Introduction
Breast cancer is one of the most common female
malignant tumors with high morbidity and mortality rate caused by
its strong metastatic ability (1,2). There are
no consensus biomarkers for early diagnosis and prognosis
assessment of breast cancer with applications in clinical practice.
Therefore, the development of breast cancer biomarkers has
attracted increased attention recently. Special AT-rich
sequence-binding protein 1 (SATB1) binds to T-rich sequences in
chromosomes to regulate the expression of downstream genes
(3,4).
The expression level of SATB1 is low in normal tissue, but is
elevated in a variety of tumors (5–7). Toll-like
receptor 4 (TLR4) is mainly expressed in immune cells, but can also
be expressed in tumor cells (8–10). In this
study, we detected the expression of SATB1 and RLR4 in 120 cases of
cancer and 53 cases of adjacent non-cancerous tissue by
immunohistochemistry. The correlation between the expression of
these two proteins and the clinical characteristics of patients
were analyzed.
Patients and methods
Patient information
A total of 120 patients diagnosed with breast cancer
in Yuhuangding Hospital of Yantai from October 2014 to October 2016
was enrolled in the study. Cancer tissue was collected after
surgical resection. At the same time, adjacent non-cancerous tissue
was collected from 53 patients. All the patients were females, and
their ages ranged from 28 to 65 years, with a mean age of 46.5±11.7
years. No patient had been treated with chemotherapy before the
study. Specimens were collected from necrotic cancer tissue and the
adjacent non-cancerous tissue within 3 cm, fixed, and embedded in
paraffin. Cancerous samples were diagnosed as breast cancer by
pathological examination. The study was approved by the Ethics
Committee of Yuhuangding Hospital of Yantai. All the patients
signed an informed consent before being enrolled in the study.
Reagents and methods
Anti-human SATB1 monoclonal antibody and rabbit
anti-human TLR4 monoclonal antibody were purchased from Abcam
(Cambridge, UK). The DAB kit and hematoxylin were purchased from
ZSBG-Bio (Beijing, China). Horseradish peroxidase-conjugated
secondary antibody was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Paraffin-embedded breast cancer samples
were cut into 4 µm sections and transferred onto glass slides.
After baking for 2 h at 90°C, tissue sections were dewaxed and
rehydrated. After that, antigen retrieval was performed by
incubating with 0.01 M sodium citrate buffer for 15 min. Endogenous
peroxidase blocker was then added and incubated at 37°C for 10 min.
After blocking with goat serum at room temperature for 20 min, the
primary antibodies of SATB1 (1:300) and TLR4 (1:300) were incubated
with the slides at 4°C overnight. After washing, secondary antibody
was added and incubated at 37°C for 1 h. DAB staining was then
performed and tissue sections were examined under the microscope to
observe the staining. After hematoxylin staining, the slides were
dehydrated, cleared, and sealed. All the operations were performed
in accordance with the manufacturer's instructions.
Determination of experimental
results
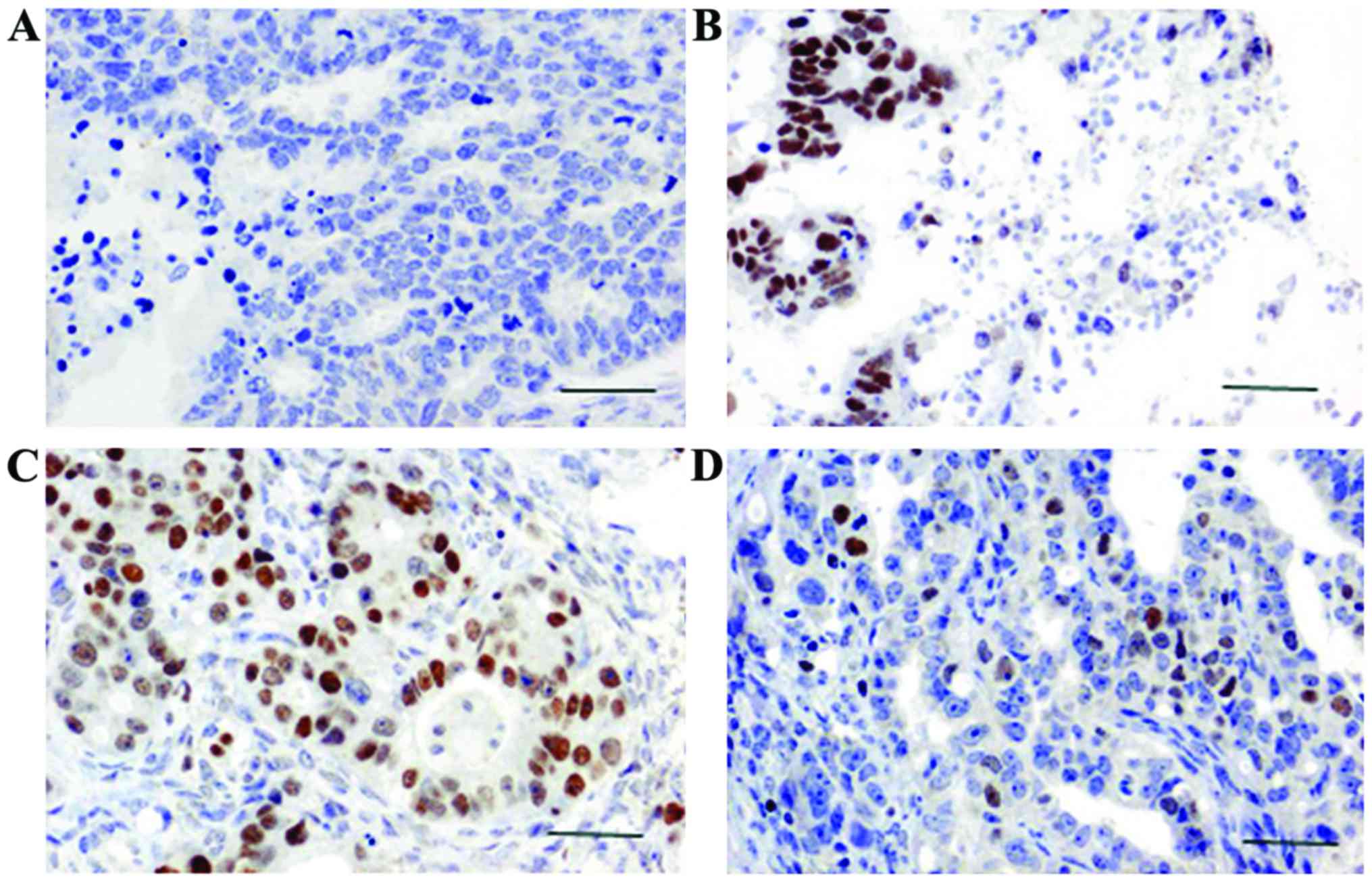
The brown or yellow granules on the slides showed
the positive expression of SATB1 and TLR4. SATB1 mainly accumulated
in the nucleus and TLR4 mainly accumulated in the cytoplasm. Using
×400 magnification in a bright field microscope (Leica, Wetzlar,
Germany), 10 distinct visual fields were selected to count the
positive cells and record the degree of staining. We also
calculated the percentage of positive cells. Scoring was performed
according to the degree of staining: no staining, 0 points; light
yellow, 1 point; yellowish-brown, 2 points; chocolate brown, 3
points. Scoring was also performed according to the percentage of
positive cells: 0–25%, 1 point; 25–65%, 2 points; 65–100%, 3
points. The product of the 2 scores greater than 3 was taken as
positive expression; values below 3 were considered negative
expression (11).
Statistical analysis
SPSS 19.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used to analyze the data. The count data were analyzed
by Chi-square test. Correlation analysis was performed by
Spearman's rank correlation analysis. P<0.05 was considered to
be statistically significant.
Results
Expression of SATB1 and TLR4 in breast
cancer and adjacent non-cancerous tissue
SATB1 expression was observed in the nucleus under a
microscope. SATB1 was positively expressed in 89 cases of breast
cancer, and the positive expression rate of SATB1 was 74.1%
(Fig. 1). Positive expression of
SATB1 was only detected in 7 cases of adjacent non-cancerous
tissue, and the positive expression rate of SATB1 was 13.21%. A
statistically significant difference in the expression of SATB1 was
found between breast cancer and adjacent non-cancerous tissue
(P<0.05; Table I).
 | Table I.Expression of SATB1 and TLR4 in breast
cancer and adjacent non-cancerous tissue. |
Table I.
Expression of SATB1 and TLR4 in breast
cancer and adjacent non-cancerous tissue.
|
|
| SATB1 | TLR4 |
|---|
|
|
|
|
|
|---|
| Groups | Cases (n) | − | + | Positive rate
(%) | − | + | Positive rate
(%) |
|---|
| Breast cancer | 120 | 31 | 89 | 74.17 | 50 | 70 | 58.33 |
| Adjacent
non-cancerous tissue | 53 | 46 | 7 | 13.21 | 5 | 48 | 90.57 |
| χ2
value |
| 37.413 |
|
26.481 |
|
|
|
| P-value |
|
0.006 |
|
0.011 |
|
|
|
TLR4 expression was detected in the cytoplasm. TLR4
was positively expressed in 70 cases of breast cancer, and the
positive rate was 58.33% (Fig. 1).
Positive expression of SATB1 was detected in 48 cases of adjacent
non-cancerous tissues, and the positive rate was 90.57%. A
statistically significant difference in the expression of TLR4 was
found between breast cancer and adjacent non-cancerous tissues
(P<0.05; Table I).
Correlation between expression of
SATB1 and TLR4
Following the immunohistochemistry results, we next
analyzed the correlation between SATB1 and TLR4. As shown in
Table II, the expression of SATB1
was negatively correlated with the expression of TLR4 (r=−0.624,
P<0.05).
 | Table II.Correlation between the expression of
SATB4 and TLR4 in breast cancer tissues. |
Table II.
Correlation between the expression of
SATB4 and TLR4 in breast cancer tissues.
|
| SATB1 |
|
|
|---|
|
|
|
|
|
|---|
| TLR4 | + | − | r value | P-value |
|---|
| + | 43 | 27 | −0.624 | 0.003 |
| − | 46 | 4 |
Correlation of SATB1 and TLR4 with the
clinical and pathological features of patients
We last examined the correlation of the expression
of SATB1 and TLR4, and the clinical and pathological features of
patients. We found that the expression levels of SATB1 and TLR4
were not significantly correlated with the age, menopause, PR
protein, and HER-2 protein expression (P>0.05). However, the
expression levels of SATB1 and TLR4 were significantly correlated
with tumor size, local lymphatic metastasis, histopathological
grade, tumor stage, and the expression of ER protein (P<0.05;
Table III).
 | Table III.Correlation of the expression of
SATB1 and TLR4 with the clinical and pathological features of
patients. |
Table III.
Correlation of the expression of
SATB1 and TLR4 with the clinical and pathological features of
patients.
|
|
| SATB1 | TLR4 |
|---|
|
|
|
|
|
|---|
| Items | Cases (n) | + | χ2
value | P-value | + | χ2
value | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
<50 | 46 | 35 | 0.25 | 0.416 | 28 | 0.74 | 0.352 |
|
≥50 | 74 | 54 |
|
| 42 |
|
|
| Menopause |
|
|
|
|
|
|
|
|
Yes | 52 | 38 | 1.36 | 0.129 | 30 | 0.62 | 0.391 |
| No | 68 | 51 |
|
| 40 |
|
|
| Tumor size |
|
|
|
|
|
|
|
| <2
cm | 44 | 29 | 5.24 | 0.017 | 23 | 8.49 | 0.013 |
| ≥2
cm | 76 | 60 |
|
| 47 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
Yes | 56 | 31 | 9.15 | 0.013 | 26 | 6.37 | 0.015 |
| No | 64 | 57 |
|
| 44 |
|
|
| Histopathological
grade |
|
|
|
|
|
|
|
| I | 38 | 19 | 11.48 | 0.007 | 18 | 7.14 | 0.015 |
| II | 53 | 44 |
|
| 36 |
|
|
|
III | 29 | 26 |
|
| 16 |
|
|
| Tumor stage |
|
|
|
|
|
|
|
| I | 32 | 18 | 12.53 | 0.007 | 16 | 9.61 | 0.011 |
| II | 57 | 43 |
|
| 33 |
|
|
| III,
IV | 31 | 28 |
|
| 21 |
|
|
| PR |
|
|
|
|
|
|
|
|
(−) | 48 | 35 | 0.94 | 0.172 | 28 | 1.74 | 0.114 |
|
(+) | 72 | 54 |
|
| 42 |
|
|
| ER |
|
|
|
|
|
|
|
|
(−) | 53 | 29 | 6.74 | 0.015 | 24 | 7.93 | 0.013 |
|
(+) | 67 | 60 |
|
| 46 |
|
|
| HER-2 |
|
|
|
|
|
|
|
|
(−) | 52 | 38 | 0.87 | 0.181 | 30 | 0.96 | 0.172 |
|
(+) | 68 | 51 |
|
| 40 |
|
|
Discussion
SATB1 is a nuclear matrix attachment-binding protein
with tissue-specific expression. The SATB1 gene is located
on chromosome 3p23 and encodes for a 763-amino acid protein
(12). SATB1 is highly expressed in
thymus where it regulates the development and maturation of T cells
(13,14). Previous studies showed that
SATB1 gene knockout in mice can inhibit the production of
CD4+ and CD8+ double positive T cells,
leading to disorders of thymus cell maturation (15). SATB1 plays a role as a ‘gene
organizer’ in the genome. SATB1 can interact with more than 1,000
proteins to specifically regulate the expression of its target
genes by chromatin remodeling and protein modification (16). SATB1 can bind the BUR region of target
genes and anchor the BUR region on the nuclear matrix to alter the
higher-order structure of the chromatin, and regulate gene
expression (17). SATB1 can also
regulate DNA binding capacity and the subcellular localization of
proteins through phosphorylation, ubiquitination, and acetylation
(18). Liu et al found that
high expression of SATB1 in breast cancer cells significantly
increased cell invasion ability (19). Clinical data from 1,318 breast cancer
patients showed that the expression level of SATB1 was negatively
correlated with survival time (20).
Our study shows that SATB1 is strongly expressed in breast cancer
and weakly expressed in adjacent non-cancerous tissue. The
expression of SATB1 was not significantly correlated with age,
menopause, and the expression of the PR and HER-2 proteins, but was
significantly correlated with tumor size, local lymphatic
metastasis, histopathological grade, tumor stage, and ER protein
expression. Our findings are consistent with previous studies
(21,22).
Toll-like receptors were first found in
Drosophila. In 1997, TLR4 homologue was identified in
humans, and so far, there are 12 members of the TLRs family
(23). The TLR4 gene is
located on chromosome 9q32-q33, and encodes for a 224-amino acid
protein. TLR4 is widely distributed on the cell surface to sense
pathogens (24). TLR4 is widely
distributed in human monocytes (25),
neutrophils (26), and epithelial
cells (27). TLR4 can recognize a
variety of pathogen-associated molecular patterns (e.g., LPS of
Gram-negative bacteria) to induce different immune responses
(28). Through binding to the
corresponding ligands and mediating intracellular signal
transduction, TLR4 plays a role as a transcription factor to
activate the expression of a variety of cell growth and
apoptosis-related factors (29,30). TLR4
can mediate MyD88-dependent pathways through the interaction with a
series of cytokines to promote tumorigenesis (31). Clinical studies also found that TLR4
was correlated with the growth and metastasis of gastric cancer
(32), ovarian cancer (33,34),
cervical cancer (35), and other
types of tumor cells. In our study, we found that TLR4 was
positively expressed in 58.33% cases of breast cancer tissues and
in 90.57% cases of adjacent non-cancerous tissue. The positive
expression rate of TLR4 in this study is consistent with previous
studies (35,36). We also found that TLR4 expression was
not significantly correlated with age, menopause, or the expression
of the PR and HER-2 proteins. However, TLR4 was significantly
correlated with tumor size, local lymphatic metastasis,
histopathological grade, tumor stage, and ER protein expression. In
addition, correlation analysis indicated that the expression level
of SATB1 was negatively correlated with the expression level of
TLR4. In conclusion, SATB1 and TLR4 are involved in the development
of breast cancer, which is of great significance for the
identification of potential therapeutic targets and prognosis of
breast cancer.
References
|
1
|
Elkin EB, Pocus VH, Mushlin AI, Cigler T,
Atoria CL and Polaneczky MM: Facilitating informed decisions about
breast cancer screening: Development and evaluation of a web-based
decision aid for women in their 40s. BMC Med Inform Decis Mak.
17:292017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crop F, Pasquier D, Baczkiewic A, Doré J,
Bequet L, Steux E, Gadroy A, Bouillon J, Florence C, Muszynski L,
et al: Surface imaging, laser positioning or volumetric imaging for
breast cancer with nodal involvement treated by helical
TomoTherapy. J Appl Clin Med Phys. 17:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu D, Zeng L, Liu F, Zhong Q, Zhang D, Cai
C, Zhang W, Wu L and Chen H: Special AT-rich DNA-binding protein-1
expression is associated with liver cancer metastasis. Oncol Lett.
12:4377–4384. 2016.PubMed/NCBI
|
|
4
|
Stephen TL, Payne KK, Chaurio RA,
Allegrezza MJ, Zhu H, Perez-Sanz J, Perales-Puchalt A, Nguyen JM,
Vara-Ailor AE, Eruslanov EB, et al: SATB1 expression governs
epigenetic repression of PD-1 in tumor-reactive T cells. Immunity.
46:51–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YC, Bu LL, Mao L, Ma SR, Liu JF, Yu GT,
Deng WW, Zhang WF and Sun ZJ: SATB1 promotes tumor metastasis and
invasiveness in oral squamous cell carcinoma. Oral Dis. 23:247–254.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JJ, Kim M and Kim HP: Epigenetic
regulation of long noncoding RNA UCA1 by SATB1 in breast cancer.
BMB Rep. 49:578–583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottimukkala KP, Jangid R, Patta I,
Sultana DA, Sharma A, Misra-Sen J and Galande S: Regulation of
SATB1 during thymocyte development by TCR signaling. Mol Immunol.
77:34–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim SG, Kim JK, Suk K and Lee WH:
Crosstalk between signals initiated from TLR4 and cell surface BAFF
results in synergistic induction of proinflammatory mediators in
THP-1 cells. Sci Rep. 7:458262017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu KC, Huang SS, Kuo YH, Ho YL, Yang CS,
Chang YS and Huang GJ: Ugonin M, a Helminthostachys
zeylanica constituent, prevents LPS-induced acute lung injury
through TLR4-mediated MAPK and NF-κB signaling pathways. Molecules.
22:E5732017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simundic T, Jelakovic B, Dzumhur A, Turk
T, Sahinovic I, Dobrosevic B, Takac B and Barbic J: Interleukin 17A
and Toll-like receptor 4 in patients with arterial hypertension.
Kidney Blood Press Res. 42:99–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kowalczyk AE, Krazinski BE, Godlewski J,
Grzegrzolka J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A,
Dziegiel P and Kmiec Z: SATB1 is down-regulated in clear cell renal
cell carcinoma and correlates with miR-21-5p overexpression and
poor prognosis. Cancer Genomics Proteomics. 13:209–217.
2016.PubMed/NCBI
|
|
12
|
Dickinson LA, Joh T, Kohwi Y and
Kohwi-Shigematsu T: A tissue-specific MAR/SAR DNA-binding protein
with unusual binding site recognition. Cell. 70:631–645. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nixon BG and Li MO: Satb1: Restraining PD1
and T cell exhaustion. Immunity. 46:3–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitagawa Y, Ohkura N, Kidani Y, Vandenbon
A, Hirota K, Kawakami R, Yasuda K, Motooka D, Nakamura S, Kondo M,
et al: Guidance of regulatory T cell development by Satb1-dependent
super-enhancer establishment. Nat Immunol. 18:173–183. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo M, Tanaka Y, Kuwabara T, Naito T,
Kohwi-Shigematsu T and Watanabe A: SATB1 plays a critical role in
establishment of immune tolerance. J Immunol. 196:563–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song G, Liu K, Yang X, Mu B, Yang J, He L,
Hu X, Li Q, Zhao Y, Cai X and Feng G: SATB1 plays an oncogenic role
in esophageal cancer by up-regulation of FN1 and PDGFRB.
Oncotarget. 8:17771–17784. 2017.PubMed/NCBI
|
|
17
|
Luan QX, Zhang BG, Li XJ and Guo MY:
MiR-129-5p is downregulated in breast cancer cells partly due to
promoter H3K27m3 modification and regulates epithelial-mesenchymal
transition and multi-drug resistance. Eur Rev Med Pharmacol Sci.
20:4257–4265. 2016.PubMed/NCBI
|
|
18
|
Yuan CL, Li L, Zhou X, Liz H and Han L:
Expression of SATB1 and HER2 in gastric cancer and its clinical
significance. Eur Rev Med Pharmacol Sci. 20:2256–2264.
2016.PubMed/NCBI
|
|
19
|
Liu X, Zheng Y, Qiao C, Qv F, Wang J, Ding
B, Sun Y and Wang Y: Expression of SATB1 and HER2 in breast cancer
and the correlations with clinicopathologic characteristics. Diagn
Pathol. 10:502015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han HJ, Russo J, Kohwi Y and
Kohwi-Shigematsu T: SATB1 reprogrammes gene expression to promote
breast tumour growth and metastasis. Nature. 452:187–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roberts MR, Sucheston-Campbell LE, Zirpoli
GR, Higgins M, Freudenheim JL, Bandera EV, Ambrosone CB and Yao S:
Single nucleotide variants in metastasis-related genes are
associated with breast cancer risk, by lymph node involvement and
estrogen receptor status, in women with European and African
ancestry. Mol Carcinog. 56:1000–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan Z, Jing W, He K, Zhang L and Long X:
SATB1 is correlated with progression and metastasis of breast
cancers: A meta-analysis. Cell Physiol Biochem. 38:1975–1983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila toll
protein signals activation of adaptive immunity. Nature.
388:394–397. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galdino H Jr, Saar Gomes R, Dos Santos JC,
Pessoni LL, Maldaner AE, Marques SM, Gomes CM, Dorta ML, de
Oliveira MA, Joosten LA, et al: Leishmania (Viannia)
braziliensis amastigotes induces the expression of TNFα and
IL-10 by human peripheral blood mononuclear cells in vitro in a
TLR4-dependent manner. Cytokine. 88:184–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan B, Chen F, Xu L, Xing J and Wang X:
HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung
injury by mediating neutrophil infiltration in mice. Sci Rep.
7:5972017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imai H, Fujita T, Kajiya M, Ouhara K,
Yoshimoto T, Matsuda S, Takeda K and Kurihara H: Mobilization of
TLR4 into lipid rafts by Aggregatibacter
Actinomycetemcomitans in gingival epithelial cells. Cell
Physiol Biochem. 39:1777–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schüller SS, Wisgrill L, Herndl E,
Spittler A, Förster-Waldl E, Sadeghi K, Kramer BW and Berger A:
Pentoxifylline modulates LPS-induced hyperinflammation in monocytes
of preterm infants in vitro. Pediatr Res. May 24–2017.(Epub ahead
of print). View Article : Google Scholar
|
|
29
|
Liu S, Wang X, Shi Y, Han L, Zhao Z, Zhao
C and Luo B: Toll-like receptor gene polymorphisms and
susceptibility to Epstein-Barr virus-associated and -negative
gastric carcinoma in Northern China. Saudi J Gastroenterol.
21:95–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haricharan S and Brown P: TLR4 has a
TP53-dependent dual role in regulating breast cancer cell growth.
Proc Natl Acad Sci USA. 112:E3216–E3225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang D, Taylor GM, Gilbert JR, Losee JE,
Sodhi CP, Hackam DJ, Billiar TR and Cooper GM: Enhanced calvarial
bone healing in CD11c-TLR4−/− and MyD88−/−
mice. Plast Reconstr Surg. 139:933e–940e. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian Y, Li X, Li H, Lu Q, Sun G and Chen
H: Astragalus Mongholicus regulate the toll-like-receptor 4
meditated signal transduction of dendritic cells to restrain
stomach cancer cells. Afr J Tradit Complement Altern Med. 11:92–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klink M, Nowak M, Kielbik M, Bednarska K,
Blus E, Szpakowski M, Szyllo K and Sulowska Z: The interaction of
HspA1A with TLR2 and TLR4 in the response of neutrophils induced by
ovarian cancer cells in vitro. Cell Stress Chaperones. 17:661–674.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang AC, Ma YB, Wu FX, Ma ZF, Liu NF, Gao
R, Gao YS and Sheng XG: TLR4 induces tumor growth and inhibits
paclitaxel activity in MyD88-positive human ovarian carcinoma in
vitro. Oncol Lett. 7:871–877. 2014.PubMed/NCBI
|
|
35
|
de Matos LG, Cândido EB, Vidigal PV,
Bordoni PH, Lamaita RM, Carneiro MM and da Silva-Filho AL:
Association between toll-like receptor and tumor necrosis factor
immunological pathways in uterine cervical neoplasms. Tumori.
103:81–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He A, Ji R, Shao J, He C, Jin M and Xu Y:
TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-κB activation contribute
to the anti-inflammatory effect of V8 in LPS-induced human cervical
cancer SiHa cells. Inflammation. 39:172–181. 2016. View Article : Google Scholar : PubMed/NCBI
|