Introduction
In recent years, the incidence of colorectal cancer
presents an increasing trend (1).
According to epidemiological statistics, there are significant
differences between colon cancer and rectal cancer in progression
and etiology (2). The incidence of
colon cancer in Shanghai has increased by 78% over ~20 years, while
the incidence of rectal cancer has increased by 6% over the same
period. Analysis has demonstrated that the Westernization in
lifestyle and diet is associated with the incidence of colorectal
cancer (3).
Increasing evidence has demonstrated that microRNAs
exhibit endogenous regulatory functions, which serve a regulatory
role in ontogeny, cellular proliferation, apoptosis and
differentiation, viral replication, reproduction, and tumors to a
certain degree. A large number of experiments have demonstrated
that the specifically expressed microRNAs are involved in the
regulation of cancer development. Recent studies have identified
that MDA-MB-231 and BT-549 breast cancer stromal cells, epithelial
cadherin transcription factors mediated by microRNA-200 family are
upregulated, which is directly associated with ZEB1 translation and
indirectly associated with the increased acetylation of histone H3
(4,5).
In certain diseases, embryo prototype mutation of
phosphatase and tensin homolog (PTEN) is >80%, of which the
substrate is a lipid generated by phosphatidylinositol 3-kinase
(PI3K) and requires protein kinase B (Akt) activation (6,7). PTEN
regulates Akt activity by controlling activated
phosphatidylinositol (3,4,5)-triphosphate (PIP3). Therefore, PTEN
mutations lead to the loss of the ability to regulate Akt and
uncontrolled cellular proliferation, which causes cancerization.
PTEN is able to dephosphorylate PIP3, antagonize PI3K activity and
reduce the concentration of PIP3 within the cells, thereby
inhibiting the activation of Akt, through which PTEN regulates
cellular activity (8). In addition,
protein phosphatase activity is associated with tumors. Fish oil
suppressed cell growth of colorectal cancer by regulating PTEN and
nuclear factor-κB signaling (9).
Debroy et al (10) suggested
that anti-microRNA-221 enhanced the radiosensitivity of colorectal
cancer cells by upregulating PTEN. Peroxisome
proliferator-activated receptor γ induced apoptosis of colorectal
cancer cells by upregulating PTEN and inhibiting PI3K activity
(11). Isayev et al (12) reported that ribonuclease inhibitor
suppresses proliferation and metastasis in colorectal cancer cells
by inhibiting the PI3K/Akt signaling pathway.
Catalpol is one of the primary active ingredients in
rehmannia, which functions as a diuretic and laxative, and exhibits
blood sugar-lowering, liver protective, anti-aging and anticancer
effects (13–16). In traditional Chinese medicine,
catalpol is believed to be Yin nourishing. Previous studies have
observed that catalpol may protect neurons from cytotoxic damage,
reducing neuronal apoptosis following cerebral ischemia (17–19). The
aim of the present study was to observe the effects of catalpol in
colorectal cancer cells, and to investigate its mechanism and
determine its therapeutic value in treating colorectal cancer.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). MTT was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Caspase-3 and
caspase-9 activity assay kits, and the bicinchoninic acid (BCA)
protein assay kit were purchased from Beyotime Institute of
Biotechnology (Haimen, China). The Annexin V-fluorescein
isothiocyanate (V-FITC)/propidium iodide (PI) Apoptosis Detection
kit was purchased from BestBio Co. (Shanghai, China). Catalpol was
purchased from Sigma-Aldrich (Merck KGaA).
Cell culture
The human colorectal cancer HCT116 cell line was
purchased from Union of Basic Medical Cell Center (Beijing, China).
HCT116 cells were cultured in DMEM containing 10% FBS with 100 U/ml
penicillin and 100 U/ml streptomycin, and cultured at 37°C in an
atmosphere containing 5% CO2.
Cell viability assays
HCT116 cells (2×104 cells/well) were
seeded in 96-well plates and cell viability was detected using MTT.
HCT116 cells were cultured with various concentrations of catalpol
(0, 25, 50 and 100 µg/ml). Following treatment for 24, 48, and 72
h, 20 µl MTT solution (0.5 mg/ml) was added into each well and
cells were incubated at 37°C for 4 h. The culture medium of each
well was subsequently removed and 150 µl dimethyl sulfoxide was
added into each well at room temperature whilst being shaken for 20
min. Absorbance was measured at 570 nm using a Bio-Rad ELISA reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Specific PI3K
inhibitors LY294002 and wortmannin were provided by Calbiochem
(Merck KGaA). The specific PI3K inhibitors LY294002 (5 µM) was
incubated at 37°C for 1 h prior to the detection in the wells.
Caspase-3 and caspase-9
activities
HCT116 cells were seeded (1×106
cells/well) in 6-well plates, and the activities of caspase-3 and
−9 were determined using caspase-3 and caspase-9 activity assay
kits. Following treatment for 48 h with catalpol (0, 25, 50 and 100
µg/ml), HCT116 cells were evaluated for hydrolysis of the peptide
substrate Ac-IETD-pNA by caspase-3 and −9, resulting in the release
of a pNA moiety. Absorbance values were measured with a microplate
reader (Bio-Rad Laboratories, Inc.) at 405 nm. The activities of
caspase-3 and −9 were expressed as nmol pNA/mg total protein.
Flow cytometric assays for Annexin
V-FITC/PI
HCT116 cells were seeded (1×106
cells/well) in 6-well plates and the apoptosis of HCT116 cells was
measured using the Annexin V-FITC/PI Apoptosis Detection kit (cat
no. 556570; BD Biosciences, San Jose, CA, USA) according to the
manufacturer' protocol. Following treatment for 48 h with catalpol
(0, 25, 50 and 100 µg/ml), HCT116 cells were washed twice with
ice-cold PBS and subsequently added to 1X binding buffer. A total
of 5 µl V-FITC was added into each well for 30 min in the dark.
Subsequently, 10 µl PI was added into each well in the dark.
Apoptosis in HCT116 cells was analyzed using flow cytometry
(FACSCalibur; BD Biosciences).
DAPI staining assay
HCT116 cells were seeded (1×106
cells/well) in 6-well plates and nucleic morphology was tested
using a DAPI staining assay. Following treatment for 48 h with
catalpol (0, 25, 50 and 100 µg/ml), PBS was used to wash HCT116
cells, and 0.5 ml paraformaldehyde (4%) was added to each well and
cultivated for 30 min at 4°C. HCT116 cells were washed twice with
PBS, and sodium citrate (0.1%) was subsequently added containing
0.1% Triton X-100 and incubated for 10 min on ice. DAPI was added
to HCT116 cells and incubated for 15 min at 4°C in the dark.
Nucleic morphology was viewed under ultraviolet light. HCT116 cells
were observed and images were captured using florescence microscopy
(Zeiss Axio Observer A1; Carl Zeiss AG, Oberkochen, Germany) at 340
nm.
Western blot analysis
HCT116 cells were seeded (1×106
cells/well) in 6-well plates, and the expressions of PTEN, PI3K,
phosphorylated (p)-Akt and Akt protein were detected via western
blot analysis. Following treatment for 48 h with catalpol (0, 25,
50 and 100 µg/ml), HCT116 cells were washed twice with ice-cold PBS
and lyzed for 30 min on ice in cell-lysis buffer (cat no. FNN0021;
Thermo Fisher Scientific, Inc.). Protein concentration was
determined using a BCA protein assay kit. Protein samples (20
µg/well) were resolved using 10% SDS-PAGE. Separated protein was
subsequently transferred onto polyvinylidene difluoride membranes
(PVDF) membrane for 2 h at 60 V. The membranes were blocked with 5%
non-fat milk powder in TBS-Tween-20 (TBST) buffer overnight at 4°C.
The PVDF was incubated with anti-PI3K (1:1,000; cat no. GW21071;
Sigma-Aldrich; Merck KGaA), anti-Akt (1:1,000; cat no. P0024-1),
anti-p-Akt (1:1,000; cat no. BM1612) (both from Boster Biological
Technology, Pleasanton, CA, USA) and anti-β-actin (1:500; cat no.
BM0627; Wuhan Boster Biological Technology, Ltd., Wuhan, China) for
2 h at room temperature. Following three washes with TBST twice for
30 mins, the membrane was incubated with anti-immunoglobulin G
secondary antibody (1:500; cat no. BA1054; Wuhan Boster Biological
Technology, Ltd.) was added prior to incubation at room temperature
for 2 h with shaking. The bands were detected using the Enhanced
Chemiluminescence Prime western blotting kit (GE Healthcare Life
Sciences, Little Chalfont, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of microRNA-200
Total RNA was extracted from HCT116 cells using
TRIzol (Tiangen Biotech Co., Ltd., Beijing, China). MicroRNA-200
was performed using the High Capacity cDNA Reverse Transcription
kit with the ABI 7500 qPCR system (both from Takara Bio, Inc.,
Tokyo, Japan), according to the manufacturer's protocol. The qPCR
was performed according to the manufacturer's protocol (cat no.
1725085; Bio-Rad Laboratories, Inc.). The PCR cycling conditions
consisted of 93°C for 3 min, then 10 cycles at: 94°C for 15 sec,
55°C for 15 sec and 72°C for 30 sec; then 20 cycles at: 89°C for 15
sec, 55°C for 15 sec and 72°C for 30 sec; and an extension cycle at
72°C for 10 min. All primers used are purchased from Sangon Biotech
(Shanghai, China). The forward and reverse primers for microRNA-200
were 5′-TGCATCATTACCAGGCAGTATTAGA-3′ and
5′-CCTCTTACCTCAGTTACAATTTATA-3′, respectively. The forward and
reverse primers for U6 were 5′-CGCTTCGGCACATATACTA-3′ and
5′-CGCTTCACGAATTTGCGTGTCA-3′, respectively. The results were
quantified using the 2−ΔΔCq method (20).
Statistical analysis
Experiments were repeated at least three times and
were analyzed using SPSS (version 17.0; SPSS, Inc., Chicago, IL,
USA). Data are expressed as the mean ± standard deviation.
Differences were tested using the Student's unpaired t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of catalpol on cellular
viability
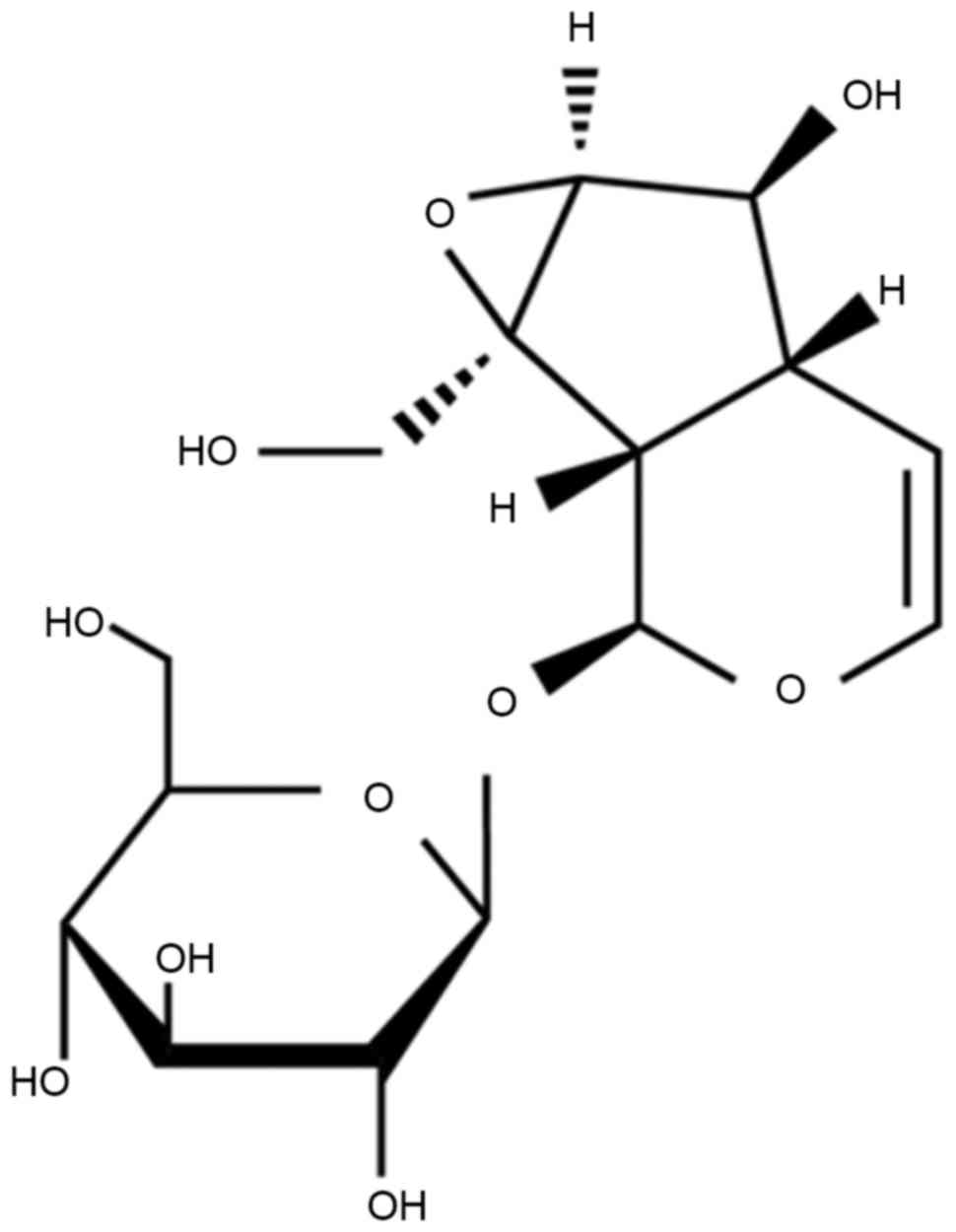
The chemical structure of catalpol is indicated in
Fig. 1. To investigate the anticancer
effect of catalpol on the viability of human colorectal cancer
cells, HCT116 cells were treated with catalpol at different
concentrations (0, 25, 50 and 100 µg/ml) for 24, 48 and 72 h,
respectively. The absorbances of HCT116 cells were detected using
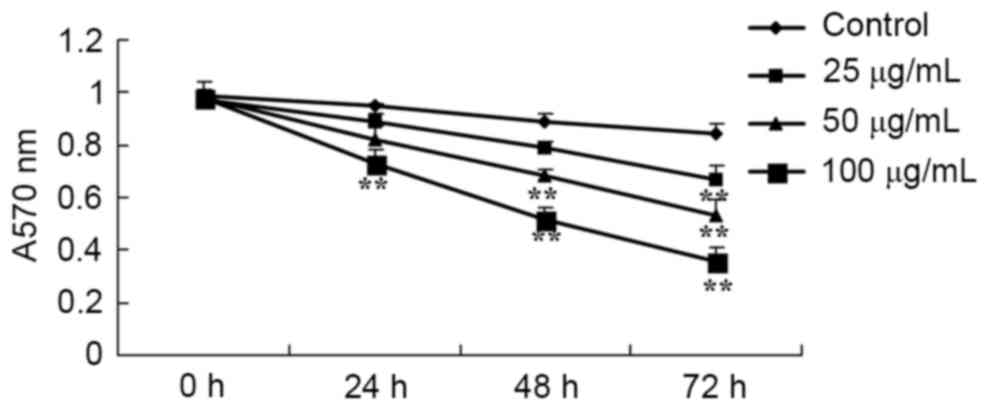
the MTT assay. As presented in Fig.
2, the viability of HCT116 cells was inhibited by treatment
with catalpol in a dose- and time-dependent manner. These data
suggest that catalpol markedly inhibits human colorectal cancer
cell viability.
Effects of catalpol on caspase-3 and
−9 activities in HCT116 cells
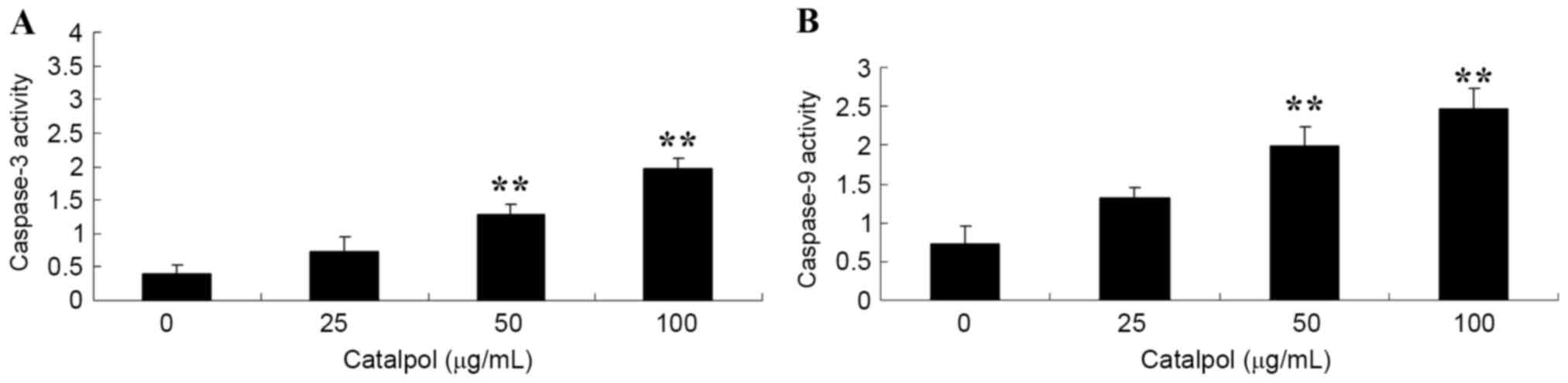
To investigate the activities of caspase-3 and −9 in
human colorectal cancer cells, HCT116 cells were treated with
different doses of catalpol (0, 25, 50 and 100 µg/ml). When the
HCT116 cells were treated for 48 h, a significant increase in the
activities of caspase-3 and −9, following treatment with 50 and 100
µg/ml catalpol, was observed compared with the respective controls
(Fig. 3).
Effects of catalpol-induced apoptosis
in HCT116 cell
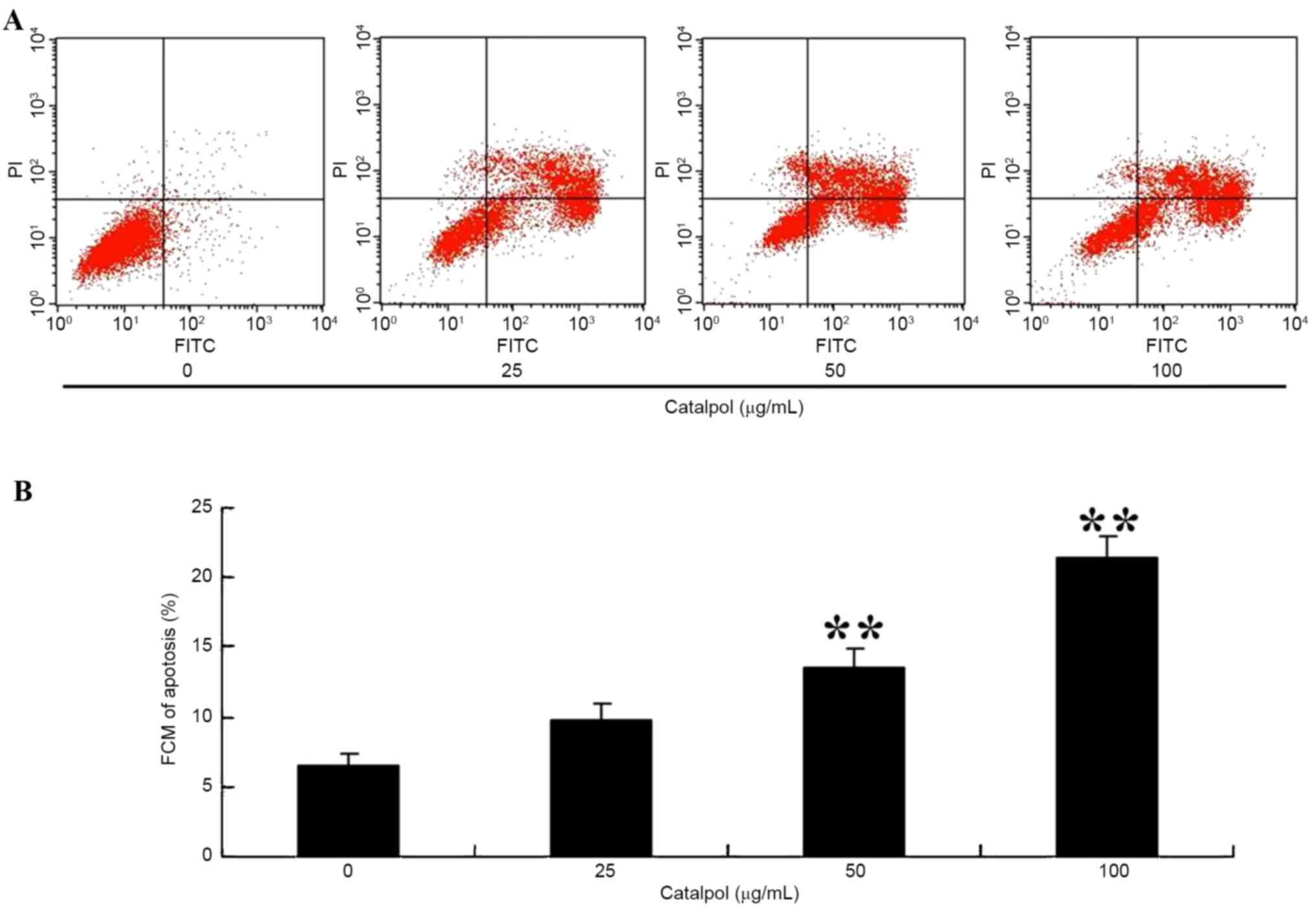
To observe apoptosis in HCT116 cells following
treatment with catalpol, the apoptosis ratio was measured using a
flow cytometric assay. As presented in Fig. 4A and B, the apoptosis percentage was
increased following 48 h treatment with 50 or 100 µg/ml
catalpol.
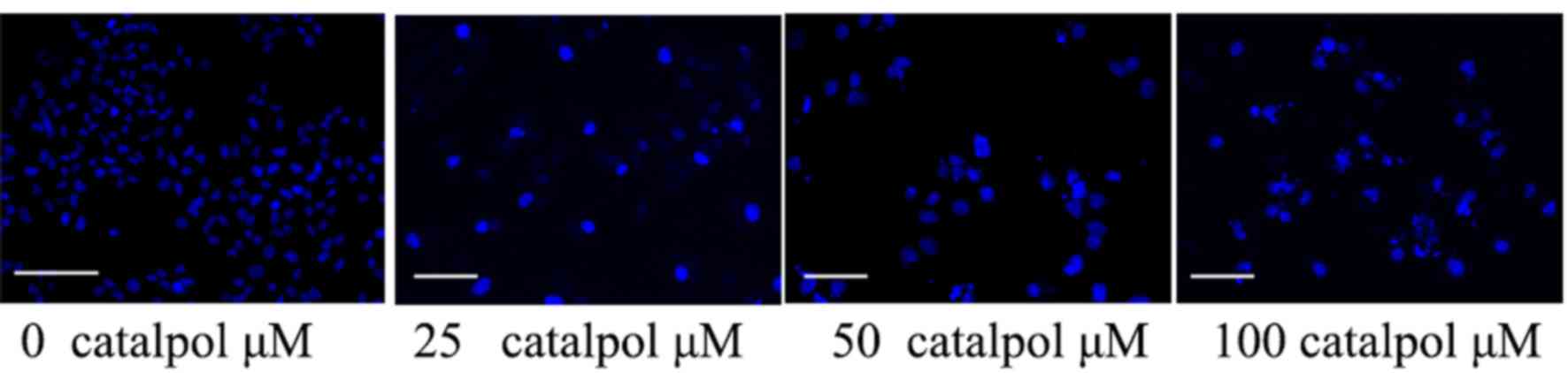
Effects of catalpol on the nucleic
morphology of HCT116 cells
The anticancer effect of catalpol on the nucleic
morphology of HCT116 cells was assessed. As presented in Fig. 5, catalpol (50 or 100 µg/ml) influenced
the nucleic morphology of HCT116 cells and accelerated HCT116 cell
nucleic apoptosis compared with the control group.
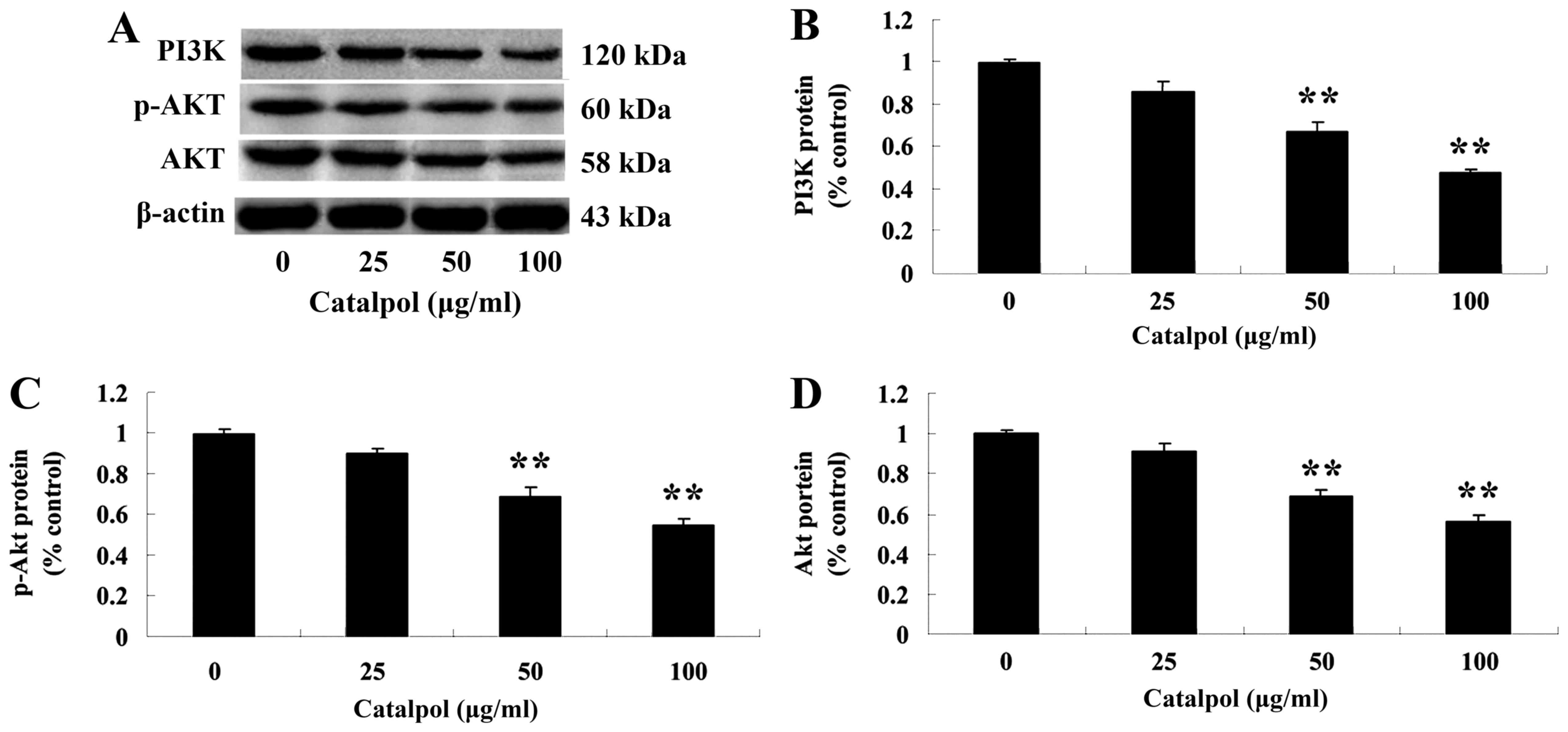
Effects of catalpol on the PI3K-Akt
signaling pathway
To further investigate the mechanism of catalpol on
the viability and apoptosis of human colorectal cancer HCT116
cells, the expression of PI3K and p-Akt protein was evaluated.
Following treatment with catalpol (0, 25, 50 or 100 µg/ml) for 48
h, the expressions of PI3K and p-Akt proteins were analyzed by
western blotting. The protein expression of PI3K and p-Akt was
markedly decreased compared with the control group (Fig. 6).
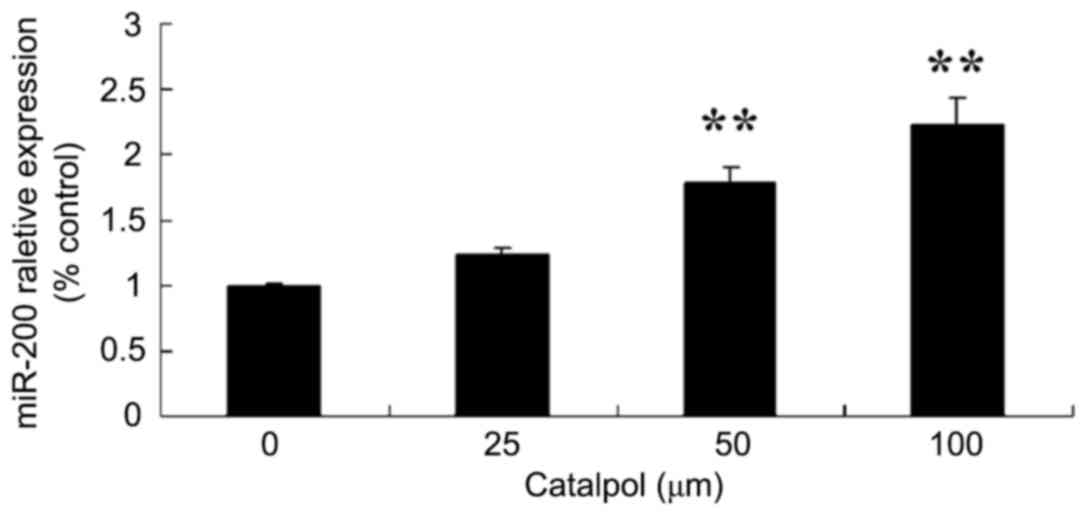
Effects of catalpol on microRNA-200
expression of HCT116 cell
To further investigate the mechanism of catalpol on
proliferation and apoptosis in human colorectal cancer HCT116
cells, microRNA-200 expression was evaluated in HCT116 cells. As
demonstrated in Fig. 7, the
expression of microRNA-200 was increased following treatment with
catalpol (0, 25, 50 and 100 µg/ml) for 48 h compared with the
control group.
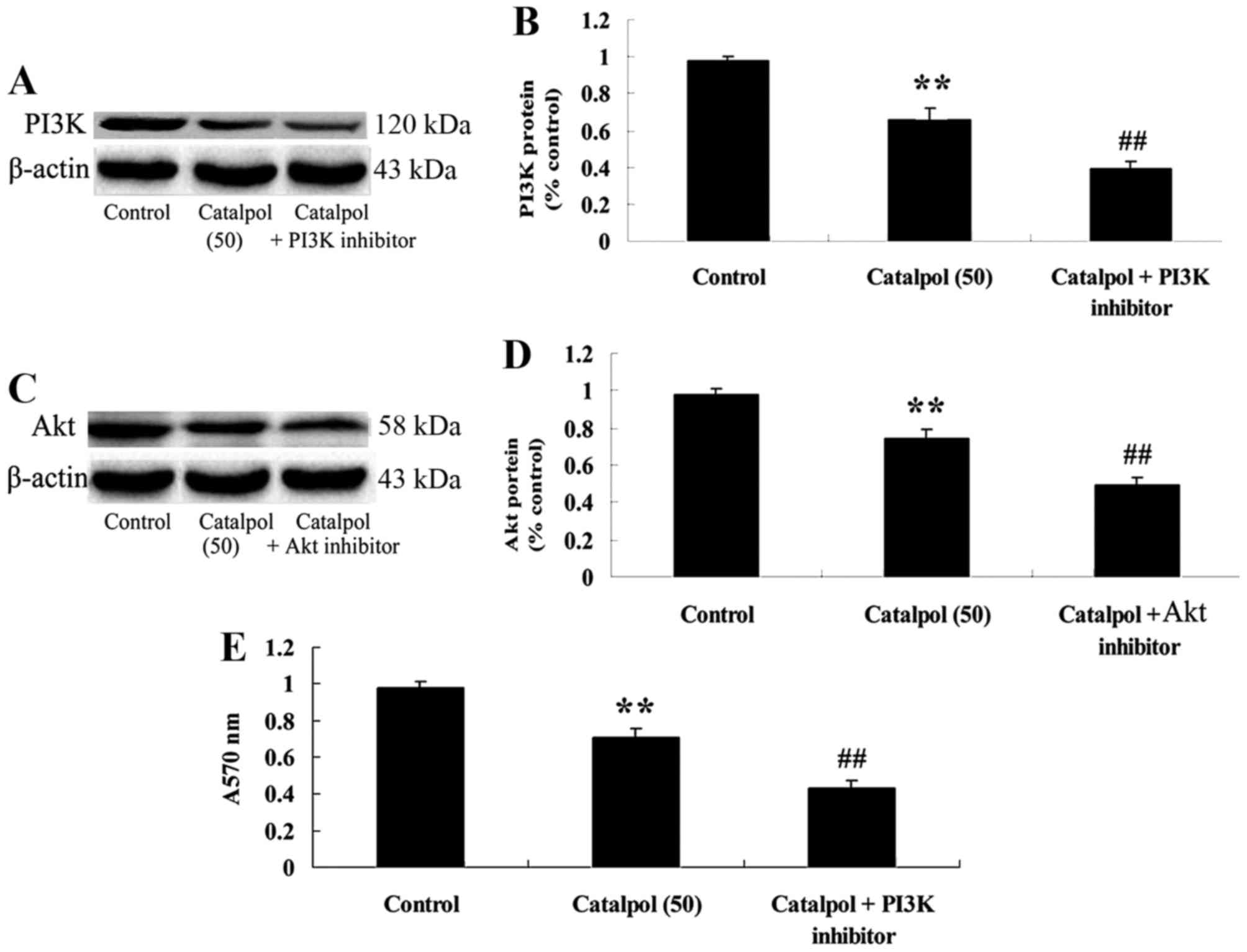
Downregulation of PI3K-Akt following
treatment with catalpol and the effect on cellular viability
To investigate the mechanism of catalpol on
viability and apoptosis in human colorectal cancer HCT116 cells,
PI3K inhibitor (LY294002, 5 µM) was added to HCT116 cells. The
results indicated that the PI3K inhibitor inhibited the PI3K-Akt
signaling pathway by suppressing PI3K and p-Akt protein expression
in HCT116 cells compared with the catalpol-treated (50 µg/ml) group
(Fig. 8A-D). In addition,
downregulation of PI3K-Akt further decreased cell viability in
HCT116 cells compared with the catalpol-treated (50 µg/ml) group
(Fig. 8E).
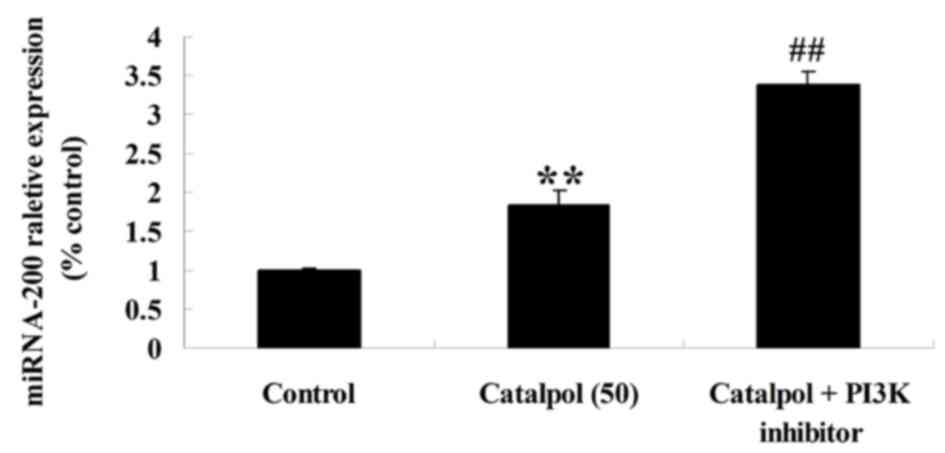
Effect of downregulation of PI3K-Akt
on microRNA-200 expression in HCT116 cells
To verify the mechanism of catalpol on the viability
and apoptosis of human colorectal cancer HCT116 cells, microRNA-200
expression in HCT116 cells was detected following downregulation of
PI3K-Akt. As presented in Fig. 9, the
downregulation of PI3K-Akt enhanced microRNA-200 expression in
HCT116 cells compared with the catalpol-treated (50 µg/ml)
group.
Discussion
Colorectal cancer is the most common malignant tumor
and it is estimated there were 1.167 million new cases in 2007
worldwide, ranking third among all malignant tumors; 603,000
succumbed to colorectal cancer in the same year (21,22).
Currently, colorectal cancer remains a major threat to human
health. In the present study, the results demonstrated that the
cell viability of human colorectal cancer cells was inhibited by
treatment with catalpol. In addition, the treatment of catalpol
increased the activities of caspase-3 and −9, increased the
apoptosis ratio, and accelerated cell nucleic apoptosis in human
colorectal cancer HCT116 cells. Lee et al (23) demonstrated that catalpol inhibited the
proliferation of ovarian cancer A2780 cells, human epidermoid
carcinoma, human rhabdomyosarcoma and transgenic murine L-cells
(17).
Increasing evidence has demonstrated that the
expression of microRNAs in certain malignant human tissues is
altered, including in lung, liver, colon, nasopharyngeal, ovarian
and breast cancer. The regulatory role of microRNAs in gastric
cancer has also been proven in an increasing number of experiments.
In human colon cancer cell lines, microRNA-200 expression is
increased and the expression of microRNA-200b decreased following
treatment with 5-fluorouracil. MicroRNA-200 inhibits
tyrosine-protein phosphatase non-receptor type 12, which
inactivates oncogenes including tyrosine-protein kinase ABL1,
proto-oncogene tyrosine-protein kinase Src and GTPase Ras. A
previous study demonstrated that microRNA-200 exhibits a regulatory
role in the proliferation of gastric cancer MGC-803 cells and that
overexpression of microRNA-200 may inhibit the proliferation of
gastric cancer MGC-803 cells (24–27). Gao
et al (28) reported that
catalpol suppresses proliferation and facilitates apoptosis in
OVCAR-3 ovarian cancer cells through activation of microRNA-200 and
downregulating matrix metalloproteinase-2 expression. In the
present study, similar results were obtained; treatment with
catalpol also promoted the expression of microRNA-200 in HCT116
cells.
The PI3K/Akt signal transduction pathway serves an
important role in cell proliferation, in which Akt is a downstream
target protein of PI3K, and continuous activation of the pathway is
associated with tumor development (29). The PI3K/Akt signaling pathway is
regulated by a variety of cytokines, in which negative regulator
molecules, including PTEN, form primary components (30). In the present study, it was observed
that catalpol reduced the expressions of PI3K and p-Akt in HCT116
cells. A PI3K inhibitor decreased the viability of human colorectal
cancer HCT116 cell following treatment with catalpol and increased
the expression of microRNA-200 in HCT116 cells. Chamnanphon et
al (18) reported that catalpol
protected oligodendrocyte survival and oligodendrocyte progenitor
differentiation through the Akt signaling pathway in rats. Sukasem
et al (31) demonstrated that
catalpol decreased peroxynitrite formation and consequently exerts
cardioprotective effects through the PI3K/Akt signaling pathway in
ischemic/reperfusion rats.
In conclusion, catalpol may be used as a natural
anticancer drug in human colorectal cancer HCT116 cells. The
present study suggests that, administration of catalpol reduced
cell viability, increased the activities of caspase-3 and −9,
increased cellular and nucleic apoptosis, suppressed the protein
expression of components of the PI3K-Akt signaling pathway, and
promoted the expression of microRNA-200 in HCT116 cells. In
addition, the present study investigated whether downregulation of
the PI3K-Akt signaling pathway was due to the effect of catalpol on
HCT116 cells. However, treatment with a PI3K inhibitor augmented
the effect of catalpol on cell viability and increased the
expression of microRNA-200 in HCT116 cells. Therefore, catalpol
promotes apoptosis in human colorectal cancer cells through the
promotion of microRNA-200 and the downregulation of the PI3K-Akt
signaling pathway. These results suggest that catalpol is a
promising seed for novel types of anti-tumor agents; however, their
molecular targets require further clarification.
Acknowledgements
The present study was supported by the Youth Fund of
The Second Hospital of Shandong University (grant no.
Y2013010024).
References
|
1
|
Pandeló José D, Bartholomeeusen K, da
Cunha RD, Abreu CM, Glinski J, da Costa TB, Bacchi Rabay AF,
Pianowski Filho LF, Dudycz LW, Ranga U, et al: Reactivation of
latent HIV-1 by new semi-synthetic ingenol esters. Virology.
462–463:328–339. 2014. View Article : Google Scholar
|
|
2
|
Liu Y, Yin Z, Zhang R, Yan K, Chen L, Chen
F, Huang W, Lv B, Sun C and Jiang X: MSCs inhibit bone
marrow-derived DC maturation and function through the release of
TSG-6. Biochem Biophys Res Commun. 450:1409–1415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar
|
|
4
|
Tryndyak VP, Beland FA and Pogribny IP:
E-cadherin transcriptional down-regulation by epigenetic and
microRNA-200 family alterations is related to mesenchymal and
drug-resistant phenotypes in human breast cancer cells. Int J
Cancer. 126:2575–2583. 2010.PubMed/NCBI
|
|
5
|
Li X, Roslan S, Johnstone CN, Wright JA,
Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ,
et al: miR-200 can repress breast cancer metastasis through
ZEB1-independent but moesin-dependent pathways. Oncogene.
33:4077–4088. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higgins MJ and Stearns V: Understanding
resistance to tamoxifen in hormone receptor-positive breast cancer.
Clin Chem. 55:1453–1455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang Y, Gong FL, Zhao GB and Li J:
Chrysin suppressed inflammatory responses and the inducible nitric
oxide synthase pathway after spinal cord injury in rats. Int J Mol
Sci. 15:12270–12279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maehr T, Vecino JL, Wadsworth S, Wang T
and Secombes CJ: Four CISH paralogues are present in rainbow trout
Oncorhynchus mykiss: Differential expression and modulation during
immune responses and development. Mol Immunol. 62:186–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S, Zhou L, Niu G, Li Y, Zhao D and
Zeng H: Differential regulation of orphan nuclear receptor TR3
transcript variants by novel vascular growth factor signaling
pathways. FASEB J. 28:4524–4533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Debroy A, Vogel SM, Soni D, Sundivakkam
PC, Malik AB and Tiruppathi C: Cooperative signaling via
transcription factors NF-κB and AP1/c-Fos mediates endothelial cell
STIM1 expression and hyperpermeability in response to endotoxin. J
Biol Chem. 289:24188–24201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z, Sun D, Yan Z, Reynolds AB,
Christman JW, Minshall RD, Malik AB, Zhang Y and Hu G: Differential
role for p120-catenin in regulation of TLR4 signaling in
macrophages. J Immunol. 193:1931–1941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isayev O, Rausch V, Bauer N, Liu L, Fan P,
Zhang Y, Gladkich J, Nwaeburu CC, Mattern J, Mollenhauer M, et al:
Inhibition of glucose turnover by 3-bromopyruvate counteracts
pancreatic cancer stem cell features and sensitizes cells to
gemcitabine. Oncotarget. 5:5177–5189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sirachainan E, Jaruhathai S, Trachu N,
Panvichian R, Sirisinha T, Ativitavas T, Ratanatharathorn V,
Chamnanphon M and Sukasem C: CYP2D6 polymorphisms influence the
efficacy of adjuvant tamoxifen in Thai breast cancer patients.
Pharmgenomics Pers Med. 5:149–153. 2012.PubMed/NCBI
|
|
14
|
Cota GF, de Sousa MR, Fereguetti TO and
Rabello A: Efficacy of anti-leishmania therapy in visceral
leishmaniasis among HIV infected patients: A systematic review with
indirect comparison. PLoS Negl Trop Dis. 7:e21952013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lyu J, Yang H, Lang J and Tan X: Tumor
necrosis factor gene polymorphisms and endometriosis in Asians: A
systematic review and meta-analysis. Chin Med J (Engl).
127:1761–1767. 2014.PubMed/NCBI
|
|
17
|
Toyama T, Yamashita H, Sugiura H, Kondo N,
Iwase H and Fujii Y: No association between CYP2D6*10 genotype and
survival of node-negative Japanese breast cancer patients receiving
adjuvant tamoxifen treatment. Jpn J Clin Oncol. 39:651–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chamnanphon M, Pechatanan K, Sirachainan
E, Trachu N, Chantratita W, Pasomsub E, Noonpakdee W, Sensorn I and
Sukasem C: Association of CYP2D6 and CYP2C19 polymorphisms and
disease-free survival of Thai post-menopausal breast cancer
patients who received adjuvant tamoxifen. Pharmgenomics Pers Med.
6:37–48. 2013.PubMed/NCBI
|
|
19
|
Pu Z, Yuan X, Zhang X, Chen Q and Xie H:
Meta-analysis on the association between CYP2D6*10 gene
polymorphism and disease free survival of breast cancer patients
receiving tamoxifen treatment in Asia. Bangladesh J Pharmacol.
9:652–662. 2014. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee K, Kim SJ, Kim D, Jo SH, Lee Joong S
and Choi SY: Prostaglandin modulates TLR3-induced cytokine
expression in human astroglioma cells. Brain Res. 1589:54–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan SM, Chadwick J, Young DL, Holmes E
and Gotlib J: Intensive serial biomarker profiling for the
prediction of neutropenic Fever in patients with hematologic
malignancies undergoing chemotherapy: A pilot study. Hematol Rep.
6:54662014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, Pant HC and Shea TB: Divergent and
convergent roles for kinases and phosphatases in neurofilament
dynamics. J Cell Sci. 127:4064–4077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armand-Labit V and Pradines A: Circulating
cell-free microRNAs as clinical cancer biomarkers. Biomol Concepts.
8:61–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng Q, Chen C, Guan H, Kang W and Yu C:
Prognostic role of microRNAs in human gastrointestinal cancer: A
systematic review and meta-analysis. Oncotarget. Mar 29–2017.(Epub
ahead of print).
|
|
26
|
Tanaka S, Hosokawa M, Yonezawa T, Hayashi
W, Ueda K and Iwakawa S: Induction of epithelial-mesenchymal
transition and down-regulation of miR-200c and miR-141 in
oxaliplatin-resistant colorectal cancer cells. Biol Pharm Bull.
38:435–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning X, Shi Z, Liu X, Zhang A, Han L,
Jiang K, Kang C and Zhang Q: DNMT1 and EZH2 mediated methylation
silences the microRNA-200b/a/429 gene and promotes tumor
progression. Cancer Lett. 359:198–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao N, Tian JX, Shang YH, Zhao DY and Wu
T: Catalpol suppresses proliferation and facilitates apoptosis of
OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and
downregulating MMP-2 expression. Int J Mol Sci. 15:19394–19405.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo JB, Feng L, Jiang WD, Liu Y, Wu P,
Jiang J, Kuang SY, Tang L, Zhang YA and Zhou XQ: The impaired
intestinal mucosal immune system by valine deficiency for young
grass carp (Ctenopharyngodon idella) is associated with decreasing
immune status and regulating tight junction proteins transcript
abundance in the intestine. Fish Shellfish Immunol. 40:197–207.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Desta Z, Stearns V, Ward B, Ho H,
Lee KH, Skaar T, Storniolo AM, Li L, Araba A, et al: CYP2D6
genotype, antidepressant use, and tamoxifen metabolism during
adjuvant breast cancer treatment. J Natl Cancer Inst. 97:30–39.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sukasem C, Sirachainan E, Chamnanphon M,
Pechatanan K, Sirisinha T, Ativitavas T, Panvichian R,
Ratanatharathorn V, Trachu N and Chantratita W: Impact of CYP2D6
polymorphisms on tamoxifen responses of women with breast cancer: A
microarray-based study in Thailand. Asian Pac J Cancer Prev.
13:4549–4553. 2012. View Article : Google Scholar : PubMed/NCBI
|