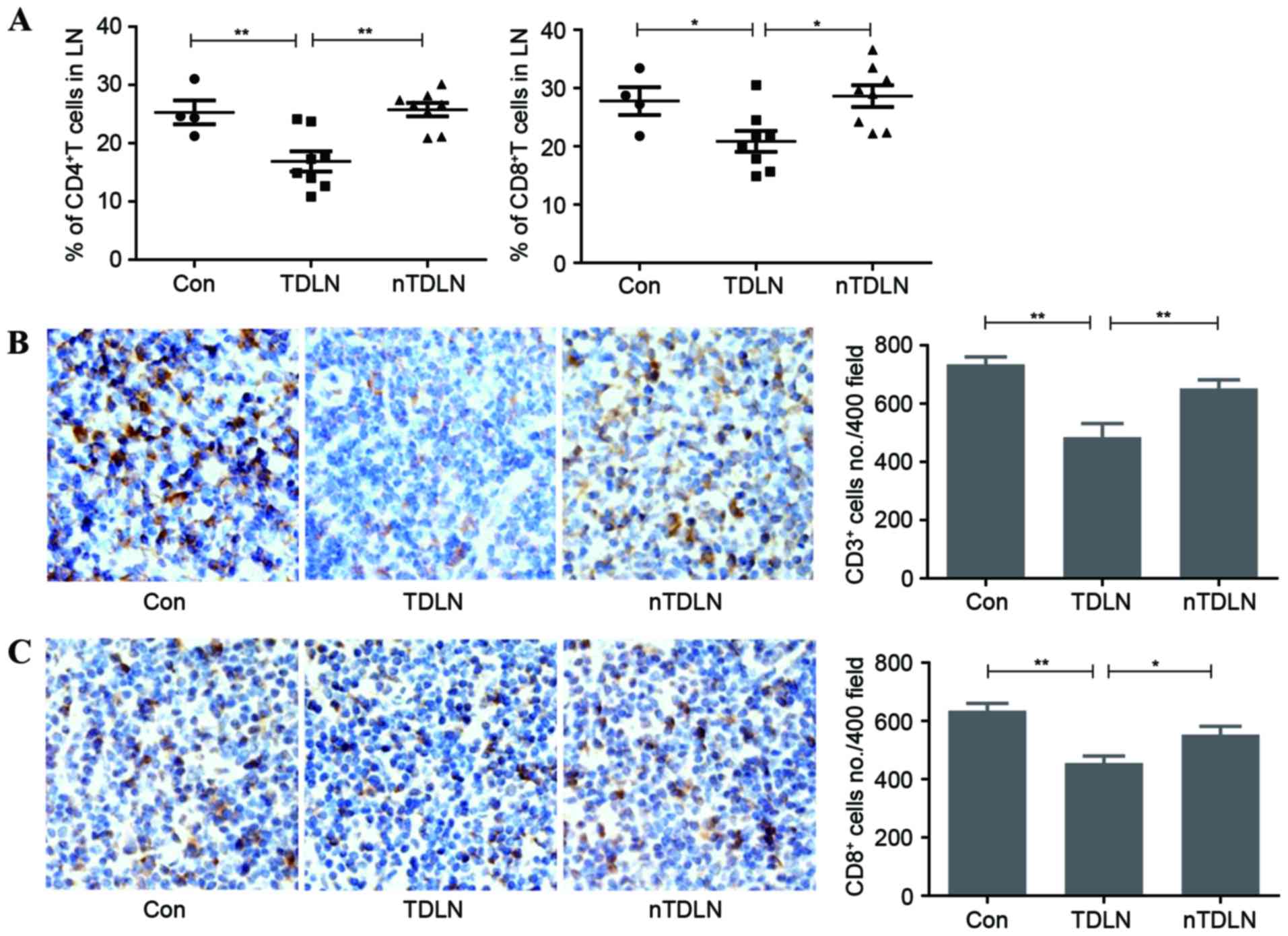
The number of T lymphocytes is reduced
in TDLNs
T cell homeostasis means that a relatively stable
number of T lymphocytes in the periphery rely on IL-7 (10). They compete for this limited survival
factor in LNs to maintain the T lymphocyte pool at a constant size
(11). The present study aimed to
identify whether the reduced level of IL-7 affects the number of
total T cells in LNs. Flow cytometric analysis demonstrated that
the number of CD4+ and CD8+ T cells decreased
significantly in TDLNs (Fig. 2A).
Immunohistochemistry results also confirmed that the amount of
CD3+ T cells had markedly fallen in TDLNs (Fig. 2B). The number of CD8+ T
cells was also calculated in the same way. In line with the
downward trend of total number of T cells, CD8+ T cells
decreased by ~15% in TDLNs (Fig. 2C).
Less T cells survived in TDLNs, indicating that less functional T
cell may be armed to fight against the tumor burdens.`
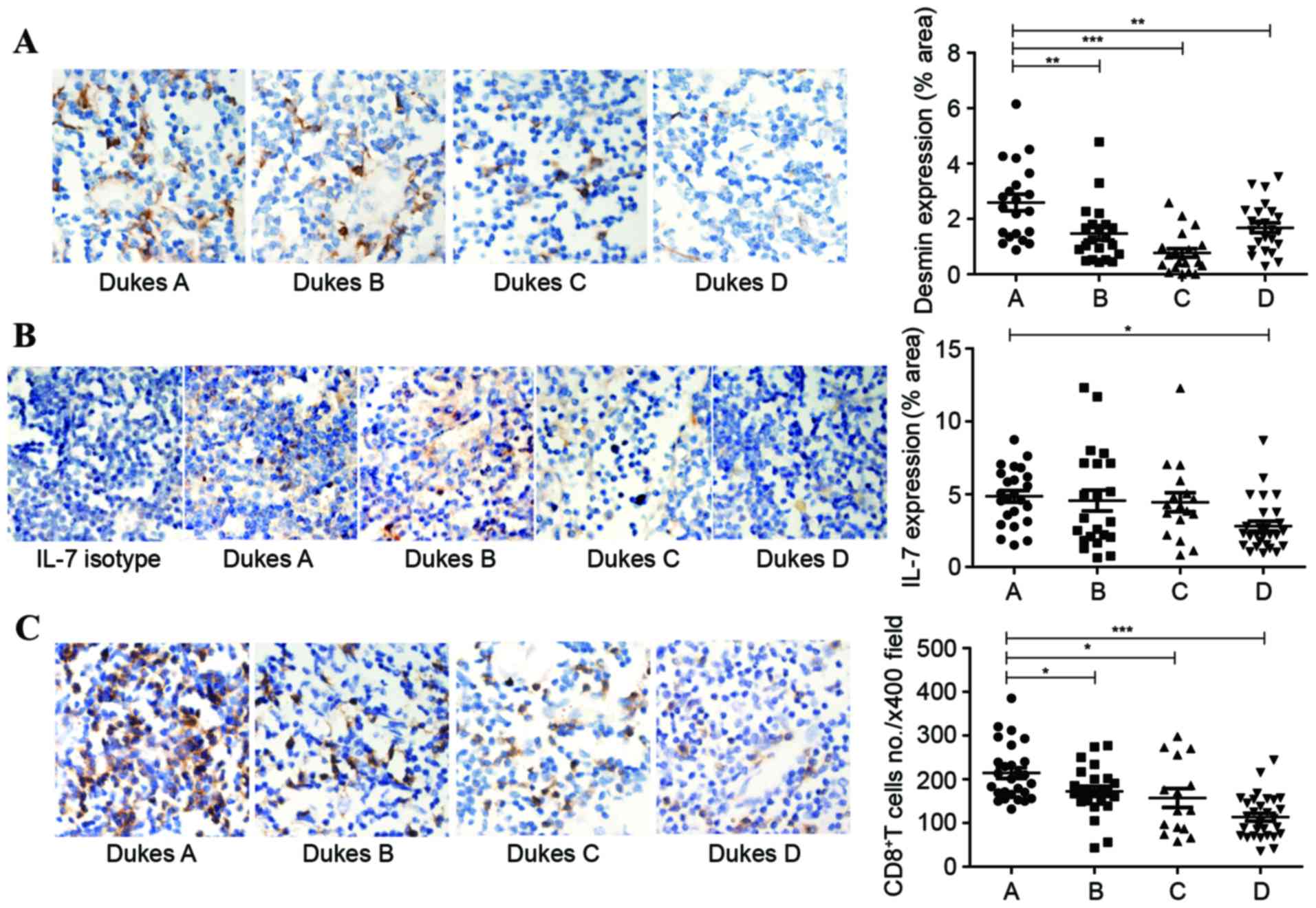
TDLNs in patients with colon
cancer
In the present study, a total of 40 patients
diagnosed with colon cancer who had received surgical excision were
selected. Each group contained 10 patients, according to their
Dukes stage (A-D). There were 1 to 4 LNs obtained from each
patient. All LNs came from mesenteric LNs which were defined as
TDLNs. Desmin was reported to be another marker of FRCs in LNs
(28). Consequently, antibodies to
desmin were used to label the FRC network in human LNs.
Immunohistochemistry was performed to check the expression of IL-7
and desmin in human LNs. The expression area of desmin shrunk by up
to 60%, accompanied with tumor progression (Fig. 3A). The percentage of area that stained
positive for IL-7 had a downward trend, and the differences between
Dukes A and D stages showed statistical significance (Fig. 3B). With less IL-7 in TDLNs, a rapid
and parallel decline of CD8+ T cells was detectable in
the Dukes B stage and accelerated markedly in the Dukes D stage
(Fig. 3C).
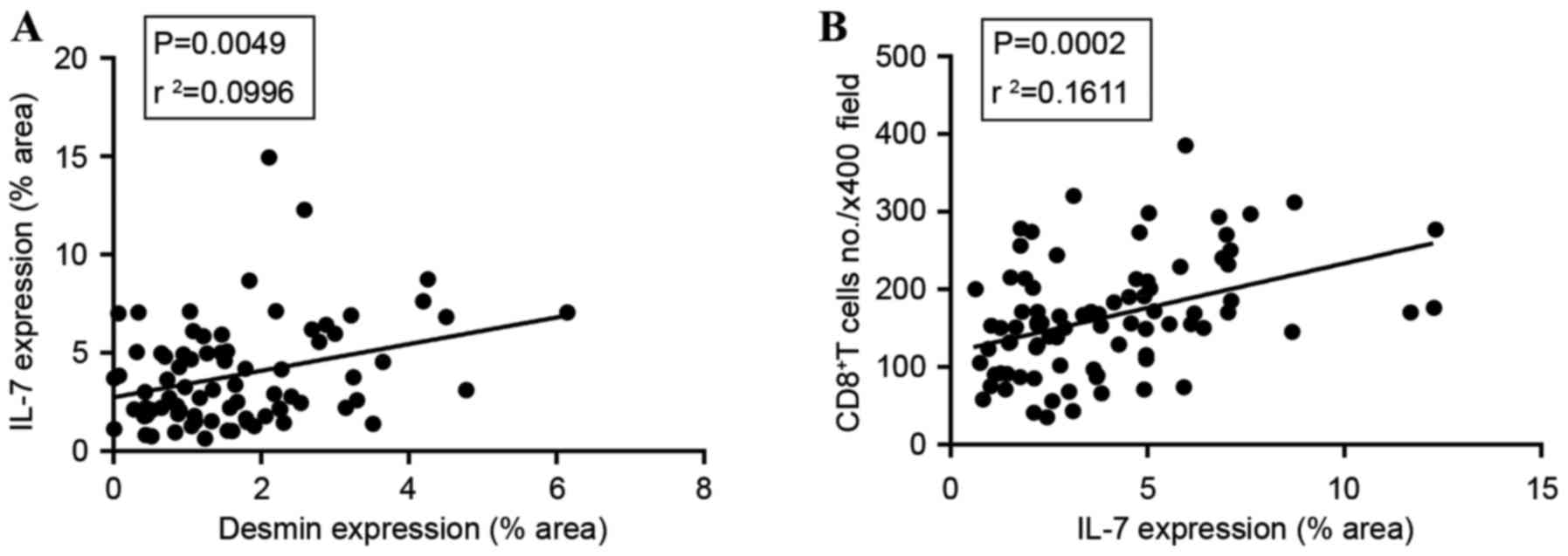
Association between desmin, IL-7 and
the number of CD8+ T cells
A significant positive association was identified
between desmin and IL-7 expression (P=0.0049; Fig. 4A). This coincided with the fact that
FRCs were the main source of IL-7. The amount of CD8+ T
cells was also significantly associated with IL-7 expression area
(P=0.0002; Fig. 4B), since IL-7 was
necessary for CD8+ T cell survival.
Discussion
Immunosuppression is a serious problem for patients
with cancer (2). Tumor growth may
result in T cell dysfunction by alterations in T cell
receptor-cluster of differentiation 3 complex and induction of T
cell tolerance (30). T cells also
produce immunosuppressive cytokines, including transforming growth
factor-β1 (TGF-β1) and granulocyte-macrophage colony-stimulating
factor, to promote tumor escape from immunological surveillance
(31,32). LN draining of pathological sites is
critical in immune function for the generation of adaptive immune
responses. However, TDLNs are often detected with tumor invasion as
early as it was first diagnosed.
FRCs in LNs orchestrate a complex network of
extracellular matrix and collagens. They facilitate immune
responses and maintain T cell homeostasis through the production of
IL-7. Previous studies of pathogen infection in vivo have
stated that a number of viruses, including LCMV and SIV, may infect
the FRCs directly, accompanied with disruption of the conduit
network (11,33,34).
Impaired FRC networks during human immunodeficiency virus infection
accounted for increased apoptosis and loss of naïve T cells
(35). A mouse tumor model of
melanomas also indicated alterations of the stromal cell network
only in inflammatory TDLNs, which profoundly altered the
distribution of T lymphocytes as a result (36). The present study demonstrated that
different tumor cells may influence the number of FRCs in TDLNs.
Loss of FRC networks reduced the major source of IL-7 and decreased
access to IL-7 (35). Consequently,
there were less T lymphocytes left in the TDLNs. This may partly
explain the weakened ability of immune surveillance of TDLNs.
Tumor cells may secrete certain types of soluble
factors that directly impede immune reactions (36). In regard to the way in which tumors
may affect FRCs, previous studies have detected higher TGF-β mRNA
expression in TDLNs (37). TGF-β is
reported to downregulate stromal IL-7 secretion in vitro
(38). Otherwise, the association
between tumor and stromal cells in LNs requires additional
study.
In conclusion, the present results indicated that
tumor cells may directly affect TDLNs by decreasing the FRC
population, leading to less IL-7 secretion. As a result, the amount
of T cells in TDLNs declined with less survival signals. This may
have partly contributed to the explanation of cancer
immunosuppressive theories.
Acknowledgements
The authors thank the Department of Pathology,
Xinqiao Hospital (Chongqing, China), for providing LN biopsy
specimens of patients, and for performing paraffin sections. The
present study was supported by the National Nature Science
Foundation of China (grant nos. 81472648 and 81500089), the
outstanding Youth Scientist Foundation of Chongqing (grant nos.
CSTC and 2008BA5035) and the National Key Basic Research Program of
China (973 program; grant nos. 010CB529404 and 2012CB526603).
Glossary
Abbreviations
Abbreviations:
|
FRC
|
fibroblastic reticular cells
|
|
LNs
|
lymph nodes
|
|
TDLNs
|
tumor draining lymph nodes
|
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao J, Zhao L, Wan YY and Zhu B: Mechanism
of action of IL-7 and its potential applications and limitations in
cancer immunotherapy. Int J Mol Sci. 16:10267–10280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malhotra D, Fletcher AL and Turley SJ:
Stromal and hematopoietic cells in secondary lymphoid organs:
Partners in immunity. Immunol Rev. 251:160–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munn DH and Mellor AL: The tumor-draining
lymph node as an immune-privileged site. Immunol Rev. 213:146–158.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turley SJ, Fletcher AL and Elpek KG: The
stromal and haematopoietic antigen-presenting cells that reside in
secondary lymphoid organs. Nat Rev Immunol. 10:813–825. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bajenoff M, Egen JG, Koo LY, Laugier JP,
Brau F, Glaichenhaus N and Germain RN: Stromal cell networks
regulate lymphocyte entry, migration, and territoriality in lymph
nodes. Immunity. 25:989–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Gao J, Li Y, Liu L, Yang Y, Guo B
and Zhu B: Disrupted homeostatic cytokines expression in secondary
lymph organs during HIV infection. Int J Mol Sci. 17:4132016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao L, Chen J, Liu L, Gao J, Guo B and
Zhu B: Essential role of TNF-alpha in development of spleen
fibroblastic reticular cells. Cell Immunol. 293:130–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Link A, Vogt TK, Favre S, Britschgi MR,
Acha-Orbea H, Hinz B, Cyster JG and Luther SA: Fibroblastic
reticular cells in lymph nodes regulate the homeostasis of naive T
cells. Nat Immunol. 8:1255–1265. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mueller SN and Germain RN: Stromal cell
contributions to the homeostasis and functionality of the immune
system. Nat Rev Immunol. 9:618–629. 2009.PubMed/NCBI
|
|
12
|
Onder L, Narang P, Scandella E, Chai Q,
Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, et
al: IL-7-producing stromal cells are critical for lymph node
remodeling. Blood. 120:4675–4683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fletcher AL, Malhotra D and Turley SJ:
Lymph node stroma broaden the peripheral tolerance paradigm. Trends
Immunol. 32:12–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JW, Epardaud M, Sun J, Becker JE,
Cheng AC, Yonekura AR, Heath JK and Turley SJ: Peripheral antigen
display by lymph node stroma promotes T cell tolerance to
intestinal self. Nat Immunol. 8:181–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dubrot J, Duraes FV, Potin L, Capotosti F,
Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W,
Swartz MA and Hugues S: Lymph node stromal cells acquire
peptide-MHCII complexes from dendritic cells and induce
antigen-specific CD4+ T cell tolerance. J Exp Med.
211:1153–1166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kittipatarin C and Khaled AR: Interlinking
interleukin-7. Cytokine. 39:75–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Surh CD and Sprent J: Homeostasis of naive
and memory T cells. Immunity. 29:848–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rathmell JC, Farkash EA, Gao W and
Thompson CB: IL-7 enhances the survival and maintains the size of
naive T cells. J Immunol. 167:6869–6876. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fry TJ and Mackall CL: The many faces of
IL-7: From lymphopoiesis to peripheral T cell maintenance. J
Immunol. 174:6571–6576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chappaz S and Finke D: The IL-7 signaling
pathway regulates lymph node development independent of peripheral
lymphocytes. J Immunol. 184:3562–3569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chappaz S, Gärtner C, Rodewald HR and
Finke D: Kit ligand and Il7 differentially regulate Peyer's patch
and lymph node development. J Immunol. 185:3514–3519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmutz S, Bosco N, Chappaz S, Boyman O,
Acha-Orbea H, Ceredig R, Rolink AG and Finke D: Cutting edge: IL-7
regulates the peripheral pool of adult ROR gamma+ lymphoid tissue
inducer cells. J Immunol. 183:2217–2221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dukes CE: The surgical pathology of rectal
cancer: President's address. Proc R Soc Med. 37:131–144.
1944.PubMed/NCBI
|
|
24
|
Fletcher AL, Malhotra D, Acton SE,
Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M and
Turley SJ: Reproducible isolation of lymph node stromal cells
reveals site-dependent differences in fibroblastic reticular cells.
Front Immunol. 2:352011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawa Y, Arima Y, Ogura H, Kitabayashi C,
Jiang JJ, Fukushima T, Kamimura D, Hirano T and Murakami M: Hepatic
interleukin-7 expression regulates T cell responses. Immunity.
30:447–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng M, Paiardini M, Engram JC, Beilman
GJ, Chipman JG, Schacker TW, Silvestri G and Haase AT: Critical
role of CD4 T cells in maintaining lymphoid tissue structure for
immune cell homeostasis and reconstitution. Blood. 120:1856–1867.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng M, Smith AJ, Wietgrefe SW, Southern
PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G,
Lifson JD, et al: Cumulative mechanisms of lymphoid tissue fibrosis
and T cell depletion in HIV-1 and SIV infections. J Clin Invest.
121:998–1008. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Astarita JL, Acton SE and Turley SJ:
Podoplanin: Emerging functions in development, the immune system,
and cancer. Front Immunol. 3:2832012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Aquino MT, Malhotra A, Mishra MK and
Shanker A: Challenges and future perspectives of T cell
immunotherapy in cancer. Immunol Lett. 166:117–133. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gorelik L and Flavell RA: Immune-mediated
eradication of tumors through the blockade of transforming growth
factor-beta signaling in T cells. Nat Med. 7:1118–1122. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Han G, Wang K, Liu G, Wang R, Xiao
H, Li X, Hou C, Shen B, Guo R, et al: Tumor-derived GM-CSF promotes
inflammatory colon carcinogenesis via stimulating epithelial
release of VEGF. Cancer Res. 74:716–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mueller SN, Matloubian M, Clemens DM,
Sharpe AH, Freeman GJ, Gangappa S, Larsen CP and Ahmed R: Viral
targeting of fibroblastic reticular cells contributes to
immunosuppression and persistence during chronic infection. Proc
Natl Acad Sci USA. 104:15430–15435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi YK, Fallert BA, Murphey-Corb MA and
Reinhart TA: Simian immunodeficiency virus dramatically alters
expression of homeostatic chemokines and dendritic cell markers
during infection in vivo. Blood. 101:1684–1691. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng M, Haase AT and Schacker TW: Lymphoid
tissue structure and HIV-1 infection: Life or death for T cells.
Trends Immunol. 33:306–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soudja SM, Henri S, Mello M, Chasson L,
Mas A, Wehbe M, Auphan-Anezin N, Leserman L, Van den Eynde B and
Schmitt-Verhulst AM: Disrupted lymph node and splenic stroma in
mice with induced inflammatory melanomas is associated with
impaired recruitment of T and dendritic cells. PloS One.
6:e226392011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imai K, Minamiya Y, Koyota S, Ito M, Saito
H, Sato Y, Motoyama S, Sugiyama T and Ogawa J: Inhibition of
dendritic cell migration by transforming growth factor-β1 increases
tumor-draining lymph node metastasis. J Exp Clin Cancer Res.
31:32012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang J, Nuccie BL, Ritterman I, Liesveld
JL, Abboud CN and Ryan DH: TGF-beta down-regulates stromal IL-7
secretion and inhibits proliferation of human B cell precursors. J
Immunol. 159:117–125. 1997.PubMed/NCBI
|