Introduction
Atypical chronic myeloid leukemia (aCML) is a rare
subtype of myelodysplastic/myeloproliferative neoplasm (MDS/MPN)
with an incidence rate of 1–2 cases for every 100 patients with
BCR-ABL1 positive CML (1). In the
World Health Organization (WHO) 2008 classification, aCML is
characterized as ‘peripheral blood leukocytosis due to increased
number of neutrophil and their precursors with prominent
dysgranulopoiesis, with absent/minimal monocytosis or basophilia’
(1). In ~40% of patients, aCML
develops into acute myeloid leukemia with median survival times
ranging between 12 and 29 months. An allogeneic hematopoietic stem
cell transplantation (AlloSCT) is the only option to treat aCML,
which is associated with poor prognoses (1,2).
Chromosomal abnormalities are exhibited by between
20 and 88% of patients with aCML, with +8, i(17q), or −7/−7q
observed most commonly (2).
Additionally, SET binding protein 1 (SETBP1) and ethanolamine
kinase 1 (ETNK1) mutations are associated with aCML, according to
previous studies (3,4). However, no specific recurrent
chromosomal or genetic abnormalities have been identified in aCML
thus far (5). Conversely, X
chromosome abnormalities occur in ~1% of patients with
hematological disorders, with i(X)(p10) considered a recurrent
chromosomal abnormality in hematological malignancies (6).
In the present study, a case of adult aCML with
i(X)(p10) and an additional cytogenetic abnormality appearing 1
year later was described. The cytogenetic abnormalities became
undetectable subsequent to the patient undergoing AlloSCT.
Case report
A 40-year-old female was referred to the Tokyo
Medical and Dental University Hospital (Tokyo, Japan), due to an
annual medical checkup revealing slight leukocytosis and anemia,
with a white blood cell count (WBC) of 12×109/l
(myelocyte, 2%; metamyelocyote, 6%) and a hemoglobin (Hb) level of
9.4 g/dl. A physical examination demonstrated no remarkable
findings. A complete blood count exhibited the following results:
WBC of 8.1×109/l (differential: Blast 0%, promyelocyte
0%, myelocyte 0%, metamyelocyte 7%, neutrophil 56%, lymphocyte 19%,
monocyte 18%), Hb level of 8.6 g/dl, a platelet count of
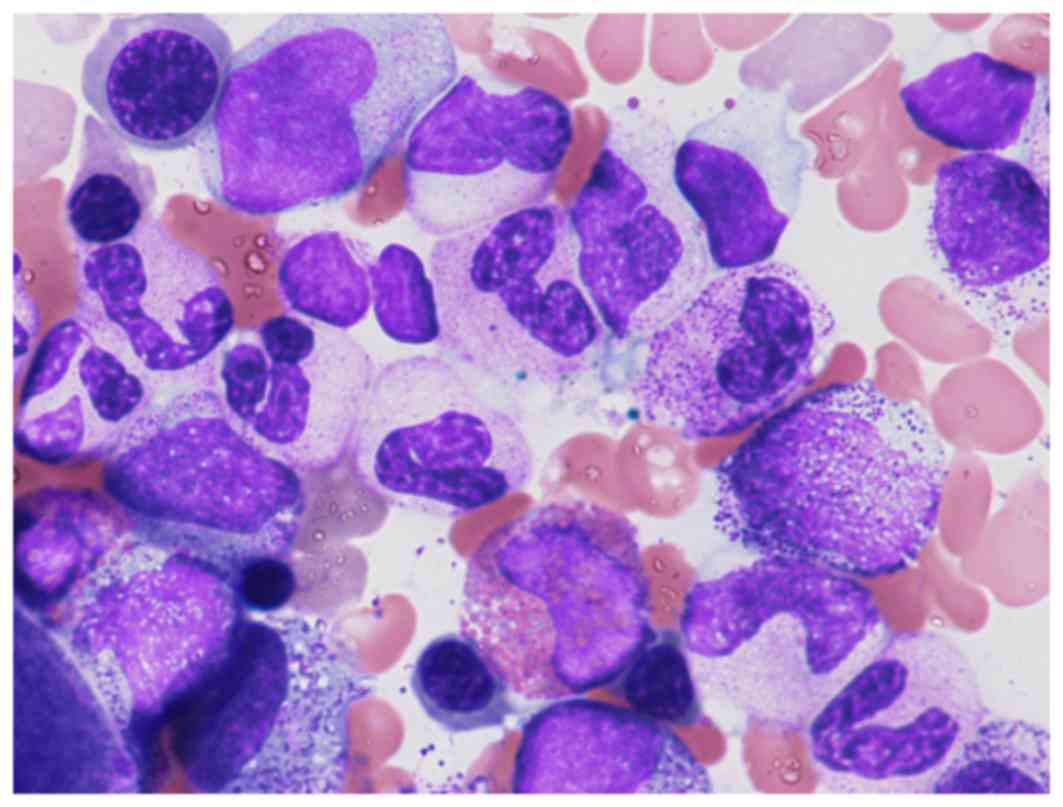
230×109/l. The bone marrow morphology revealed 4%
blasts, granulocyte proliferation with dysplasia and slight
dysplasia in the megakaryocytic lineage identified by May-Giemsa
staining with Giemsa and May-Grünwald solutions (Muto Pure
Chemicals Co., Ltd., Tokyo, Japan), as presented in Fig. 1.
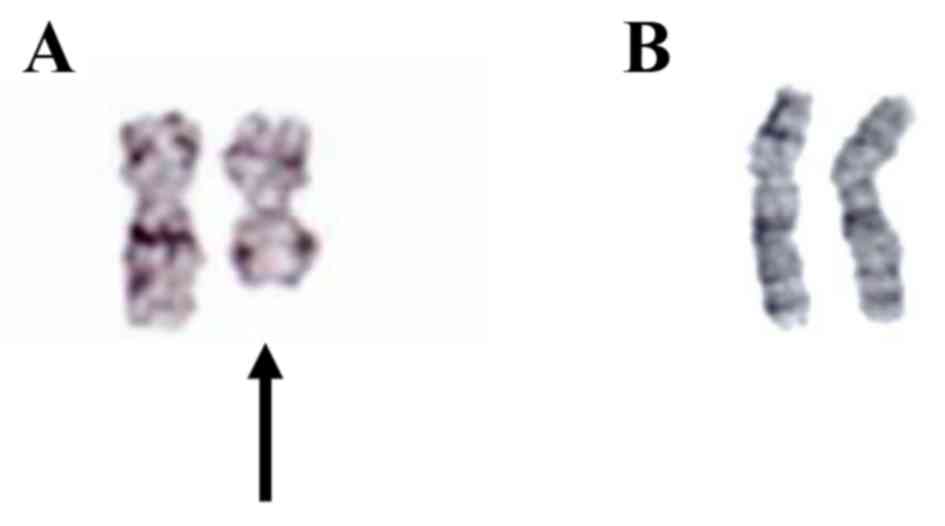
A G-banded cytogenetic analysis of the bone marrow
cells revealed 46, X, i(X)(p10) in all metaphases analyzed, as
illustrated in Fig. 2A, and
subsequent chromosomal analysis of phytohemagglutinin-stimulated
peripheral blood cells revealed the normal female karyotype 46, XX,
as demonstrated in Fig. 2B.
Fluorescent in situ hybridization (FISH) analysis did not
detect the BCR/ABL fusion gene, and the results of the molecular
genetic analyses were negative for the Janus kinase 2 (JAK2) V617F
mutation, and mutations in granulocyte colony-stimulating factor
receptor (CSF3R), SETBP1, and ETNK1.
The absolute monocyte count of the patient was
>1×109/l, and the percentage of monocytes was
<10%. Furthermore, the percentage of the neutrophil precursors
promyelocytes, myelocytes and metamyelocytes was >10% throughout
the clinical course of the patient. Based on these clinical and
hematological findings, the diagnosis was aCML.
Initially, the patient did not receive any therapy,
but exhibited a rapid increase in WBC to 43×109/l in the
6 months following initial diagnosis, as demonstrated in Fig. 3. Subsequently, hydroxycarbamide
therapy was initiated. However, the treatment did not induce an
adequate hematological response. Furthermore, an additional
cytogenetic abnormality, t(10;17)(p13;q21), was detected in 2/20
bone marrow cells analyzed 1 year following initial diagnosis.
Therefore, the patient received AlloSCT from a human leukocyte
antigen-matched sibling donor. Prior to this, the patient received
3.2 mg/kg/day intravenous busulfan, in 4 doses, between days 5 and
2 prior to AlloSCT treatment, 30 mg/m2/day fludarabine
between days 6 and 2 prior to AlloSCT treatment and total body
irradiation using 400 cGy/day in 2 doses on the day of treatment
initiation. Graft vs. host disease prophylaxis comprised a
continuous infusion of cyclosporine A (2 mg/kg, from 1 day prior to
AlloSCT treatment) and a short course of methotrexate (10
mg/m2 on day 1 following AlloCST treatment, and 7
mg/m2 on days 3 and 6 following AlloCST treatment). The
patient was successfully engrafted with the donor cells on day 20
of transplantation. Post-transplant cytogenetic analysis revealed a
normal male karyotype with full donor chimerism as measured by FISH
analysis, showing the XY pattern in >99% of the bone marrow
cells.
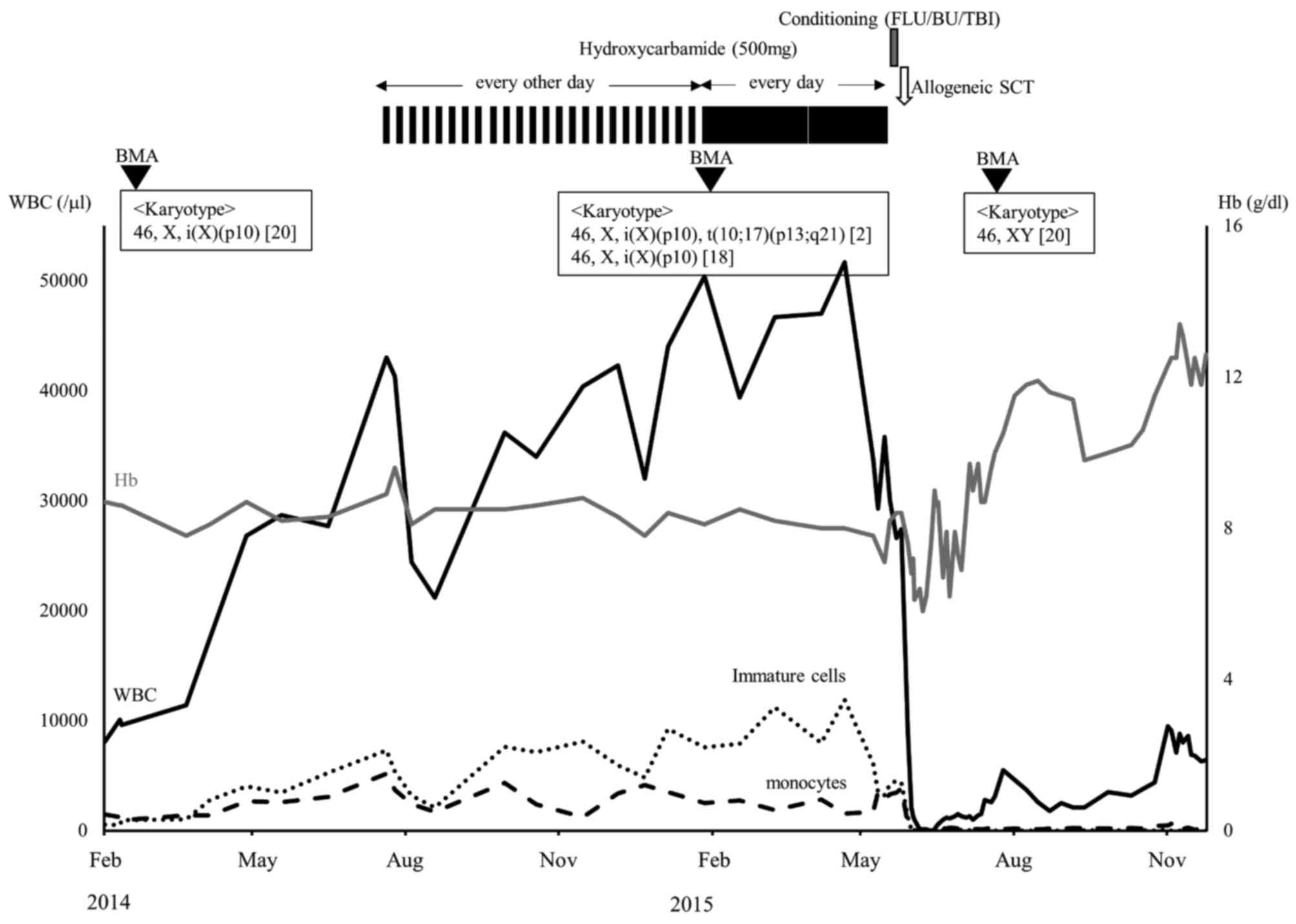 | Figure 3.Clinical course of the patient. Solid
black line, white blood cells; solid gray line, hemoglobin; dotted
line, immature cells, promyelocytes, myelocytes and metamyelocytes;
dashed line, monocytes; FLU, fludarabine; BU, busulfan; TBI, total
body irradiation; SCT, stem cell transplantation; BMA, bone marrow
aspiration. |
Discussion
Although recurrent chromosomal and genetic
abnormalities are frequently observed, none are specific to aCML
(7). Conversely, a number of genetic
mutations have been reported with diverse frequencies: JAK2V617F at
4–8%; CSF3R at <10%; SETBP1 at 25% and ETNK1 at 8.8%. According
to previous studies, mutations in SETBP1 or ETNK1 are strongly
associated with aCML (3,4,7,8). In the present study, these mutations
were not detected by the direct sequencing methods performed in
previous studies. Therefore, the present case did not demonstrate
the chromosomal abnormalities or genetic mutations previously
reported in aCML (3,4,8).
X chromosome abnormalities occur in ~1% of patients
with hematological disorders (6). At
present, 26 cases with well-characterized i(X)(p10) have been
reported, as demonstrated in Table I
(6,9–12). All
except one of the patients were female. Although the majority of
patients exhibited myeloid malignancies, the present study is the
first case of a patient with aCML exhibiting i(X)(p10) to be
reported to date. It is notable that i(X)(p10) has been
demonstrated to be the sole chromosomal abnormality or the
abnormality in a stem line in ~50% of previously published studies
(6), which suggests that it may serve
an initial or primary role in leukemogenesis. However, detailed
clinical courses of patients with i(X)(p10) have not been
investigated to date. The patient of the present study exhibited
i(X)(p10) as the sole chromosomal abnormality at the point of
diagnosis of aCML, and acquired the additional t(10;17)(p13;q21)
abnormality during the subsequent progression of the disease. This
clinical course is compatible with the hypothesis that i(X)(p10)
may serve a primary role in leukemogenesis. Furthermore,
t(10;17)(p13;q21), which is rare recurrent chromosome abnormality
in myeloid leukemia (13), is
potentially associated with disease progressions such as the
additional cytogenetic abnormalities observed in BCR/ABL-1 positive
CML.
 | Table I.Previously published cases of
hematological malignancies with i(X)(p10). |
Table I.
Previously published cases of
hematological malignancies with i(X)(p10).
| Patient | Age | Gender | Disease | Karyotype | (Refs.) |
|---|
| 1 | 74 | F | AML |
47,X,i(X)(p10),+i(X)(p10)/48,idem,+8/48,idem,+20 | (18) |
| 2 | 79 | F | AML |
47,X,i(X)(p10),+i(X)(p10) | (19) |
| 3 | 76 | F | MDS | 46,X,i(X)(p10) | (20) |
| 4 | 32 | F | CML |
47,XX,t(9;22)(q34;q11),+22,47,X,i(X)(p10),
t(9;22),+22,48,X,i(X)(p10),+i(X)(p10),t(9;22),+22 | (21) |
| 5 | 26 | F | HL |
81-85,XX,-X,i(X)(p10),del(1)(p21),+i(2)(p10)x2, del(3)(q21),del(4)(q?25),i(4)(p10),i(4)(q10),+5,-6,-7,del(7)(q32), i(7)(q10),del(9)(q21q31),der(12)t (3;12)(q21;q22),
−13,-13,-15,+16,del(17)(p11),-18,-18,-20,add(20)(q13), −22,-22,-22,i(22)(q10),+mar | (22) |
| 6 | 75 | F | CMML |
46,X,i(X)(p10)/46,idem,del(20)(q11q13) | (23) |
| 7 | 65 | F | ALL |
47,X,i(X)(p10),add(2)(p?),add(14)(q?),-19,+22,+r/47, idem,del(6)(q?),add(16)
(q24) | (24) |
| 8 | 33 | M | ALL |
46,X,+i(X)(p10),-Y/46,idem,del(17)(p12p13)/46,idem, del(7)(q32q36),del(17) | (25) |
| 9 | 18 | F | HL |
59-83,XXX,-X,i(X)(p10),-1,+2,add(2)(q37)x3,+3,-6, del(7)(q12q22),-8,del(8) (q24),-9,-10,-11,-11,del(11)(q12q13),
+12,-13,-13,-14,-15,-16,-17,-17,-18, add(20)(q13), +del(20)(q11q13),-21,+4mar | (26) |
| 10 | 50 | F | CML |
46,X,i(X)(p10),t(9;22)(q34;q11),i(17) (q10)/50,idem,+1,+8,+13,+19 | (27) |
| 11 | 3 | F | ALL |
48,XX,+i(X)(p10),+21c | (28) |
| 12 | ? | F | CMML | 46,X,i(X)(p10) | (29) |
| 13 | 74 | F | AML | 46, X, i(X)(p10)
[5]/46, XX [7] | (30) |
| 14 | 62 | F | MDS | 46,X,i(X)(p10) | (6) |
| 15 | 62 | F | AML | 46,X,i(X)(p10) |
|
| 16 | 17 | F | ALL | 45,X,-X,r(20)/46,X,i(X)(p10),r(20) |
|
| 17 | 73 | F | MDS |
46,X,i(X)(p10),del(5)(q13q33) |
|
| 18 | 32 | F | AML |
47-50,XX,+i(X)(p10)x2,+8,+9 |
|
| 19 | 49 | F | MDS | 46,X,i(X)(p10) |
|
| 20 | 76 | F | MDS | 46,X,i(X)(p10) or
del(X)(q24)?c |
|
| 21 | 80 | F | MDS | 46,X,i(X)(p10) |
|
| 22 | 38 | F | MDS | 46,X,i(X)(p10) |
|
| 23 | 10 | F | ALL | 52, XX, i(X)(p10),
+4, −7, ins(7;?)(q22;?), t(10;21)(q22;q22), +14, +der(15)t(9;15)(q12;p11.2), +21, +21, mar | (9) |
| 24 | 68 | F | t-AML | 46, XX,
del(20)(q11) [5]/45, X, i(X)(p10),
−7, del(20)(q11) [20] | (10) |
| 25 | ? | F | AML | 46, XX,
inv(3)(q21;q26) [1]/45, idem, −7
[11]/45, idem, i(X)(p10), −1 [8] | (11) |
| 26 | ? | F | CMML | 46, X,
i(X)(p10) | (12) |
| 27 | 40 | F | aCML | 46, XX,
i(X)(p10) | Present case |
Similar characteristics have been observed for
patients exhibiting i(X)(p10) and idic(X)(q13), which is the most
common X chromosome-related abnormality with ~30 cases reported
(14). This abnormality occurs in
females of advanced age (range, 55–87 years) with myeloid
malignancies, including aCML, and is often observed as the sole
abnormality (15). There are also
similar structural abnormalities of the X chromosome in i(X)(p10)
and idic(X)(q13) mutations, with the break points at the centromere
and Xq13, respectively (6). Based on
the clinical and cytogenetic similarities, it is hypothesized that
the mutations may share a common mechanism for leukemogenesis. In
this regard, previous studies have revealed that the gene dosage
effect due to the simultaneous gain of Xp and loss of Xq may serve
a crucial role for idic(X)(q13), which does not result in formation
of a fusion gene (6,14). Notably, the loss of Xq by idic(X)(q13)
and i(X)(p10) results in the deletion of the X-inactive specific
transcript (XIST) gene located at Xq13.2. XIST transcribes the long
non-coding RNA XIST. The transcribed long non-coding RNA spreads
along the X chromosome and serves an important role in the
initiation of X inactivation in female cells. Additionally, there
is considerable evidence that XIST RNA serves other important
functions in the differentiation, proliferation and genome
maintenance of human cells. Furthermore, loss of XIST RNA
expression has been found in female breast, ovarian and cervical
cancer cell lines, thus implicating the dysregulation of XIST in
oncogenesis (16). Notably, a
previous study demonstrated that the deletion of XIST in the
hematopoietic cells in mice results in the development of MDS/MPN
with 100% penetrance (17). Thus, a
loss of XIST may serve a crucial role in the leukemogenesis of
idic(X)(q13) or i(X)(p10) (6,14). Additional genetic and molecular
analyses of i(X)(p10) and idic(X)(q13) in patients with MDS/MPN are
required to establish the association of a loss of XIST with
MDS/MPN in humans.
References
|
1
|
Vardiman JW, Bennett JM, Bain BJ, Brunning
RD and Thiele J: Atypical chronic myeloid leukaemia, BCR-ABL1
negativeSwerdlow SH, Campo E, Lee Harris N, et al: WHO
Classification of Tumors of Haematopoietic and Lymphoid Tissues.
Lyon, France: IARC Press; pp. 80–81. 2008
|
|
2
|
Wang SA, Hasserjian RP, Fox PS, Rogers HJ,
Geyer JT, Chabot-Richards D, Weinzierl E, Hatem J, Jaso J,
Kanagal-Shamanna R, et al: Atypical chronic myeloid leukemia is
clinically distinct from unclassifiable
myelodysplastic/myeloproliferative neoplasms. Blood. 123:2645–2651.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piazza R, Valletta S, Winkelmann N,
Redaelli S, Spinelli R, Pirola A, Antolini L, Mologni L, Donadoni
C, Papaemmanuil E, et al: Recurrent SETBP1 mutations in atypical
chronic myeloid leukemia. Nat Genet. 45:18–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gambacorti-Passerini CB, Donadoni C,
Parmiani A, Pirola A, Redaelli S, Signore G, Piazza V, Malcovati L,
Fontana D, Spinelli R, et al: Recurrent ETNK1 mutations in atypical
chronic myeloid leukemia. Blood. 125:499–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gotlib J, Maxson JE, George TI and Tyner
JW: The new genetics of chronic neutrophilic leukemia and atypical
CML: Implications for diagnosis and treatment. Blood.
122:1707–1711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adeyinka A, Smoley S, Fink S, Sanchez J,
Van Dyke DL and Dewald G: Isochromosome (X)(p10) in hematologic
disorders: FISH study of 14 new cases show three types of
centromere signal patterns. Cancer Genet Cytogenet. 179:25–30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoi K and Cross NC: Molecular pathogenesis
of atypical CML, CMML and MDS/MPN-unclassifiable. Int J Hematol.
101:229–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maxson JE, Gotlib J, Pollyea DA,
Fleischman AG, Agarwal A, Eide CA, Bottomly D, Wilmot B, McWeeney
SK, Tognon CE, et al: Oncogenic CSF3R mutations in chronic
neutrophilic leukemia and atypical CML. N Engl J Med.
368:1781–1790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharathkumar A, DeCamillo D, Bhambhani K,
Cushing B, Thomas R, Mohamed AN, Ravindranath Y and Taub JW:
Children with hyperdiploid but not triple trisomy (+4,+10,+17)
acute lymphoblastic leukemia have an increased incidence of
extramedullary relapse on current therapies: A single institution
experience. Am J Hematol. 83:34–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Preiss BS, Bergmann OJ, Friis LS, Sørensen
AG, Frederiksen M, Gadeberg OV, Mourits-Andersen T, Oestergaard B
and Kerndrup GB: AML Study Group of Southern Denmark: Cytogenetic
findings in adult secondary acute myeloid leukemia (AML): Frequency
of favorable and adverse chromosomal aberrations do not differ from
adult de novo AML. Cancer Genet Cytogenet. 202:108–122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lugthart S, Gröschel S, Beverloo HB,
Kayser S, Valk PJ, van Zelderen-Bhola SL, Jan Ossenkoppele G,
Vellenga E, van den Berg-de Ruiter E, Schanz U, et al: Clinical,
molecular, and prognostic significance of WHO type inv
(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities
in acute myeloid leukemia. J Clin Oncol. 28:3890–3898. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen B, Ma Y, Xu X, Wang X, Qin W, Ji M
and Lin G: Analyses on clinical characteristic and prognoses of 41
patients with chronic myelomonocytic leukemia in China. Leuk Res.
34:458–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh SH, Park TS, Cho SY, Kim MJ, Huh J, Kim
B, Song SA, Lee JY, Jun KR, Shin JH, et al: Acute myeloid leukemia
associated with t(10;17)(p13-15;q12-21) and phagocytic activity by
leukemic blasts: A clinical study and review of the literature.
Cancer Genet Cytogenet. 202:43–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paulsson K, Haferlach C, Fonatsch C,
Hagemeijer A, Andersen MK, Slovak ML and Johansson B: MDS
Foundation: The idic(X)(q13) in myeloid malignancies: Breakpoint
clustering in segmental duplications and association with TET2
mutations. Hum Mol Genet. 19:1507–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oscier DG: Atypical chronic myeloid
leukaemia, a distinct clinical entity related to the
myelodysplastic syndrome? Br J Haematol. 92:582–586. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weakley SM, Wang H, Yao Q and Chen C:
Expression and function of a large non-coding RNA gene XIST in
human cancer. World J Surg. 35:1751–1756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yildirim E, Kirby JE, Brown DE, Mercier
FE, Sadreyev RI, Scadden DT and Lee JT: Xist RNA is a potent
suppressor of hematologic cancer in mice. Cell. 152:727–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagemeijer A, Hählen K and Abels J:
Cytogenetic follow-up of patients with nonlymphocytic leukemia. II.
Acute nonlymphocytic leukemia. Cancer Genet Cytogenet. 3:109–124.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fitgerald PH, Morris CM, Fraser GJ, Giles
LM, Hamer JW, Heaton DC and Beard ME: Nonrandom cytogenetic changes
in New Zealand patients with acute myeloid leukemia. Cancer Genet
Cytogenet. 8:51–66. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knuutila S, Teerenhovi L and Borgström GH:
Chromosome instability is associated with hypodiploid clones in
myelodysplastic syndromes. Hereditas. 101:19–30. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selleri L, Emilia G, Temperani P,
Grassilli E, Zucchini P, Tagliafico E, Bonati A, Venezia L, Ferrari
S, Torelli U, et al: Philadelphia-positive chronic myelogenous
leukemia with typical bcr/abl molecular features and atypical,
prolonged survival. Leukemia. 3:538–542. 1989.PubMed/NCBI
|
|
22
|
Schlegelberger B, Weber-Matthiesen K,
Himmler A, Bartels H, Sonnen R, Kuse R, Feller AC and Grote W:
Cytogenetic findings and results of combined immunophenotyping and
karyotyping in Hodgkin's disease. Leukemia. 8:72–80.
1994.PubMed/NCBI
|
|
23
|
Nacheva E, Holloway T, Carter N, Grace C,
White N and Green AR: Characterization of 20q deletions in patients
with myeloproliferative disorders or myelodysplastic syndromes.
Cancer Genet Cytogenet. 80:87–94. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Temperani P, Giacobbi F, Gandini G,
Torelli U and Emilia G: Chromosome rearrangements at telomeric
level in hematologic disorders. Cancer Genet Cytogenet. 83:121–126.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martineau M, Clark R, Farrell DM, Hawkins
JM, Moorman AV and Secker-Walker LM: Isochromosomes in acute
lymphoblastic leukaemia: i(21q) is a significant finding. Genes
Chromosomes Cancer. 17:21–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Falzetti D, Crescenzi B, Matteuci C,
Falini B, Martelli MF, Van Den Berghe H and Mecucci C: Genomic
instability and recurrent breakpoints are main cytogenetic findings
in Hodgkin's disease. Haematologica. 84:298–305. 1999.PubMed/NCBI
|
|
27
|
Barbouti A, Johansson B, Höglund M,
Mauritzson N, Strömbeck B, Nilsson PG, Tanke HJ, Hagemeijer A,
Mitelman F and Fioretos T: Multicolor COBRA-FISH analysis of
chronic myeloid leukemia reveals novel cryptic balanced
translocations during disease progression. Genes Chromosomes
Cancer. 35:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baker JM, Coppes MJ and Roland B: A case
of Down syndrome with acute lymphoblastic leukemia and
isochromosome Xp. Cancer Genet Cytogenet. 147:75–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MacGrogan D, Kalakonda N, Alvarez S,
Scandura JM, Boccuni P, Johansson B and Nimer SD: Structural
integrity and expression of the L3MBTL gene in normal and malignant
hematopoietic cells. Genes Chromosomes Cancer. 41:203–213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao L, Wang X, Ryder J, Ji M, Chen Y, Chen
H, Sun H, Yang Y, Du X, Kerzic P, et al: Prospective study of 174
de novo acute myelogenous leukemias according to the WHO
classification: Subtypes, cytogenetic features and FLT3 mutations.
Eur J Haematol. 77:35–45. 2006. View Article : Google Scholar : PubMed/NCBI
|