Introduction
Liver cancer is responsible for primary malignancy
of the liver (1–3) and is currently the third pre-eminent
cause of cancer deaths around the world, with over 500,000 people
affected by the disease (4,5). Liver cancer cases mostly occur in Africa
and Asia, where the high number of hepatitis B and hepatitis C
cases strongly predisposes to the development of liver disease and
the subsequent development of liver cancer (6,7).
Consequently, comprehensive elucidation of the mechanisms governing
recurrence of liver cancer and metastasis are urgently needed.
MicroRNAs (miRNAs) are endogenous non-coding RNAs,
which play vital roles in tumor development and tumorigenesis
(8–10). In some human cancers, miRNAs are often
unregulated and have an oncogenic function, while most miRNAs are
downregulated and may possess a tumor-suppressive activity
(11–13). Emerging evidence suggests that the
abnormal expression of miRNAs is involved in the invasion and
metastasis, during the progression of various human cancers
(14,15). miR-346 has been reported as an oncomir
in various human cancers, including cutaneous squamous cell
carcinoma (16), cervical cancer
(17), prostate cancer (18), nasopharyngeal carcinoma (19), lung cancer (20) and breast cancer (21).
The present study aimed to investigate the
expression and the molecular regulatory mechanism of miR-346 in
liver cancer. We demonstrated that miR-346 is upregulated in liver
cancer cell lines. Furthermore, ectopic expression or inhibition of
miR-346 could accelerate or block proliferation, migration, and
invasion abilities of liver cancer cells, respectively.
Furthermore, we further identified FBXL2 as a functional target of
miR-346 and demonstrated FBXL2 involve in the effects of increased
miR-346 on promoting proliferation, migration, and invasion. Our
findings, for the first time, suggest a fundamental role for
miR-346 in liver cancer.
Materials and methods
Cell lines and cell culture
Human liver cancer cell lines MHCC-97H, SMMC-7221
(22), HepG2, Huh-7, and Hep3B, the
normal liver cell lines THLE-2 and THLE-3 were obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA). Cell
lines were cultivated in Dulbecco's modified Eagle's medium (Gibco,
USA) supplied with 10% fetal bovine serum (FBS, Gibco, USA) at 37°C
in a humidified atmosphere containing 5% CO2.
RNA extraction, reverse transcription
and real-time PCR
Total RNA from cultured cells was extracted using
the Trizol reagent (Takara, Japan) according to the manufacturer's
instructions. TaqMan microRNA assays (Applied Biosystems, Foster
City, CA, USA) were used to determine the expression levels of
miR-346 after reverse transcribing by sequence-specific primers
(Applied Biosystems), and U6 small nuclear RNA was used as an
internal control. Three independent experiments were performed to
analyze the relative gene expression.
Western blot analysis
Western blot was performed according to the method
described previously (23). The
following antibodies were used for analysis: anti-FBXL2 (1:1,000,
ab17018, Abcam, Cambridge, MA, USA); GAPDH (1:5,000, HRP-60004,
Proteintech Group, Chicago, IL, USA) was served as the loading
control.
MiR-346 mimics and inhibitors
MiRNA-346 mimics, miR-346 inhibitors, small
interference RNA for FBXL2, and their negative control RNA and
their negative control RNA oligonucleotides were purchased from
GenePharma (Shanghai, China). For overexpression experiments, the
human FBXL2 cDNA were cloned into the pcDNA3.1 vector (Clonetech).
The liver cancer cell line cells were transfected using
Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instruction. Scramble siRNA were used as the
control.
Cell proliferation assay
1×104 cells per well were incubated in 96-well
culture plates in 100 µl of medium. CCK-8 (Dojindo Laboratories,
Japan) was used according to the manufacturer's instructions.
Plates were incubated at 37°C for 2 h, and the absorbance at 450 nm
was then measured. Proliferation rates were determined at days 1,
2, 3, 4 and 5 post-transfection.
Migration and invasion assay
Cells were seeded into Transwell chambers (Corning,
Corning, NY, USA) in the upper chambers in medium containing 10%
FBS. 16 h later, the filters were stained with crystal violet. For
invasion assay, chambers were coated with Matrigel (BD Biosciences,
San Jose, CA, USA). Cell migration and invasion were assessed by
counting the number of cells that had penetrated through the
filter. These experiments were repeated three times.
Statistical analysis
All statistical analysis were performed using
SPSS17.0 (SPSS Statistics, Chicago, IL, USA) software. Data were
expressed as mean ± SD and analyzed using the Student's t-test.
Paired t-test was used for paired samples. P<0.05 was considered
to indicate a statistically significant difference.
Results
MiR-346 is highly expressed in human
liver cancer cell lines
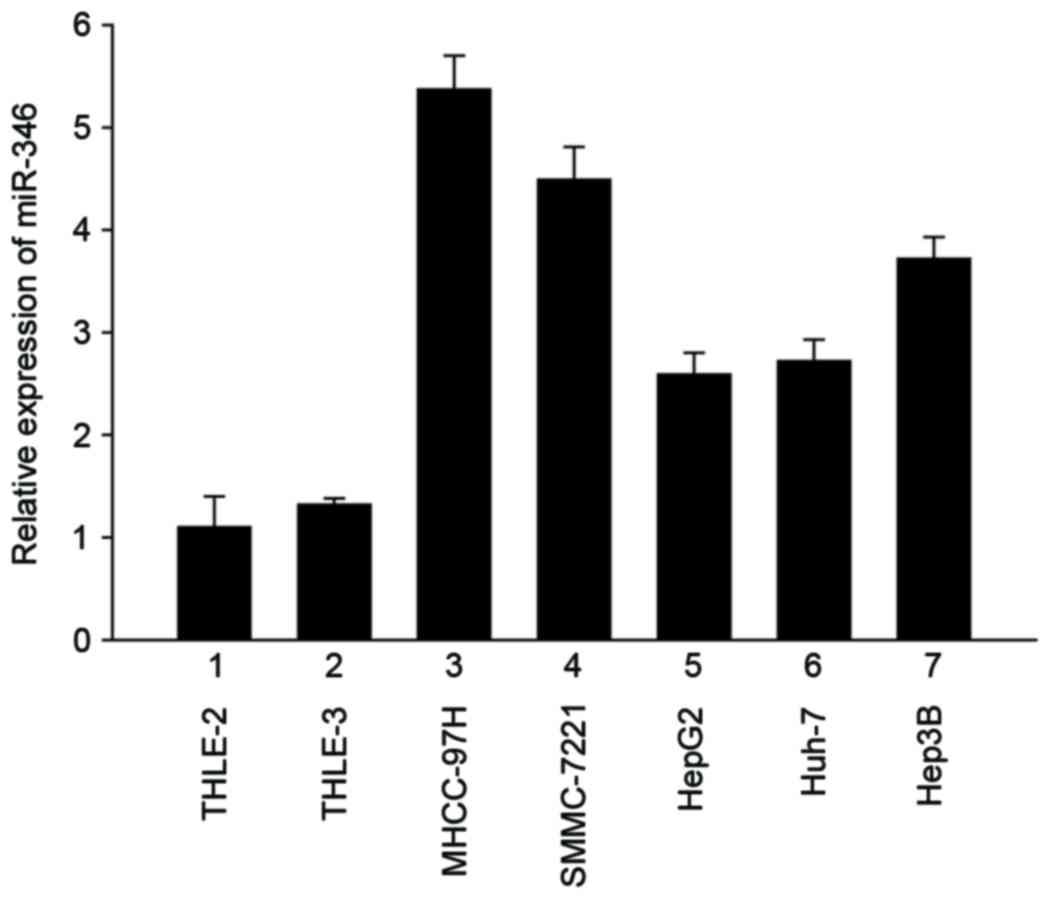
To determine the role of miR-346 in liver cancer,
real-Time PCR was performed to show that miR-346 was overexpressed
in five liver cancer cell lines, including MHCC-97H, SMMC-7221,
HepG2, Huh-7, and Hep3B, compared with the normal liver cell lines
THLE-2 and THLE-3 (Fig. 1). This
result demonstrated that, compared with normal human liver cells,
miR-346 was significantly upregulated in human liver cancer cell
lines.
Overexpression of miR-346 promotes the
proliferation, migration, and invasion of liver cancer cells
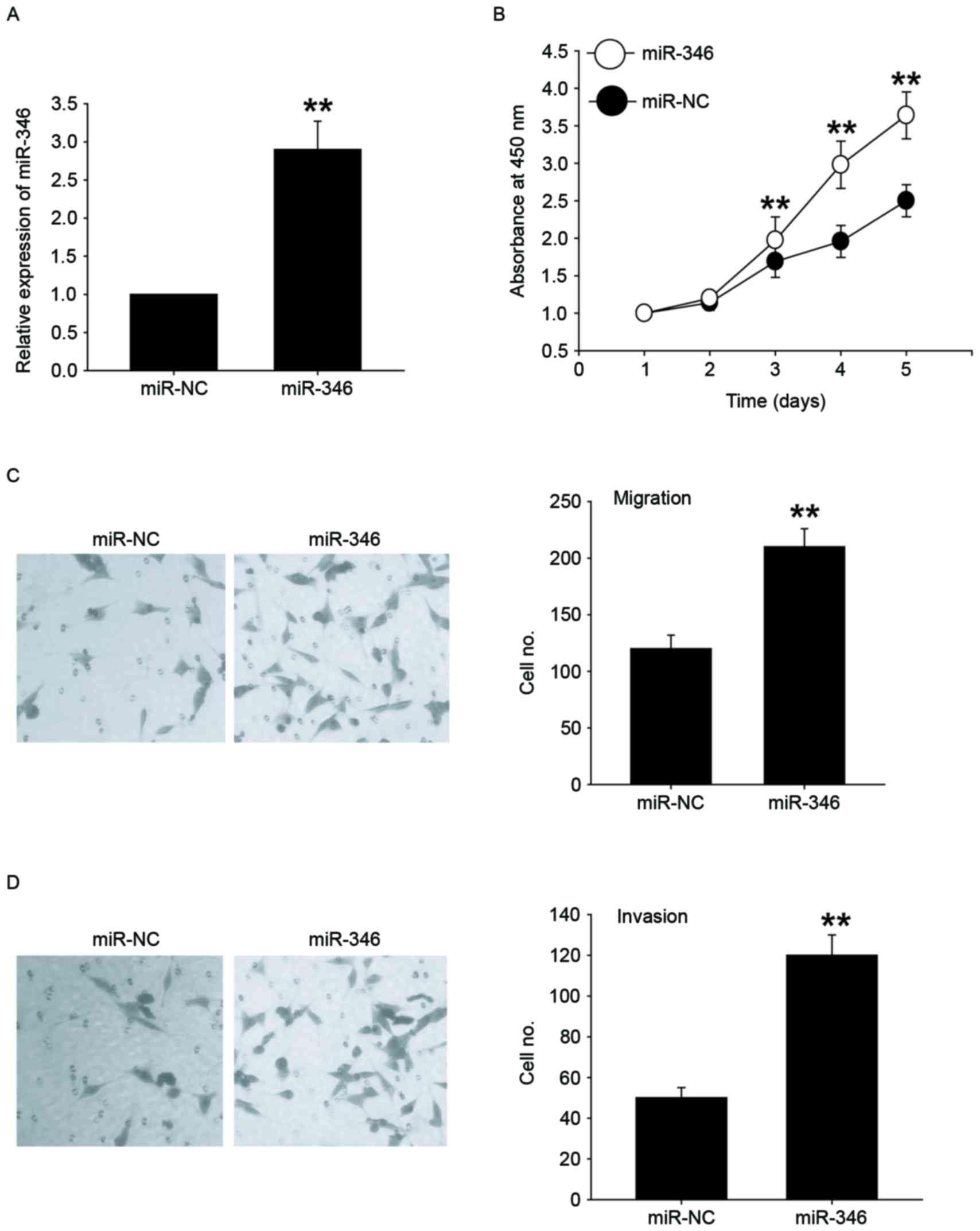
We transfected miR-346 mimics into HepG2 cells with
lower expression of miR-346 to investigate the role of miR-346
(Fig. 2A). CCK-8 analysis showed that
overexpression of miR-346 significantly promoted the cell
proliferation (Fig. 2B). Next, we
explored the potential roles of miR-346 in migration and invasion
of HepG2 cells by using Transwell migration and invasion assays.
miR-346 overexpression significantly increased cell migration
(Fig. 2C) and invasion (Fig. 2D) in HepG2 cell line. Together, these
results indicated that miR-346 overexpression promoted cell
proliferation, migration, and invasion.
Downregulation of miR-346 inhibits the
proliferation, migration, and invasion of liver cancer cells
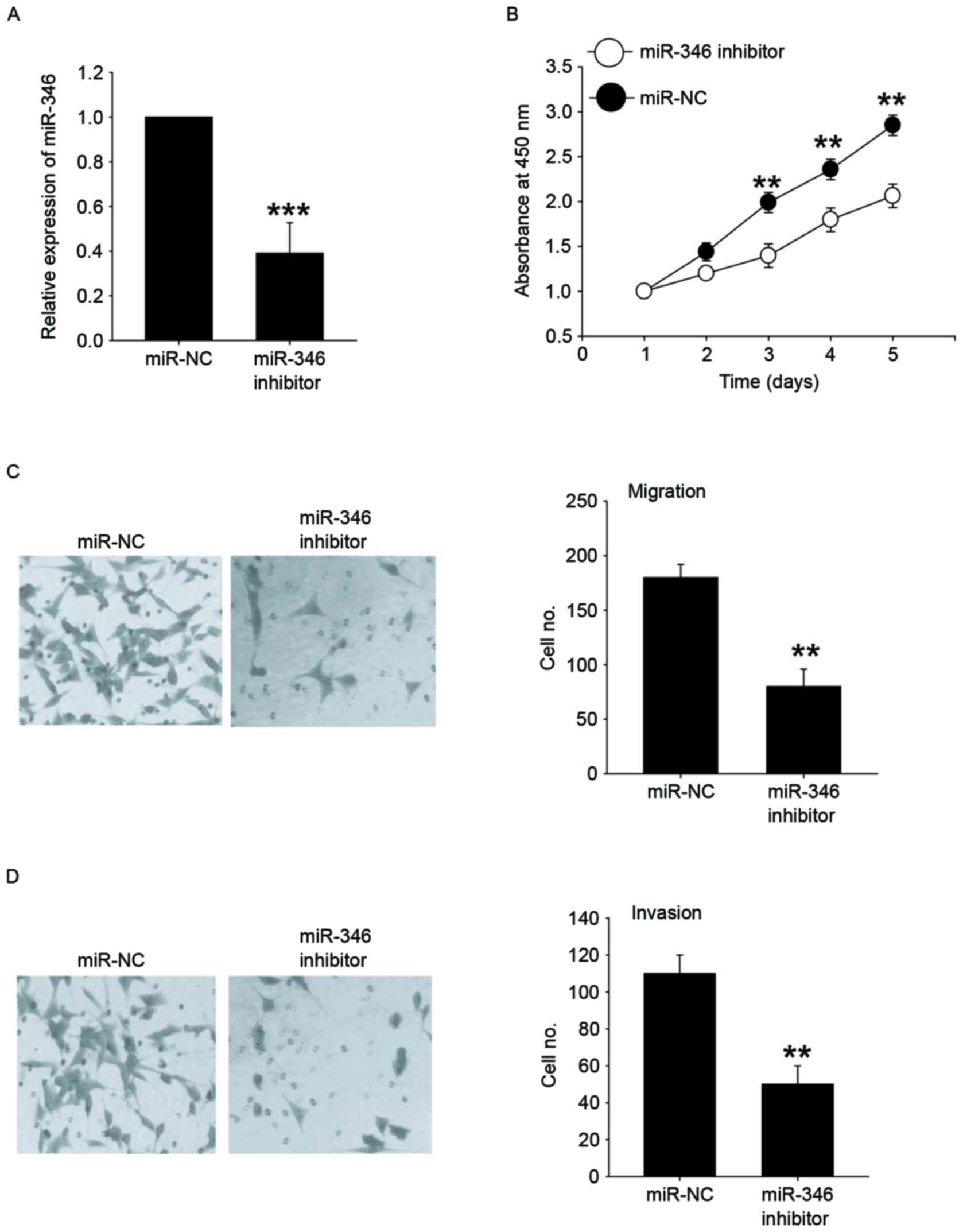
Next, MHCC-97H cells with higher expression of
miR-346 were successfully transfected with the miR-346 inhibitors
(Fig. 3A). The CCK-8 assay
demonstrated that inhibition of miR-346 significantly reduced the
cellular growth of MHCC-97H cells (Fig.
3B). Furthermore, MHCC-97H cells were transfected with miR-346
inhibitors and then analyzed for their metastatic potential using
Transwell assays. Our results showed that the migration and
invasion abilities in the miR-346 downexpression group was
significantly reduced when compared with the control group
(Fig. 3C and D). These results
demonstrated that downregulation of miR-346 could inhibit the
proliferative and metastatic potential of liver cancer cells.
FBXL2 was a direct and functional
target of miR-346 in liver cancer cells
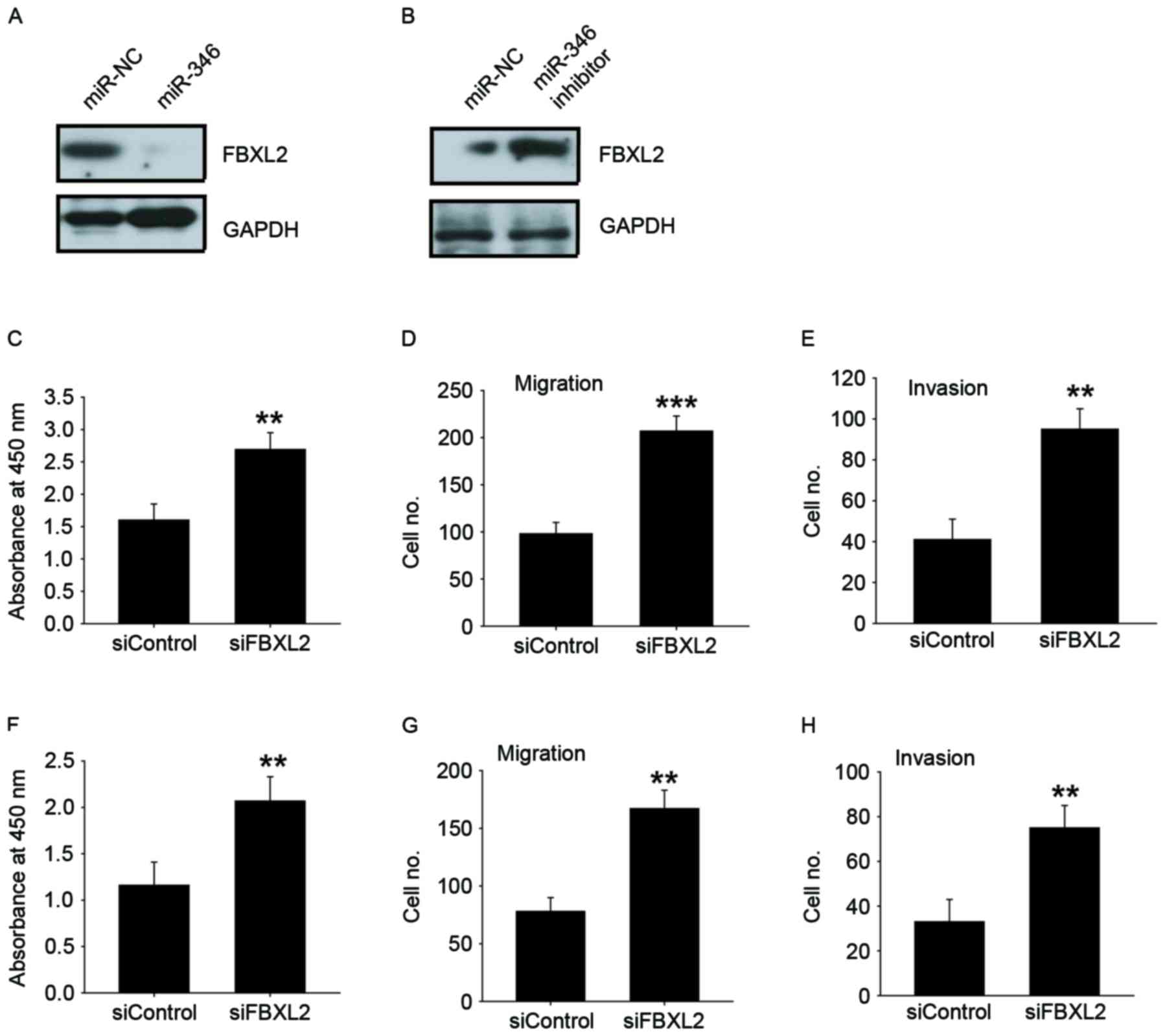
FBXL2 was the theoretical target gene of miR-346,
determined by analysis using publicly available algorithm Target
Scan. Western blot analysis indicated that overexpression of
miR-346 inhibited FBXL2 expression in HepG2 cells with lower
expression of miR-346 (Fig. 4A); in
contrast, knockdown of miR-346 increased FBXL2 expression in
MHCC-97H cells with higher expression of miR-346 (Fig. 4B). We found that FBXL2 silencing could
promote cell proliferation, migration, and invasion in HepG2
(Fig. 4C-E) and in Huh-7 cells
(Fig. 4F-H). These results showed
that FBXL2 has an opposite effect on cell proliferation, migration,
and invasion, compared to miR-346, suggesting that it may be a
functional target of miR-346 in liver cancer cells.
miR-346 promotes the proliferation,
migration, and invasion of liver cancer cells via FBXL2
To verify whether miR-346 promotes proliferation and
metastasis by targeting FBXL2, gain-and-loss assays were performed.
We found that simultaneous inhibition of FBXL2 expression in
MHCC-97H cells with miR-346 inhibition can restore their
proliferation and metastatic capacity (Fig. 5A-C), while simultaneous overexpression
of both FBXL2 expression in HepG2 cells with miR-346 overexpression
eliminated the role of miR-346 in proliferation and metastasis
(Fig. 5D-F). These results indicate
that miR-346 may promote proliferation, migration, and invasion via
FBXL2.
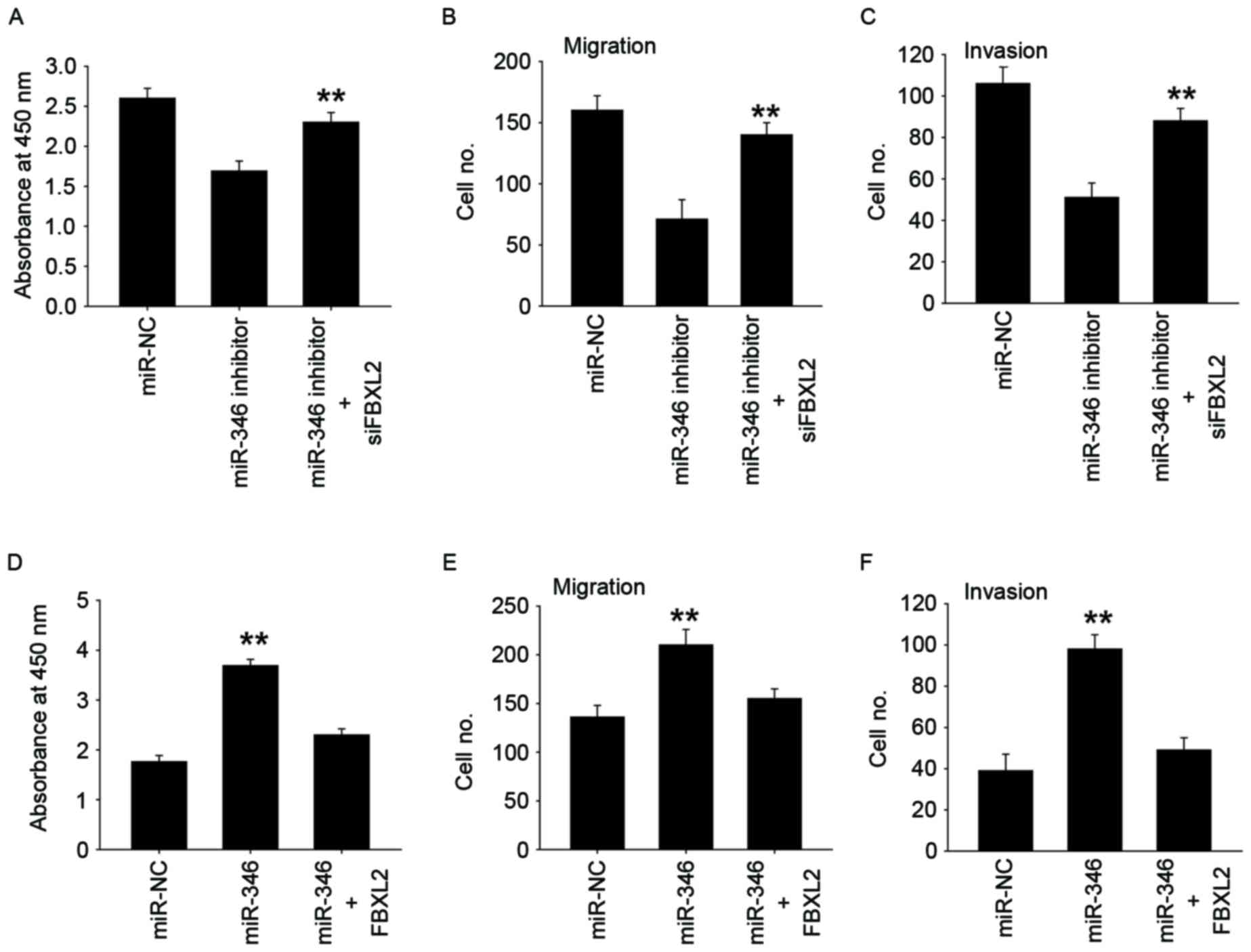 | Figure 5.miR-346 promotes the proliferation,
migration, and invasion of liver cancer cells via FBXL2. CCK-8
assay (A), Transwell-migration assay (B), Transwell-invasion assay
(C) were performed to evaluate the reversal by FBXL2 siRNA of
miR-346 inhibitors induced inhibition of cell proliferation,
migration, and invasion in MHCC-97H cells. CCK-8 assay (D),
Transwell-migration assay (E), Transwell-invasion assay (F) were
performed to evaluate the reversal by FBXL2 overexpression of
miR-346 mimics induced increase of cell proliferation, migration,
and invasion in HepG2 cells. All data are shown as mean ± SD from
three independent experiments. **P<0.01. |
Discussion
The molecular mechanisms underlying liver cancer
development, however, remain largely unknown (24,25).
Increasing data findings have indicated miRNAs to be critical
regulators of cancer-related processes, but it is still unknown the
molecular mechanisms by which miRNAs modulate the behavior of
cancer cells (26,27). miR-346 has been reported as an oncomir
because it facilitates cell growth and metastasis in various human
cancers (16–21). This is the first study that indicates
that ectopically expressed miR-346 can promote migration,
proliferation, and invasion of liver cancer cells.
In the present study, we found that miR-346 is
upregulated in liver cancer cells; this suggests that it might
contribute to the development and ultimately progression of liver
disease. It was also found that overexpression of miR-346 promoted,
migration, proliferation, and invasion of the cells, whereas the
knockdown of miR-346 inhibited cell migration, proliferation, and
invasion. miR-346 promotes the proliferation, migration, and
invasion of liver cancer cells via FBXL2 which is a tumor
suppressor and is also a direct target of miR-346.
FBXL2, which is an SCF (Skp1-Cullin-F-box) E3 ligase
component, was initially found as maintenance of cellular
homeostasis (28). Then, Chen et
al suggested that FBXL2 impaired cell proliferation by
mediating cyclin D3 polyubiquitination and degradation (29). Accumulating evidence has demonstrated
that FBXL2 plays the tumor suppressor role in different kinds of
cancers. For example, FBXL2 exerted human lung tumor
suppressor-like activity by ubiquitin-mediated degradation of
cyclin D3 resulting in cell cycle arrest (30). It also inhibited leukemic cell
proliferation by targeting cyclin D2 (31). FBXL2 also decreased gastric cancer
proliferation by promoting degradation of forkhead box M1 (32).
The present study, therefore, demonstrates that
miR-346 is markedly upregulated in liver cancer cells. Also, FBXL2
is a direct target gene of miR-346 and overexpression of miR-346
reduced the expression of FBXL2 and promoted the migration,
proliferation, and invasion of liver cancer cells, whereas the
downregulation of miR-346 had the opposite effect. Further
investigation is required to fully characterize the biological
function of miR-346 and its clinical relevance in the development
of liver cancer. Although the particular mechanisms are not yet
fully understood, the present study suggests that miR-346 may play
a significant role in regulating the migration, proliferation, and
invasion of liver cancer cells, and indicates that miR-346 may
represent a potential therapeutic target for liver cancer.
Acknowledgments
The present study was supported by the Department of
Hepatobiliary Surgery, Chinese PLA General Hospital.
References
|
1
|
Inokawa Y, Inaoka K, Sonohara F, Hayashi
M, Kanda M and Nomoto S: Molecular alterations in the
carcinogenesis and progression of hepatocellular carcinoma: Tumor
factors and background liver factors. Oncol Lett. 12:3662–3668.
2016.PubMed/NCBI
|
|
2
|
Fernández-Rodríguez CM and
Gutiérrez-García ML: Prevention of hepatocellular carcinoma in
patients with chronic hepatitis B. World J Gastrointest Pharmacol
Ther. 5:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamed O, Kimchi ET, Sehmbey M, Gusani NJ,
Kaifi JT and Staveley-O'Carroll K: Impact of genetic targets on
cancer therapy: Hepatocellular cancer. Adv Exp Med Biol. 779:67–90.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niu ZS, Niu XJ and Wang WH: Genetic
alterations in hepatocellular carcinoma: An update. World J
Gastroenterol. 22:9069–9095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baffy G: Hepatocellular carcinoma in
non-alcoholic fatty liver disease: Epidemiology, pathogenesis and
prevention. J Clin Transl Hepatol. 1:131–137. 2013.PubMed/NCBI
|
|
6
|
de Martel C, Maucort-Boulch D, Plummer M
and Franceschi S: World-wide relative contribution of hepatitis B
and C viruses in hepatocellular carcinoma. Hepatology.
62:1190–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakayama H and Takayama T: Management
before hepatectomy for hepatocellular carcinoma with cirrhosis.
World J Hepatol. 7:2292–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahdian-Shakib A, Dorostkar R, Tat M,
Hashemzadeh MS and Saidi N: Differential role of microRNAs in
prognosis, diagnosis and therapy of ovarian cancer. Biomed
Pharmacother. 84:592–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin VY and Chu KM: MiRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krauskopf J, Verheijen M, Kleinjans JC, de
Kok TM and Caiment F: Development and regulatory application of
microRNA biomarkers. Biomark Med. 9:1137–1151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu KW, Fang WL, Huang KH, Huang TT, Lee
HC, Hsieh RH, Chi CW and Yeh TS: Notch1 pathway-mediated
microRNA-151-5p promotes gastric cancer progression. Oncotarget.
7:38036–38051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi RU, Miyazaki H, Takeshita F,
Yamamoto Y, Minoura K, Ono M, Kodaira M, Tamura K, Mori M and
Ochiya T: Loss of microRNA-27b contributes to breast cancer stem
cell generation by activating ENPP1. Nat Commun. 6:73182015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Li N, Dong Y, Li S, Xu L, Li X, Li
Y, Li Z, Ng SS, Sung JJ, et al: miR-34a-5p suppresses colorectal
cancer metastasis and predicts recurrence in patients with stage
II/III colorectal cancer. Oncogene. 34:4142–4152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Bryan S, Dong S, Mathis JM and Alahari
SK: The roles of oncogenic miRNAs and their therapeutic importance
in breast cancer. Eur J Cancer. 72:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen B, Pan W, Lin X, Hu Z, Jin Y, Chen H,
Ma G, Qiu Y, Chang L, Hua C, et al: MicroRNA-346 functions as an
oncogene in cutaneous squamous cell carcinoma. Tumour Biol.
37:2765–2771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo J, Lv J, Liu M and Tang H: miR-346
Up-regulates Argonaute 2 (AGO2) protein expression to augment the
activity of other MicroRNAs (miRNAs) and contributes to cervical
cancer cell malignancy. J Biol Chem. 290:30342–30350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Wang S, Zhang W, Qiu J, Shan Y,
Yang D and Shen B: Screening key microRNAs for castration-resistant
prostate cancer based on miRNA/mRNA functional synergistic network.
Oncotarget. 6:43819–43830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan HL, Li L, Li SJ, Zhang HS and Xu W:
miR-346 promotes migration and invasion of nasopharyngeal carcinoma
cells via targeting BRMS1. J Biochem Mol Toxicol. 30:602–607. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun CC, Li SJ, Yuan ZP and Li DJ:
MicroRNA-346 facilitates cell growth and metastasis, and suppresses
cell apoptosis in human non-small cell lung cancer by regulation of
XPC/ERK/Snail/E-cadherin pathway. Aging (Albany NY). 8:2509–2524.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Luo LJ, Zhang L, Wang DD, Yang SJ,
Ding L, Li J, Chen D, Ma R, Wu JZ and Tang JH: MiR-346 promotes the
biological function of breast cancer cells by targeting SRCIN1 and
reduces chemosensitivity to docetaxel. Gene. 600:21–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Cao Y, Zhang J, Lei M, Deng X, Zahid
KR, Liu Y, Liu K, Yang J, Xiong G, et al: Determination of
glutathione in apoptotic SMMC-7221 cells induced by xylitol
selenite using capillary electrophoresis. Biotechnol Lett.
38:761–766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng CW, Wang HW, Chang CW, Chu HW, Chen
CY, Yu JC, Chao JI, Liu HF, Ding SL and Shen CY: MicroRNA-30a
inhibits cell migration and invasion by downregulating vimentin
expression and is a potential prognostic marker in breast cancer.
Breast Cancer Res Treat. 134:1081–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brito AF, Abrantes AM, Tralhão JG and
Botelho MF: Targeting Hepatocellular Carcinoma: What did we
Discover so Far? Oncol Rev. 10:3022016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tornesello ML, Buonaguro L, Izzo F and
Buonaguro FM: Molecular alterations in hepatocellular carcinoma
associated with hepatitis B and hepatitis C infections. Oncotarget.
7:25087–25102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie T, Huang M, Wang Y, Wang L, Chen C and
Chu X: MicroRNAs as regulators, biomarkers and therapeutic targets
in the drug resistance of colorectal cancer. Cell Physiol Biochem.
40:62–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohammadi A, Mansoori B and Baradaran B:
The role of microRNAs in colorectal cancer. Biomed Pharmacother.
84:705–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen BB, Coon TA, Glasser JR and
Mallampalli RK: Calmodulin antagonizes a calcium-activated SCF
ubiquitin E3 ligase subunit, FBXL2, to regulate surfactant
homeostasis. Mol Cell Biol. 31:1905–1920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen BB, Glasser JR, Coon TA and
Mallampalli RK: FBXL2 is a ubiquitin E3 ligase subunit that
triggers mitotic arrest. Cell Cycle. 10:3487–3494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen BB, Glasser JR, Coon TA and
Mallampalli RK: F-box protein FBXL2 exerts human lung tumor
suppressor-like activity by ubiquitin-mediated degradation of
cyclin D3 resulting in cell cycle arrest. Oncogene. 31:2566–2579.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen BB, Glasser JR, Coon TA, Zou C,
Miller HL, Fenton M, McDyer JF, Boyiadzis M and Mallampalli RK:
F-box protein FBXL2 targets cyclin D2 for ubiquitination and
degradation to inhibit leukemic cell proliferation. Blood.
119:3132–3141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li LQ, Pan D, Chen H, Zhang L and Xie WJ:
F-box protein FBXL2 inhibits gastric cancer proliferation by
ubiquitin-mediated degradation of forkhead box M1. FEBS Lett.
590:445–452. 2016. View Article : Google Scholar : PubMed/NCBI
|