Introduction
Ovarian cancer (OC) is the fifth leading cause of
cancer death for women in the United States (1). Nearly two-thirds of OC patients will
eventually suffered from malignant ascites (MA), which would
negatively affect their quality of life and survival due to
ascites-associated symptoms (AAS) (2). These symptoms include abdominal
distention, abdominal pain, nausea, anorexia, vomiting, fatigue,
dyspnea and weariness. Although there are several therapeutic
strategies for MA, it is still prone to relapse. Once refractory
ascites occurred, it is difficult to treat patients with OC
(3). Therefore, it is urgent to find
a novel and effective therapy for refractory MA in OC.
Adoptive cell transfer (ACT) is an emerging
technique used to treat malignant carcinoma. During this process, T
cells are firstly collected from one patient, expanded
exponentially in vitro, and then re-infused to the patient
to help the immune system targeting tumor cells. A seminal study by
Rosenberg et al (4)
demonstrated that tumor-infiltrating lymphocytes (TILs) extracted
from freshly resected melanomas can be expanded in vitro and
mediate specific lysis of autologous tumor cells. In their study,
adoptive transfer of TILs led to objective regression of metastatic
melanoma in 11 of 22 patients.
Additional studies showed that lymphodepletion prior
to adoptive transfer of TILs led to 50–72% objective response rates
(4–7).
However, while tumor reactive lymphocytes which are isolated from
ascites (8) or solid tumors (9–12) are an
efficient and less toxic treatment method for OC, the reports on
the intraperitoneal (IP) administration of tumor-associated
lymphocytes (TALs) to treat MA arising from OC are rare. TALs are a
population of antigen-specific cytotoxic cells which are easier
obtained compared with TILs (13).
Furthermore, previous studies (14–16) not
only demonstrated that tumor reactive lymphocytes can be easierly
purified from MA than from solid tissue of OC, but also showed that
TALs which extracted from ascites had more significant NK activity
against K562 cells than that from a series of disaggregated solid
tumors. Therefore, we carefully analyzed the toxicity and efficacy
of TALs combined with or without chemotherapy in MA caused by
OC.
Patients and methods
Patient cohort
Between January 2001 and December 2011, 32 patients
with MA arising from OC were enrolled in this study at Nanfang
Hospital of Southern Medical University (Guangzhou, China). All
patients met the following inclusion criteria: Had measurable MA by
ultrasound and computed tomography (maximal depth, ≥3.0 cm), and
recovered from any toxic effects. Other requirements included: Age
≥18 years, Karnofsky performance status (KPS) score ≥40, life
expectancy of 6 weeks or more, adequate bone marrow function, no
serious damage to liver or kidneys, and no active infection.
Patients that had intestinal dysfunction or uncorrectable
obstruction, significant adhesions preventing free flow of fluid,
prior introperitoneal therapy with recombinant interleukin-2
(rIL-2), prior introperitoneal chemotherapy unless free of
extensive adhesions or significant medical/psychiatric disorders
were excluded. All patients gave informed consent according to the
Ethics Committee of Southern Medical University in China.
TALs preparation
MA from 19 of 32 patients, who received TALs
immunotherapy, were collected under sterile conditions in
centrifuge tubes containing preservative-free heparin (10 U/ml).
Ascitic fluid samples were centrifuged at 600 g for 5 min, cell
pellets containing tumor cells and lymphocytes were resuspended in
Earle's balanced salt solution (EBSS), and passed through a 100 µm
cell strainer to remove desquamation. Single cell suspensions were
washed twice with RPMI 1640 and then cultured in RPMI 1640
supplemented with 10% human AB serum and 1,000 IU ml−1
rIL-2 (Double-aigrettes Pharmacy, Ltd., China). After overnight
incubation, non-adhesive lymphocytes were passed through a 70 µm
cell strainer to remove large aggregates of erythrocytes, tumor
cells, macrophages, fibrocytes, and cell debris. If the total
lymphocyte number exceeded 1×107 cells, the remaining
cell suspensions which resuspended in EBSS were layered onto a 100%
Ficoll-Hypaque density cushion and centrifuged at 800 g for 30 min.
Then, the lymphocytes cells were removed from the mid layer of
Ficoll-Hypaque and EBSS. After washed twice with EBSS, the
lymphocytes cells were activated and amplified by RPMI 1640 with
10% human AB serum and 1,000 IU ml−1 rIL-2. As
previously described, the TALs can be easily distinguished from
tumor cells by using morphology and size, if there were few tumor
cells or macrophages adhered to the flask, we can collect the
suspension TALs by gentle pipetting while leaving tumor cells and
macrophages adherent (5,6). The procedure was repeated two to three
times until TALs purity reached 100%. The complete medium and rIL-2
in media were supplemented according to the growth rate of TALs.
When the total number of TALs exceeded 1×109 after in 1-
to 2-week culture, the phenotype of TALs were determined by flow
cytometry. Then the TALs were administered to the peritoneal cavity
with 250 ml sterile saline. If the total number was less than
1×107, ficoll separation was postponed and the cells
continued to be cultured with rIL-2.
FACS analysis
Flow cytometry was performed on cultured TALs
immediately before the patient received their first IP injection.
Briefly, single-cell TALs suspensions (1×105 cells) were
labeled with CD8-PE/CD4-FITC/CD3-PE-Cy5 and their homeotype
negative controls (BD Biosciences, San Jose, CA, USA),
respectively.
Treatment design
Eight patients with MA caused by OC received IP
injections of TALs in the TALs group, 13 patients received
intravenous (IV) chemotherapy alone in the chemotherapy group, and
11 patients were treated with combined IP TALs and IV chemotherapy
in the combination group. In chemotherapy group, 4 patients had a
previously untreated OC and 9 patients had recurrent OC after
surgery with or without chemotherapy. In the combination group, 5
patients had previously untreated OC and the remaining 6 patients
had recurrent OC after chemotherapy. No patients were resistant to
platinum. IV chemotherapy prior to IP TALs was administered as
follows on day 1: Liposome-paclitaxel (135 mg/m2) plus
carboplatin (AUC 5) for at least two cycles. After recovery from
toxicity due to the chemotherapy at least 5 days, the 19 patients
in TALs and combination group were given IP injections of TALs.
Response assessment
Response assessments were done according to World
Health Organization (WHO) and performed 3 weeks after 2 cycles of
therapy or sooner in the event that there was evidence of clinical
deterioration. Patients were considered to be in complete remission
(CR) if the ascites disappeared or receded (100 ml; maximal depth,
<2 cm) for 4 weeks. Partial remission (PR) involved a ≥50%
decrease in ascites volume lasting for 4 weeks. Stable disease (SD)
required a ≤25% increase or ≤50% decrease in ascites volume.
Patients were considered to have progressive disease (PD) if the
ascites volume increased by ≥25%. Combined CR and PR plus SD was
defined as the MA controlled rate. The tumor response was assessed
according to the criteria set forth by WHO (17). CR plus PR was defined as an objective
response rate.
Follow-up evaluation
Patients were followed every month for 6 months,
then every 2 months until December 2013. Complete medical
histories, KPS scores (100-point), AAS (anorexia, insomnia,
dyspnea, nausea, vomiting, abdominal pain, abdominal distention,
fatigue, weariness), routine blood examinations, CA125 and albumin
levels were conducted at enrollment (pre-treatment) and 3 weeks
after the first cycle of therapy (post-treatment). A 5-point scale
determined the severity of AAS (0, not at all; 1, very little; 2,
somewhat; 3, moderately; 4, very much). Abdominal ultrasonography
was repeated prior to each cycle of therapy. Survival data were
obtained from the day of administration of TALs or chemotherapy
until the death of patients or last contact when the patient is
still alive. Time to progression (TTP) was defined as the time from
treatment to PD or death. Overall survival (OS) was defined as the
time from treatment to death.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). The Pearson Chi-square and one-way
ANOVA compared the clinical parameters between patient cohorts.
Scores and serum markers before and after treatment were compared
by a paired t-test. Survival rates between the combination group
and chemotherapy or TALs group were compared using the Kaplan-Meier
method and log-rank test P<0.05 was considered statistically
significant and data were presented as the mean ± SD.
Results
The clinical characteristics of all 32 patients are
illustrated in Table I. All patients
had pathological staging according to the International Federation
of Gynecologists and Obstetricians (FIGO). There were no
statistical significances in clinical stage, histological subtype,
histological grade or cytology of ascites between or within the
three groups (P>0.05). The mean KPS score of 32 patients was
60.9±12.3 at enrollment, which was improved significantly to
73.4±15.6 after the first cycle of therapy (P<0.001). The mean
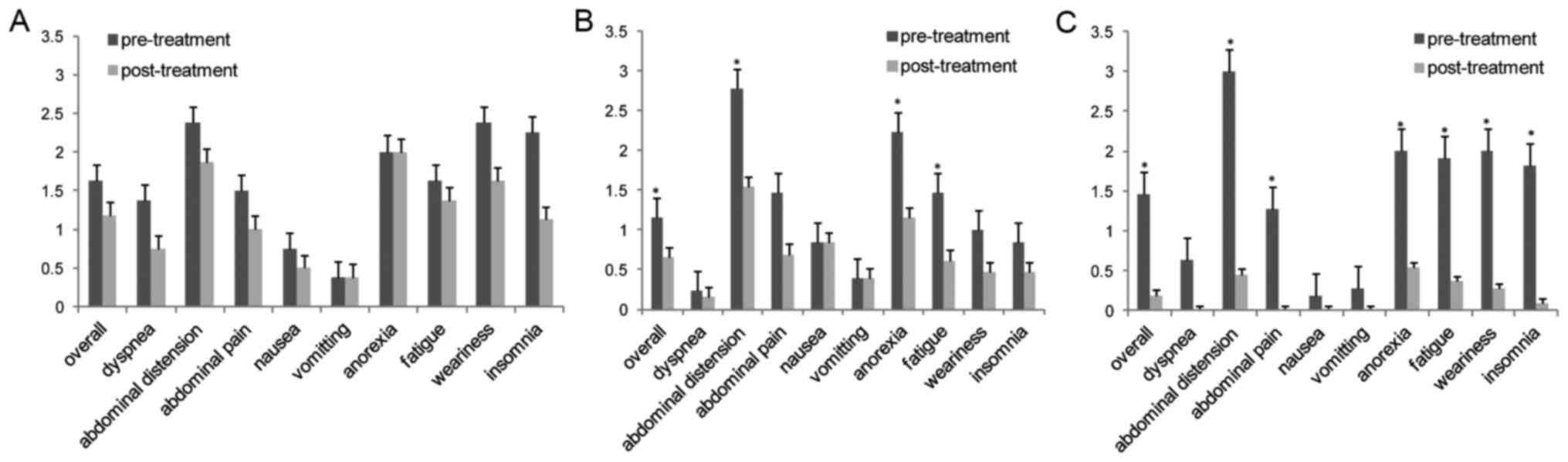
score of AAS significantly decreased from 1.4±0.6 to 0.6±0.7
(P<0.001). Serum CA125 and albumin levels significantly changed
from 797.2±998 to 407.2±631 (P=0.005) and 33.5±4.2 to 35.9±6.3
(P=0.04), respectively.
 | Table I.Clinical and treatment
characteristics of patients. |
Table I.
Clinical and treatment
characteristics of patients.
|
|
|
|
|
| Maximun depth of
ascites (cm) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Patient | Age (years) | Histology | Stage/grade | Ascites
cytology | Pre- | Post- | Previous
chemotherapy |
|---|
| A1 | 38 | Serous | III/G2 | − | 9 | 10.5 | Surgery,
TCa, PC, GP, endostar,
radiotherapy |
| A2 | 56 | Serous | IV/G2 | + | 12 | <2 | Surgery, DC,
CC |
| A3 | 56 | Serous | IV/G2 | + | 14.8 | 12.5 | Surgery, PC,
EP |
| A4 | 45 | Serous | IV/G2 | + | 8 | 11.3 | Surgery,
TCc |
| A5 | 69 | Serous | IV/G1 | + | 10 | 13 | Surgery,
TCc |
| A6 | 74 | Transitional
cell | III/G3 | + | 14 | <2 | Surgery, CC |
| A7 | 35 | Mucus | IIb/G1 | + | 6.5 | 7.1 | Surgery, PC |
| A8 | 55 | Serous | III/G3 | − | 9.5 | 2.8 | Surgery,
TCa, PC |
| B1 | 51 | Endometroid | III/G2 | + | 15 | <2 | Surgery,
TCa |
| B2 | 65 | Serous | IV/G2 | + | 12.5 | <2 | None |
| B3 | 60 | Serous | III/G3 | + | 15 | <2 | None |
| B4 | 42 | Serous | IIIc/G2 | + | 11.3 | 4.3 | Surgery,
TCb, CD |
| B5 | 44 | Adenocarcinoma | III/G3 | + | 6 | 3 | Surgery, DC,
VIP |
| B6 | 71 | Carcinosarcoma | IIIc/G3 | + | 14 | 2.1 | Surgery, CP |
| B7 | 54 | Adenocarcinoma | IV/G3 | + | 13 | 0 | None |
| B8 | 50 | Serous | IV/G3 | − | 12 | 7 | None |
| B9 | 47 | Serous | III/G2 | + | 10 | 5 | PC |
| B10 | 69 | Serous | IIIc/G3 | + | 11.3 | 3.2 | Xeloda |
| B11 | 76 | Serous | IIIc/G1 | + | 16 | 0 | None |
| C1 | 47 | Adenocarcinoma | IV/G3 | + | 8.2 | 0 | None |
| C2 | 54 | Adenocarcinoma | IV/G3 | + | 10 | 7 | None |
| C3 | 57 | Serous | III/G1 | + | 13 | <2 | Surgery, PC |
| C4 | 61 | Serous | IIIc/G2 | + | 11 | <2 | Surgery, PC |
| C5 | 49 | Serous | IV/G3 | + | 11 | 14.4 | Surgery |
| C6 | 73 | Serous | IIIc/G2 | + | 8.8 | 12.4 | None |
| C7 | 50 | Adenocarcinoma | IV/G3 | + | 10.5 | 8 | Surgery,
TCa, GP |
| C8 | 56 | Serous | IIIc/G2 | + | 10.1 | 10 | Surgery, DC |
| C9 | 43 | Serous | IIIc/G1 | + | 13.4 | 7 | Surgery, CC |
| C10 | 57 | Adenocarcinoma | IIIc/G3 | − | 10.7 | 14 | Surgery, PC |
| C11 | 63 | Serous | IV/G2 | − | 6.4 | 0 | Surgery,
TCc |
| C12 | 49 | Adenocarcinoma | IV/G2 | + | 6.6 | 0 | None |
| C13 | 52 | Serous | IIIc/G2 | + | 6 | <2 | Surgery, PC |
The TALs were successfully separated from ascites in
patients who were received TALs therapy. With the time extension,
the cellular morphology of TALs changed from initial round to
round, branching and rods. The TALs will form sample colony growth
when it at exuberant growth period (Fig.
1B). Before the first TALs therapy, the CD3+,
CD4+/CD3+ and CD8+/CD3+
T lymphocyte populations of TALs were also detected by flow
cytometry (Fig. 1A).
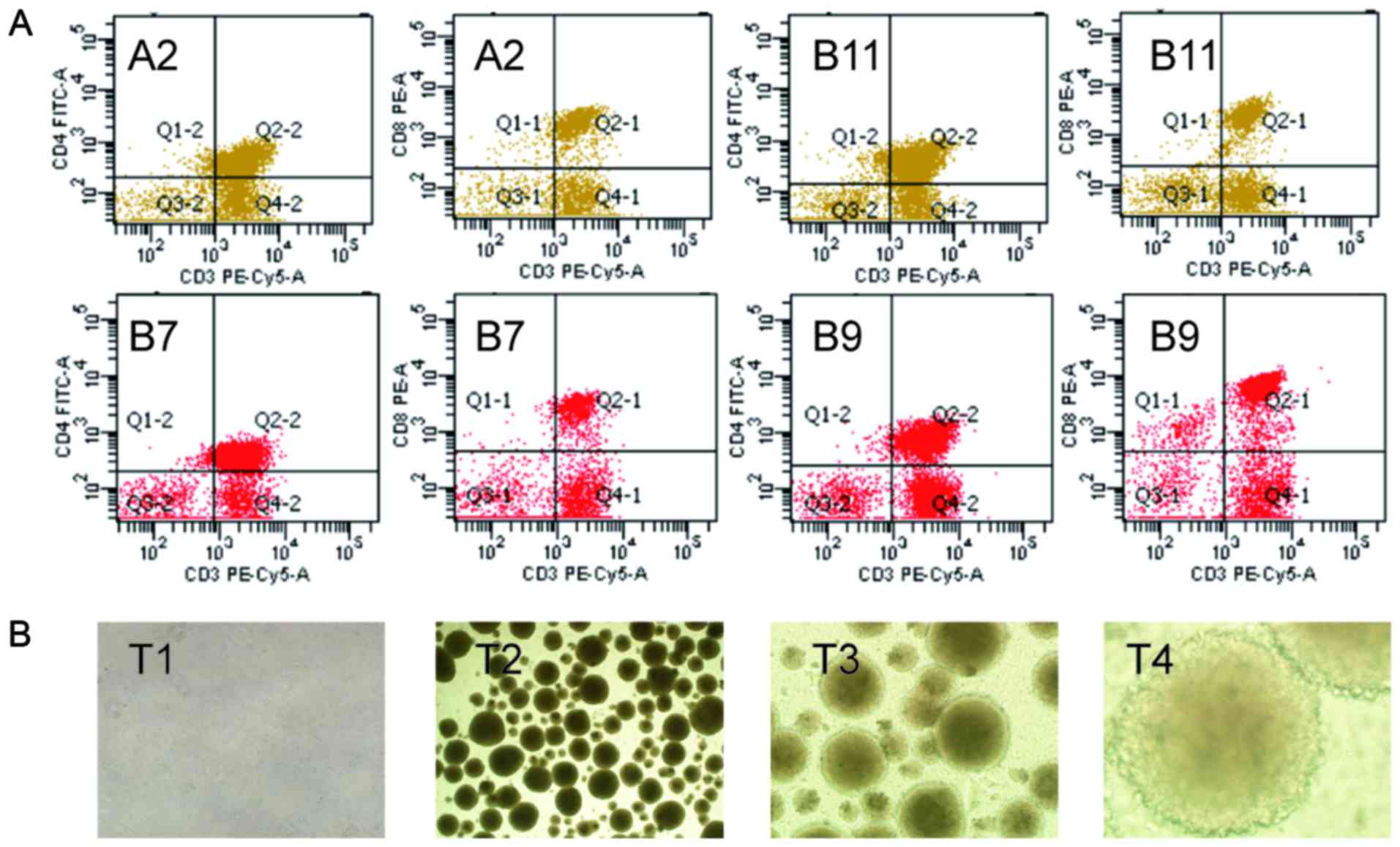 | Figure 1.The immune phenotype and
morphological observation of TALs. (A) The CD3+,
CD4+/CD3+ and CD8+/CD3+
T lymphocyte populations of TALs were detected by flow cytometry in
A2, B7, B9 and B11 patients when they received TALs therapy. (B)
The morphological observation of TALs in patient A2. TALs,
tumor-associated lymphocytes; T1, the TALs when first separation,
×100; T2, the TALs after 10 days' cultivation, ×100; T3, the TALs
after 10 days' cultivation, ×200; T4, the TALs after 10 days'
cultivation, ×400. |
In the TALs group, the mean ages of TALs ranged from
7 to 15 days (10.25±2.81), and the treatment doses of IP TALs
ranged from 9×109 to 3×1010 cells (2.4±1.1
×1010). CD3+, CD4+/CD3+
and CD8+/CD3+ T lymphocyte populations were
89.2±5.5, 37.9±11.5 and 45.5±13.4, respectively (Table II). However, there was no difference
between the populations of CD4+/CD3+ and
CD8+/CD3+ T lymphocytes. There were no
significant changes in KPS and AAS scores or serum CA125 and
albumin levels before or after treatment (Fig. 2A and Table
III). According to the response criteria subscribed above, the
MA controlled rate (CR+PR+SD) was 62.5% (5/8 patients), with a CR
of 25% (2/8), a PR of 12.5% (1/8) and a SD of 25% (2/8). PD was
observed in 3 patients (37.5%) (Table
IV).
 | Table II.Characteristics of TALs and survival
of patients in TALs group and combination group. |
Table II.
Characteristics of TALs and survival
of patients in TALs group and combination group.
|
|
|
|
|
|
| % of positive
cells |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient | TALsa age (days) | TALs dose
(×109) | No. of courses | Respb | Respc | CD3 | CD4 | CD8 | TTP (months) | OS (months) |
|---|
| A1 | 9 | 3, 4, 2, 2 | 4 | PD | PD | 95.5 | 40.2 | 47.8 | − | 6 |
| A2 | 9 | 7, 11, 14, 5,
2 | 5 | CR | PR | 80.6 | 41.2 | 22.9 | 5 | 5 |
| A3 | 8 | 10, 7 | 2 | SD | PD | 90.0 | 40.5 | 60.5 | 2 | 12 |
| A4 | 7 | 11, 9, 10, 8 | 4 | PD | PD | 87.4 | 21.3 | 40.2 | − | 8 |
| A5 | 10 | 12, 10, 7 | 3 | PD | PD | 84.5 | 44.8 | 37.1 | − | 4 |
| A6 | 14 | 13, 10, 6 | 3 | CR | PR | 86.4 | 40.4 | 39.8 | 2 | 7 |
| A7 | 10 | 9, 13 | 2 | SD | SD | 93.3 | 54.2 | 51.1 | 1 | 13 |
| A8 | 15 | 6, 3 | 2 | PR | SD | 96.0 | 20.4 | 64.3 | 3 | 9 |
| B1 | 11 | 12, 10, 7 | 3 | CR | PR | 90.6 | 40.2 | 66.8 | 17 | 23 |
| B2 | 11 | 9, 8, 11 | 3 | CR | PR | 87.0 | 18.9 | 54.6 | 10 | 18 |
| B3 | 18 | 14, 14, 10, 8,
8 | 5 | CR | PR | 79.6 | 26.3 | 50.5 | 17 | 68 |
| B4 | 8 | 8, 12, 11, 11 | 4 | PR | SD | 80.4 | 29.3 | 47.5 | 14 | 25 |
| B5 | 9 | 14, 11, 10 | 3 | PR | PD | 87.4 | 29.9 | 50.0 | 6 | 16 |
| B6 | 10 | 7, 7 | 2 | CR | CR | 82.3 | 37 | 56.5 | 15 | 36 |
| B7 | 16 | 13, 12, 12 | 3 | CR | PR | 75.7 | 49.2 | 18.7 | 12 | 25 |
| B8 | 8 | 9, 7, 8, 3 | 4 | SD | SD | 94.7 | 37.2 | 49.3 | 8 | 22 |
| B9 | 10 | 1, 1, 1 | 3 | PR | CR | 87.5 | 41.4 | 41.2 | 13 | 32 |
| B10 | 7 | 1, 1, 1, 1 | 4 | PR | SD | 84.3 | 14.8 | 44.6 | 9 | 13 |
| B11 | 7 | 1, 1, 1, 1 | 4 | CR | PR | 78.7 | 52.1 | 21.0 | 32 | 43 |
 | Table III.Treatment effects on patients between
the three groups. |
Table III.
Treatment effects on patients between
the three groups.
|
| KPS score (mean ±
SD) | CA125 (U/ml) (mean
± SD) | ALB (g/l) (mean ±
SD) |
|---|
|
|
|
|
|
|---|
|
| Pre- | Post- | P-value | Pre- | Post- | P-value | Pre- | Post- | P-value |
|---|
| Combination
group | 60.9 (9.4) | 81.8 (7.5) | <0.001 | 477.2 (370.5) | 250.2 (279) | 0.061 | 31.2 (4.0) | 36±5.3 | 0.033 |
| Chemotherapy
group | 63.1 (14.4) | 74.6 (16.1) | 0.012 | 1,294.9
(1,399.7) | 559.3 (917.8) | 0.021 | 35.1 (3.6) | 37.7±7.7 | 0.175 |
| TALs group | 57.5 (12.8) | 60.0 (15.1) | 0.626 | 428.3 (282.6) | 375.9 (354.1) | 0.393 | 34.2 (4.4) | 32.7±3.9 | 0.279 |
 | Table IV.Comparison of patient outcomes
between the three groups. |
Table IV.
Comparison of patient outcomes
between the three groups.
|
| TTP (months) | OS (months) | Acites
response | Tumor response |
|---|
|
|
|
|
|
|
|---|
|
| Median (95%
CI) | P-value | Median (95%
CI) | P-value | CR+PR (%) | P-value | CR+PR (%) | P-value |
|---|
| Combination
group | 13 (8.7–17.3) | NA | 25 (21.9–28.1) | NA | 10 (90.9) | NA | 7 (63.6) | NA |
| TALs group | 1 (0–2.8) | <0.001 | 7 (4.2–9.8) | <0.001 | 3
(37.5) | 0.009 | 2 (25.0) | 0.002 |
| Chemotherapy
group | 6 (2.5–9.5) | 0.027 | 18 (13.3–22.7) | 0.135 | 7
(53.8) | 0.047 | 4 (30.8) | 0.107 |
In the chemotherapy group, only symptoms of
abdominal distension, anorexia, and fatigue significantly improved
after carboplatin-based chemotherapy (all P<0.05) (Fig. 2B). As shown in Table IV, KPS scores and serum CA125
significantly changed after treatment from 63.1±14.4 to 74.6±16.1
(P=0.012) and 1294.9±1399.7 to 559.3±917.8 (P=0.021), respectively.
Changes in serum albumin levels revealed no significant
improvement. The MA controlled rate was 76.9%, with a CR of 46.2%
(6/13), a PR of 7.6% (1/13), and a SD of 23.1% (3/13) (Table IV).
In the combined TALs and chemotherapy group,
treatment doses of IP TALs (7 to 18 days old) ranged from
3×109 to 5.4×1010 cells. The CD3+,
CD4+/CD3+ and CD8+/CD3+
T lymphocyte populations were 84.4±5.7, 34.2±11.7 and 45.5±14.3,
respectively (Table II). All had no
statistical significance between the TALs group and the combined
therapy group. After the combined treatment, the mean score of
overall AAS and six specific AAS (insomnia, weariness, fatigue,
anorexia, abdominal pain, and abdominal distension) significantly
improved (P<0.05) (Fig. 2C).
Although serum CA125 levels decreased from 477.20±370.46 to
250.23±278.97 U/ml, there was no statistical significance
(P=0.061). However, serum albumin levels significantly improved
from 31.20±3.96 to 36.0±5.30 g/l (P=0.033). The MA controlled rate
was 100%, with a CR of 54.5% (6/11), a PR of 36.4% (4/11), and a SD
of 9.1% (1/11) (Tables III and
IV).
Median TTP was significantly different in the
combination group (13 months) compared to the TALs group (1 months,
P<0.001), and the chemotherapy group (6 months, P=0.027). The
median OS of patients in combination group (25 months) was
significantly longer than the TALs group (7 months, P<0.001),
but not the chemotherapy group (18 months, P=0.135). The objective
response rate of MA was 90.9% in the combination group, which was
higher than the TALs group (37.5%, P=0.009) and chemotherapy group
(53.8%, P=0.047). However, tumor objective response was achieved in
7 of 11 patients (63.6%) in the combination group, 2 of 8 patients
(25%) in the TALs group, and 4 of 13 patients (30.8%) in
chemotherapy group. But the significance difference existed only in
the combination group and TALs group (P=0.002) (Table IV).
There were no cases of treatment-related toxicities
with IP TALs administration. Twelve patients receiving IV
chemotherapy alone or combined with IP TALs experienced grades 1–3
bone marrow suppression as well as grades 1 and 3 vomiting. All
side-effects were managed with routine medical treatments.
Discussion
The aim of this study was to assess the efficacy and
toxicity of TALs in combination with or without chemotherapy in OC
patients with MA. Our results indicated that combining therapy of
TALs and chemotherapy is safe, feasible, and more effective than
chemotherapy or TALs alone in controlling MA and improving quality
of life in OC patients.
TALs is a unique subtype of TILs, and can be served
as a suitable model for the study of TILs (18). Unlike the LAK and TILs, the effector
TALs coexist with tumor target cells in a defined environment
presented by MA. Previous study (19)
showed that the non-specific cytotoxic potential of TALs against
autologous tumor can be increased by incubation with IL-2. Melioli
et al (20) reported that most
TALs isolated from MA secondary to OC consist predominantly of T
cells and almost lack of B and NK cells. A study by Ioannides et
al (21) also demonstrated that
CD3+CD4+ TALs isolated from OC ascites can be
propagated in large numbers and for long time intervals as T-cell
lines in vitro.
Previous clinical studies used adoptive cell therapy
of young ‘unselected’ TILs to treat a variety of cancer, which are
directly isolated from solid tumors without assessing the
percentage of myeloid-derived suppressor cells (MDSCs) or
macrophages (6,7,22–26). Other studies also demonstrated that
the efficacy and tumor response rates of patients were similar in
both ‘selected’ TILs group and ‘unselected’ TILs group (27,28). In
addition, Allavena et al (29)
reported that suppression by mature macrophages dose not play a
major role in the determination of the low reactivity of the TALs
from ascites of OC patient in contrast to peripheral blood
lymphocytes of the same patient. Furthermore, a pilot clinical
trial conducted by Freedman and Platsoucas (12) suggested that both ‘unselected’ TILs
and ‘unselected’ TALs plus rIL-2 could be safely administered
intraperitoneally to patients with OC. Similarly, in our study, we
collected young (7 to 18 days old) ‘unselected’ TALs from ascites
without screening for the presence of MDSC or macrophages. Our
study also confirmed that TALs can be easily expanded to a
therapeutic dose after a short incubation with rIL-2. Nevertheless,
the data about adoptive cell transfer of TALs therapy in OC is very
little. Hence, we hope more clinical trials will be conducted in
the future, focusing on the young ‘unselected’ TALs to treat
various solid tumors.
Data from our study corroborated results from Han
et al (13) and showed that
TALs are easily isolated and rapidly expanded from MA before
chemotherapy. Under high dose rIL-2 conditions, there was no
difference in the percentage of CD4+/CD3+ or
CD8+/CD3+ TALs in this trial. It is possible
that ovarian CD8+ TALs require different growth
conditions from those needed for CD4+ TALs or the
CD8+ TALs are outgrown by faster-growing CD4+
T cells. Nonetheless, the response rates had no significant
difference in this study whether patients received TALs that
contained predominantly CD4+ or CD8+ T cells.
However, the critical point of adoptive transfer of tumor-reactive
lymphocytes was not reasonable in combination with chemotherapy and
other treatment methods. Although chemotherapy had an
immunosuppressive effect on immunotherapy, it can reduce the number
of MDSCs, activate dendritic cells and cytotoxic T cells (30,31)
Previous studies also indicated that immune ablation is an
effective preconditioning regimen that can increase T-cell
responses after adoptive transfer (32–36) and
suggests that chemotherapeutic agents can be used in combination
with adoptive cell therapy. Furthermore, most adoptive TILs
therapies used to treat melanoma patients occurs after
lymphodepleting chemotherapy (5,22,23).
Adoptive cell transfer of TALs followed by
chemotherapy demonstrated higher response rates and longer OS than
use of TALs or chemotherapy alone. We found that a single use of
TALs therapy provided a short duration response that lasted 1 to 5
months and was slightly less effective at controlling MA and
improving quality of life. Compared with a single use of
chemotherapy, the combination TALs therapy and chemotherapy not
only showed higher response rates and longer OS, but also induced
fewer side-effects in OC patients. TALs may play a role in reducing
the toxicities associated with chemotherapy and help rebuild the
immune system.
However, the inadequacy of this study was lack to
detect the percentages of MDSCs or
CD4+CD25+FoxP3+ regulatory T cells
(Tregs) cells in TALs, and further analysis their clinic effect on
OC patients. Whatever, there is little study to support that the
TILs or TALs which had no MDSCs, macrophages and Tregs have more
clinic effect on patients than the ‘unselected’ lymphocytes.
Although MDSCs are a heterogeneous group of immature myeloid cells
that negatively regulate the immune responses during tumor
progression, inflammation and infection, it is still unclear what
subset of MDSC may be responsible for T cell suppression and what
the specific nature of MDSC-suppression is, i.e., antigen dependent
or independent. To the best of our knowledge, the low reactivity of
TALs can be due to suppressive cytokine environment within the
ascites (37). The lower reactivity
of TALs can be also caused by higher DNA damage which occurs in
those lymphocytes more than that in peripheral blood lymphocytes
(18). In addition, mature
macrophages do not play a major role in the low reactivity of the
TALs (29). However, some groups have
identified Treg infiltration to be a biomarker of good clinical
outcome in ovarian carcinoma (38),
highlighting the complexity of Tregs as biomarker. Other studies
also demonstrated that CD4+ T lymphocytes increases
proportionally to the effector T cells in cancer, thus Tregs could
be associated with improved outcome (39,40).
Furthermore, the help given by given CD4+ T lymphocytes
during the priming of CD8+ cytotoxic T lymphocytes
confers a key feature of immunological memory (41). Pace et al also suggested that
Tregs are important regulators of the homeostasis of
CD8+ T cell priming and played a critical role in the
induction of high-avidity responses and effective memory (42). According to the clinical results,
Hinrich and Rosenberg suggest that Treg cells may be important in
TILs therapy but that Treg cells from the reconstituting host
rather than from the infused cell product may suppress antitumor
responses (43). Although MDSCs are a
heterogeneous group of immature myeloid cells that negatively
regulate the immune responses during tumor progression,
inflammation and infection, it is still unclear what subset of
MDSCs may be responsible or the dominant mechanism for T cell
suppression. So, there remains a significant gap in our
understanding of their phenotypical and functional
heterogeneity.
Overall, the data demonstrate that chemotherapy can
be safely administered before TALs therapy and provide impressive
response rates in the treatment of MA. However, more studies are
needed to combine a variety of non-proven modalities in an effort
to find an effective combination to combat OC.
Acknowledgements
We would like to thank the study participants and
hospital staff for their contributions to the present study. This
study was in part supported by Grants from the Guangdong Province
Science and Technological Program in China (grant no.
2011B031800042), and a Project funded by China Postdoctoral Science
Foundation (grant no. 2016M602674).
References
|
1
|
Xie J, Poole EM, Terry KL, Fung TT, Rosner
BA, Willett WC and Tworoger SS: A prospective cohort study of
dietary indices and incidence of epithelial ovarian cancer. J
Ovarian Res. 7:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cella D, Neubauer N, Thomas J, Kutner J
and Seiden MV: The FACIT-AI, a new tool for assessing symptoms
associated with malignant ascites. Gynecol Oncol. 128:187–190.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kipps E, Tan DS and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenberg SA, Packard BS, Aebersold PM,
Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp
CA, et al: Use of tumor-infiltrating lymphocytes and interleukin-2
in the immunotherapy of patients with metastatic melanoma. A
preliminary report. N Engl J Med. 319:1676–1680. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dudley ME, Wunderlich JR, Yang JC, Sherry
RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE,
Mavroukakis SA, et al: Adoptive cell transfer therapy following
non-myeloablative but lymphodepleting chemotherapy for the
treatment of patients with refractory metastatic melanoma. J Clin
Oncol. 23:2346–2357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Besser MJ, Shapira-Frommer R, Treves AJ,
Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E,
Chermoshniuk N, et al: Clinical responses in a phase II study using
adoptive transfer of short-term cultured tumor infiltration
lymphocytes in metastatic melanoma patients. Clin Cancer Res.
16:2646–2655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dudley ME, Yang JC, Sherry R, Hughes MS,
Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et
al: Adoptive cell therapy for patients with metastatic melanoma:
Evaluation of intensive myeloablative chemoradiation preparative
regimens. J Clin Oncol. 26:5233–5239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrini S, Biassoni R, Moretta A, Bruzzone
M, Nicolin A and Moretta L: Clonal analysis of T lymphocytes
isolated from ovarian carcinoma ascitic fluid. Int J Cancer.
36:337–343. 1985.PubMed/NCBI
|
|
9
|
Freedman RS, Tomasovic B, Templin S,
Atkinson EN, Kudelka A, Edwards CL and Platsoucas CD: Large-scale
expansion in interleukin-2 of tumor-infiltrating lymphocytes from
patients with ovarian carcinoma for adoptive immunotherapy. J
Immunol Methods. 167:145–160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki Y, Takakuwa K, Kodama S, Tanaka K,
Takahashi M, Tokunaga A and Takahashi T: Use of adoptive transfer
of tumor-infiltrating lymphocytes alone or in combination with
cisplatin-containing chemotherapy in patients with epithelial
ovarian cancer. Cancer Res. 51:1934–1939. 1991.PubMed/NCBI
|
|
11
|
Fujita K, Ikarashi H, Takakuwa K, Kodama
S, Tokunaga A, Takahashi T and Tanaka K: Prolonged disease-free
period in patients with advanced epithelial ovarian cancer after
adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer
Res. 1:501–507. 1995.PubMed/NCBI
|
|
12
|
Freedman RS and Platsoucas CD:
Immunotherapy for peritoneal ovarian carcinoma metastasis using ex
vivo expanded tumor infiltrating lymphocytes. Cancer Treat Res.
82:115–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han X, Papadopoulos AJ, Ruparelia V,
Devaja O and Raju KS: Tumor lymphocytes in patients with advanced
ovarian cancer: Changes during in vitro culture and implications
for immunotherapy. Gynecol Oncol. 65:391–398. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mantovani A, Allavena P, Sessa C, Bolis G
and Mangioni C: Natural killer activity of lymphoid cells isolated
from human ascitic ovarian tumors. Int J Cancer. 25:573–582. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozenci V, Miller AM, Palmborg A, Egevad L,
Jaremko GA, Kälkner KM and Pisa P: Presence and specificity of
tumor associated lymphocytes from ascites fluid in prostate cancer.
Prostate. 65:20–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santin AD, Hermonat PL, Ravaggi A, Bellone
S, Roman JJ, Smith CV, Pecorelli S, Radominska-Pandya A, Cannon MJ
and Parham GP: Phenotypic and functional analysis of
tumor-infiltrating lymphocytes compared with tumor-associated
lymphocytes from ascitic fluid and peripheral blood lymphocytes in
patients with advanced ovarian cancer. Gynecol Obstet Invest.
51:254–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Xing SS, Guo SB, Jin W and Zhang
W: Oxidative DNA damage of lymphocytes in peripheral blood and
ascites in cancer patients. Current Oncol. 19:(Suppl 2). eS10–eS14.
2012. View Article : Google Scholar
|
|
19
|
Apiranthitou-Drogari M, Paganin C,
Bernasconi S, Losa G, Maneo A, Colombo N, Mantovani A and Allavena
P: In search of specific cytotoxic T lymphocytes infiltrating or
accompanying human ovarian carcinoma. Cancer Immunol Immunother.
35:289–295. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Melioli G, Ferrari I, Casartelli G and
Ragni N: Lymphocytes isolated from the peritoneal fluid of women
with advanced ovarian carcinoma differ significantly from
autologous peripheral blood lymphocytes. Gynecol Oncol. 48:301–307.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ioannides CG, Platsoucas CD, Rashed S,
Wharton JT, Edwards CL and Freedman RS: Tumor cytolysis by
lymphocytes infiltrating ovarian malignant ascites. Cancer Res.
51:4257–4265. 1991.PubMed/NCBI
|
|
22
|
Ullenhag GJ, Sadeghi AM, Carlsson B,
Ahlström H, Mosavi F, Wagenius G and Tötterman TH: Adoptive T-cell
therapy for malignant melanoma patients with TILs obtained by
ultrasound-guided needle biopsy. Cancer Immunol Immunother.
61:725–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellebaek E, Iversen TZ, Junker N, Donia M,
Engell-Noerregaard L, Met Ö, Hölmich LR, Andersen RS, Hadrup SR,
Andersen MH, et al: Adoptive cell therapy with autologous tumor
infiltrating lymphocytes and low-dose Interleukin-2 in metastatic
melanoma patients. J Transl Med. 10:1692012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong JJ, Rosenberg SA, Dudley ME, Yang JC,
White DE, Butman JA and Sherry RM: Successful treatment of melanoma
brain metastases with adoptive cell therapy. Clin Cancer Res.
16:4892–4898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pilon-Thomas S, Kuhn L, Ellwanger S,
Janssen W, Royster E, Marzban S, Kudchadkar R, Zager J, Gibney G,
Sondak VK, et al: Efficacy of adoptive cell transfer of
tumor-infiltrating lymphocytes after lymphopenia induction for
metastatic melanoma. J Immunother. 35:615–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itzhaki O, Hovav E, Ziporen Y, Levy D,
Kubi A, Zikich D, Hershkovitz L, Treves AJ, Shalmon B, Zippel D, et
al: Establishment and large-scale expansion of minimally cultured
‘young’ tumor infiltrating lymphocytes for adoptive transfer
therapy. J Immunother. 34:212–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tran KQ, Zhou J, Durflinger KH, Langhan
MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA and Dudley
ME: Minimally cultured tumor-infiltrating lymphocytes display
optimal characteristics for adoptive cell therapy. J Immunother.
31:742–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dudley ME, Gross CA, Langhan MM, Garcia
MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE,
et al: CD8+ enriched ‘young’ tumor infiltrating
lymphocytes can mediate regression of metastatic melanoma. Clin
Cancer Res. 16:6122–6131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Allavena P, Introna M, Mangioni C and
Mantovani A: Inhibition of natural killer activity by
tumor-associated lymphoid cells from ascites ovarian carcinomas. J
Natl Cancer Inst. 67:319–325. 1981.PubMed/NCBI
|
|
30
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in
tumor-bearing animals and enhances antitumor immune activity. Clin
Cancer Res. 11:6713–6721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu WM, Fowler DW, Smith P and Dalgleish
AG: Pre-treatment with chemotherapy can enhance the antigenicity
and immunogenicity of tumours by promoting adaptive immune
responses. Br J Cancer. 102:115–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
North RJ: Cyclophosphamide-facilitated
adoptive immunotherapy of an established tumor depends on
elimination of tumor-induced suppressor T cells. J Exp Med.
155:1063–1074. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheever MA, Greenberg PD and Fefer A:
Specificity of adoptive chemoimmunotherapy of established syngeneic
tumors. J Immunol. 125:711–714. 1980.PubMed/NCBI
|
|
34
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gattinoni L, Powell DJ Jr, Rosenberg SA
and Restifo NP: Adoptive immunotherapy for cancer: Building on
success. Nat Rev Immunol. 6:383–393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ballen KK, Colvin G, Dey BR, Porter D,
Westervelt P, Spitzer TR and Quesenberry PJ: Cellular immune
therapy for refractory cancers: Novel therapeutic strategies. Exp
Hematol. 33:1427–1435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giuntoli RL II, Webb TJ, Zoso A, Rogers O,
Diaz-Montes TP, Bristow RE and Oelke M: Ovarian cancer-associated
ascites demonstrates altered immune environment: Implications for
antitumor immunity. Anticancer Res. 29:2875–2884. 2009.PubMed/NCBI
|
|
38
|
Leffers N, Gooden MJ, de Jong RA,
Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG,
Daemen T and Nijman HW: Prognostic significance of
tumor-infiltrating T-lymphocytes in primary and metastatic lesions
of advanced stage ovarian cancer. Cancer Immunol Immu. 58:449–459.
2009. View Article : Google Scholar
|
|
39
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Friedman KM, Prieto PA, Devillier LE,
Gross CA, Yang JC, Wunderlich JR, Rosenberg SA and Dudley ME:
Tumor-specific CD4+ melanoma tumor-infiltrating
lymphocytes. J Immunother. 35:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Disis ML, Bernhard H and Jaffee EM: Use of
tumour-responsive T cells as cancer treatment. Lancet. 373:673–683.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pace L, Tempez A, Arnold-Schrauf C,
Lemaitre F, Bousso P, Fetler L, Sparwasser T and Amigorena S:
Regulatory T cells increase the avidity of primary CD8+
T cell responses and promote memory. Science. 338:532–536. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hinrichs CS and Rosenberg SA: Exploiting
the curative potential of adoptive T-cell therapy for cancer.
Immunol Rev. 257:56–71. 2014. View Article : Google Scholar : PubMed/NCBI
|