Introduction
Ewing's sarcoma (ES) is the second most common
malignant bone tumor in children and the fourth most common overall
(1,2).
ES is most common during the second decade of life, with the median
age at diagnosis ranging from 13 to 19 years (3). Pelvic ES accounted for 19.9% of cases in
the Mayo Clinic series (4) and 21% in
the chapter written by Ginsberg et al (5), who discussed the principles and practice
of ES. The numerous advances made in diagnostic imaging and
multimodality therapy over the past few decades mean that the
overall 5-year survival rate of ES has increased from 10% in the
1970s (6) to 55–75% at the turn of
the century (7). Studies have
demonstrated that 5-year survival is improved in patients treated
with surgical resection and chemotherapy vs. patients treated with
chemotherapy and radiation or with radiation alone (8,9).
However, patients with pelvic ES have a poorer
prognosis compared with those that have lesions in their
extremities. The 5-year survival rate for these patients is
markedly lower (35%) (8). Pelvic
resections are classified by region according to studies by
Enneking and Dunham (10), and
O'Connor and Sim (11): Type I refers
to resection of the ilium, type II to resection of the
peri-acetabular region, type III to resection of the pubis or
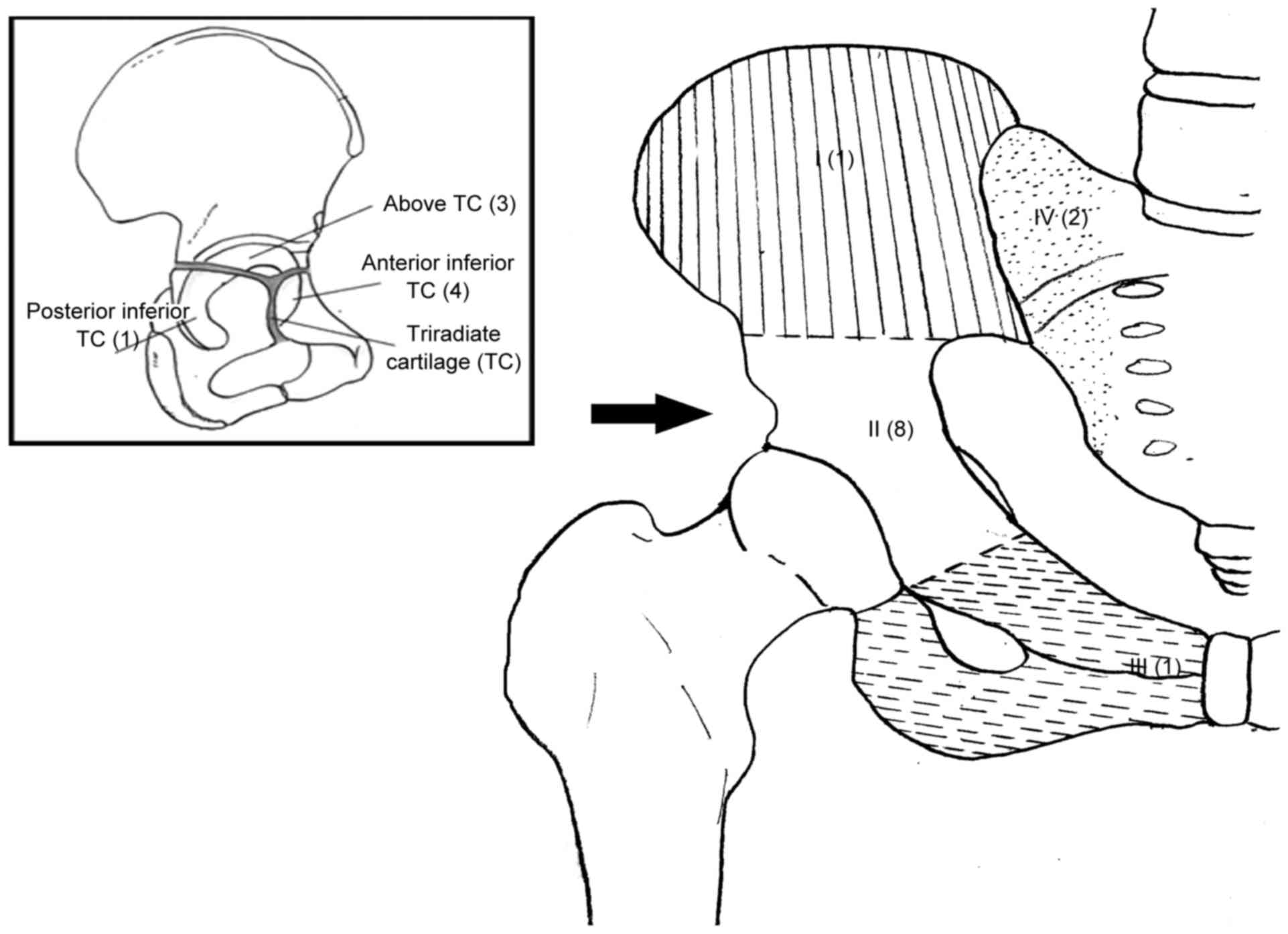
ischium and type IV to resection of the lateral mass of the sacrum.
The resultant defects can cause substantial functional impairment.
Various reconstructions, including fibular struts, hip
transposition, arthrodesis and prosthetic replacements have been
established. However, high rates of early and late complications
have been reported (12,13).
Unlike type I and type III resections, type II
resections usually require excision of the entire acetabulum and
lead to impaired hip function in adults. However, in skeletally
immature children and adolescents, the pelvis has its own intrinsic
structure. The triradiate cartilage closes at 16 to 18 years of
age, thinning out prior to this (14,15). This
physis could act as a barrier to tumor extension, although it is
not impenetrable. Prior to closure, it may represent an oncological
margin for tumor resection, particularly for tumors that do not
contact or partially contact the physis (16). As in metaphyseal tumors of long bones,
in which an unaffected physis allows a transepiphyseal resection
for joint-sparing, periacetabular tumors can be excised using
trans-acetabular osteotomy based on triradiate cartilage. This can
maximally preserve the unaffected acetabular components, which
contribute to the growth of the acetabulum (15). Winkelmann (17) removed the sarcomas (types I and II)
using a similar strategy. Sales de Gauzy et al (1) excised the periacetabular ES (types II
and III) beyond the triradiate cartilage without reconstruction in
2 children. Nevertheless, there have been few reports in the
literature documenting the surgical strategy for pelvic ES in
children and adolescents, particularly for periacetabular
lesions.
The present study retrospectively reviews the
resection of pelvic ES and the following reconstructions in 12
children and adolescents in order to present intermediate-term
results of using this surgical strategy as part of multimodality
therapy.
Patients and methods
Patient demographics
Between January 2001 and October 2013, 12 children
and adolescents with pelvic ES were treated surgically at the
Department of Orthopedic Surgery, Xi-Jing Hospital (Xi'an, China).
This series comprised 9 males and 3 females, with a mean age of
12.7 years (range, 7–16 years). Tumor sites were assigned according
to the Enneking and Dunham classification (10). In total, 3 patients had type I (ilium)
lesions, 2 of which extended into the sacrum (type I+IV); 8 had
type II (periacetabular) lesions (3 above, 4 anterior inferior and
1 posterior inferior to the triradiate cartilage; termed type IIA,
type IIB and type IIC, respectively, in this study); and 1 had a
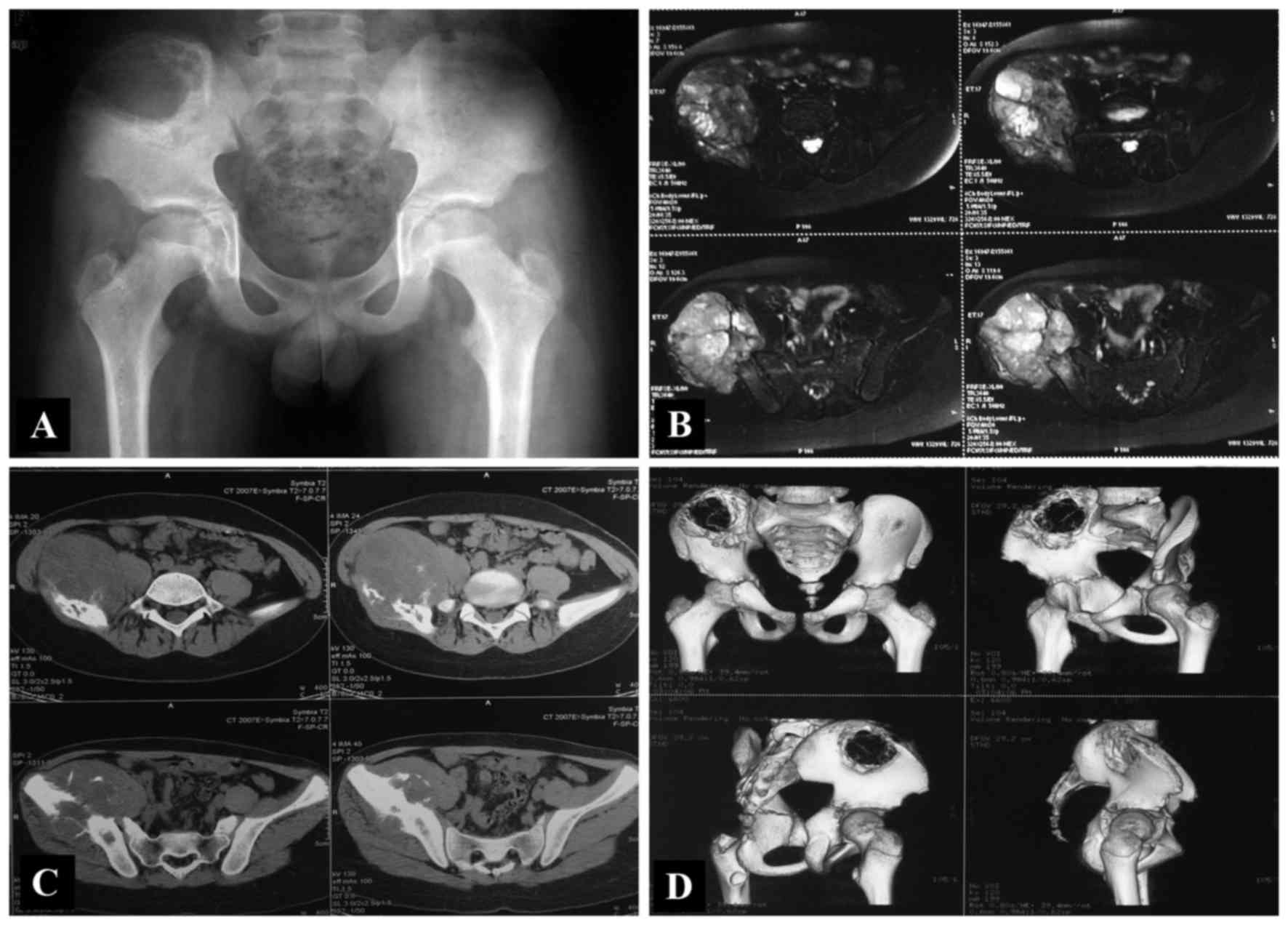
type III (ischiopubic) lesion (Fig.
1). Staging studies, including plain film, magnetic resonance
imaging (MRI), chest computed tomography (CT) and bone
scintigraphy, were performed. All patients were non-metastatic at
presentation.
Treatment protocol
All patients received vincristine (1.5
mg/m2 once a day), doxorubicin (30 mg/m2
every 2 days) and ifosfamide (2,000 mg/m2 every 3 days)
neoadjuvant chemotherapy for 12 weeks prior to surgery. No
preoperative radiotherapy was performed. The response to
chemotherapy was assessed by the Response Evaluation Criteria in
Solid Tumors (RECIST) (18).
Surgeries were performed between weeks 8 and 10 after the
initiation of chemotherapy. Thereafter, chemotherapy was continued
in all patients. Postoperative radiotherapy was administered to
patients who received excisions with marginal margins (i.e., a
margin through tissue that has gross or histological evidence of
reaction to the tumor) or contaminated margins. Patients treated
with Intensity Modulated Radiation Therapy were prescribed a dose
of 45 Gy in 15 fractions (3 Gy/day for 15 days).
Depending on the location and extent of the tumor,
four types of excision were performed singly or in combination.
Part of the ilium and the surrounding gluteal muscles (type I) were
excised in 1 patient with iliac lesions. Another 2 patients with
iliac lesions required excision of a lateral mass of the sacrum
besides the ilium and gluteal muscles (type I+IV). The following
excisions were performed on 8 periacetabular lesions: 3 patients
underwent resection of the upper component of the acetabulum and
neighboring ilium (type I+IIA); 4 patients required removal of the
anterior inferior component of the acetabulum, as well as all or
part of the pubis and ischium (type IIB+III); 1 patient had the
whole of the inferior component of the acetabulum, with the pubis
and ischium, resected (type IIB+IIC+III); and 1 patient had a pubic
lesion and underwent partial excision of the pubis (type III)
(Table I).
 | Table I.Demographic and clinical data. |
Table I.
Demographic and clinical data.
| Case | Sex/age, years | Site of lesion | PO CT/RT | Type of
resection | Type of
reconstruction | LR/Met | CANS | FPM | Patient status (years
after surgery) | Functional score | Radiological
score |
|---|
| 1 | F/7 | Type I+IIA | Yes/No | I+IIA | Allograft, plate | No/No | No | − | ANED (4) | 27 | 93 |
| 2 | M/11 | Type I+IV | Yes/Yes | I+IV | Allograft, screw,
rod | Yes/Yes | No | + | AWD (2) | 18 | 76 |
| 3 | M/12 | Type I | Yes/No | I | Allograft, screw,
rod | No/No | Yes | − | ANED (3) | 28 | 91 |
| 4 | M/13 | Type I+IIA | Yes/Yes | I+IIA | Allograft,
plate | No/Yes | No | + | DOD (2) | 24 | 78 |
| 5 | M/13 | Type IIB+III | Yes/No | IIB+III | Allograft,
plate | No/No | Yes | − | ANED (5) | 26 | 93 |
| 6 | F/13 | Type III | Yes/No | III |
Non-reconstruction | No/No | Yes | − | ANED (3.5) | 28 | 93 |
| 7 | M/15 | Type IIB+C+III | Yes/No | IIB+C+III | Allograft,
plate | No/No | No | − | ANED (3) | 27 | 93 |
| 8 | F/16 | Type IIB+III | Yes/Yes | IIB+III | Allograft,
plate | No/No | No | + | ANED (6) | 26 | 92 |
| 9 | M/16 | Type I+IV | Yes/Yes | I+IV | Allograft, screw,
rod | No/No | Yes | + | ANED (2) | 25 | 94 |
| 10 | M/11 | Type IIB+III | Yes/No | IIB+III | Allograft,
plate | No/No | No | − | ANED (3) | 27 | 93 |
| 11 | M/10 | Type I+IIA | Yes/No | I+IIA | Allograft,
plate | No/Yes | Yes | − | AWD (3) | 28 | 93 |
| 12 | M/13 | Type IIB+III | Yes/No | IIB+III | Allograft,
plate | No/No | No | − | ANED (2.5) | 28 | 93 |
As aforementioned, a ‘marginal margin’ is a margin
through tissue that has gross or histological evidence of a
reaction to the tumor. If there is an intact fascial boundary
between the tumor and surgical resection margin, the margin is
defined as ‘wide’ (19). A marginal
margin typically occurred in certain areas of the specimen where
the tumor grew very close to the neurovascular or genitourinary
structures. In the present study, surgical margins were classified
as wide in 8 patients and as marginal in 4. The excised tumor was
examined for safe margin by an experienced pathology technician.
Samples from different sides of the removed tissue (proximal,
distal, lateral, medial, top and bottom) were embedded in Optimal
Cutting Temperature Compound, frozen, sectioned into 10 µm thick
slices and then stained with hematoxylin and eosin for 8 min at
room temperature. During surgery, 2 patients were observed to have
contaminated margins (into the lesion) and required further
excision to yield negative margins. After 2008, 5 patients received
precise resection using a computer-assisted navigation system
(CANS; Stryker Pacific, Ltd., Hong Kong, China). The images from
CT, MRI and bone scintigraphy were integrated in CANS and a
three-dimensional (3D) tumor model was generated. This model was
used for preoperative planning and navigation-guided resection, as
reported previously (20). Of the 5
patients undergoing CANS, 1 underwent a type I excision, 1
underwent a type I+IV excision, 1 underwent a type IIB+III
excision, 1 underwent a type I+IIA excision and 1 underwent a type
III excision (Table I).
The reconstructions were individualized on the basis
of variables that included patient age, functional demands and
tumor extension (Table I). The
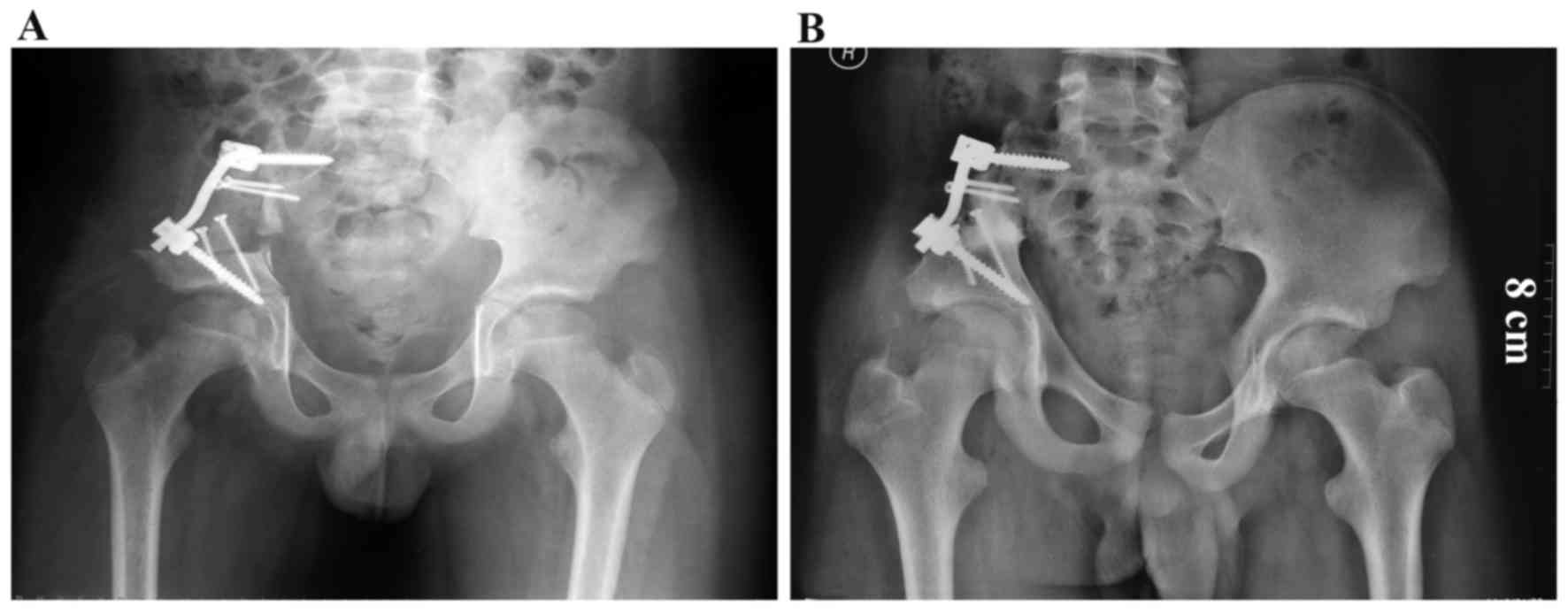
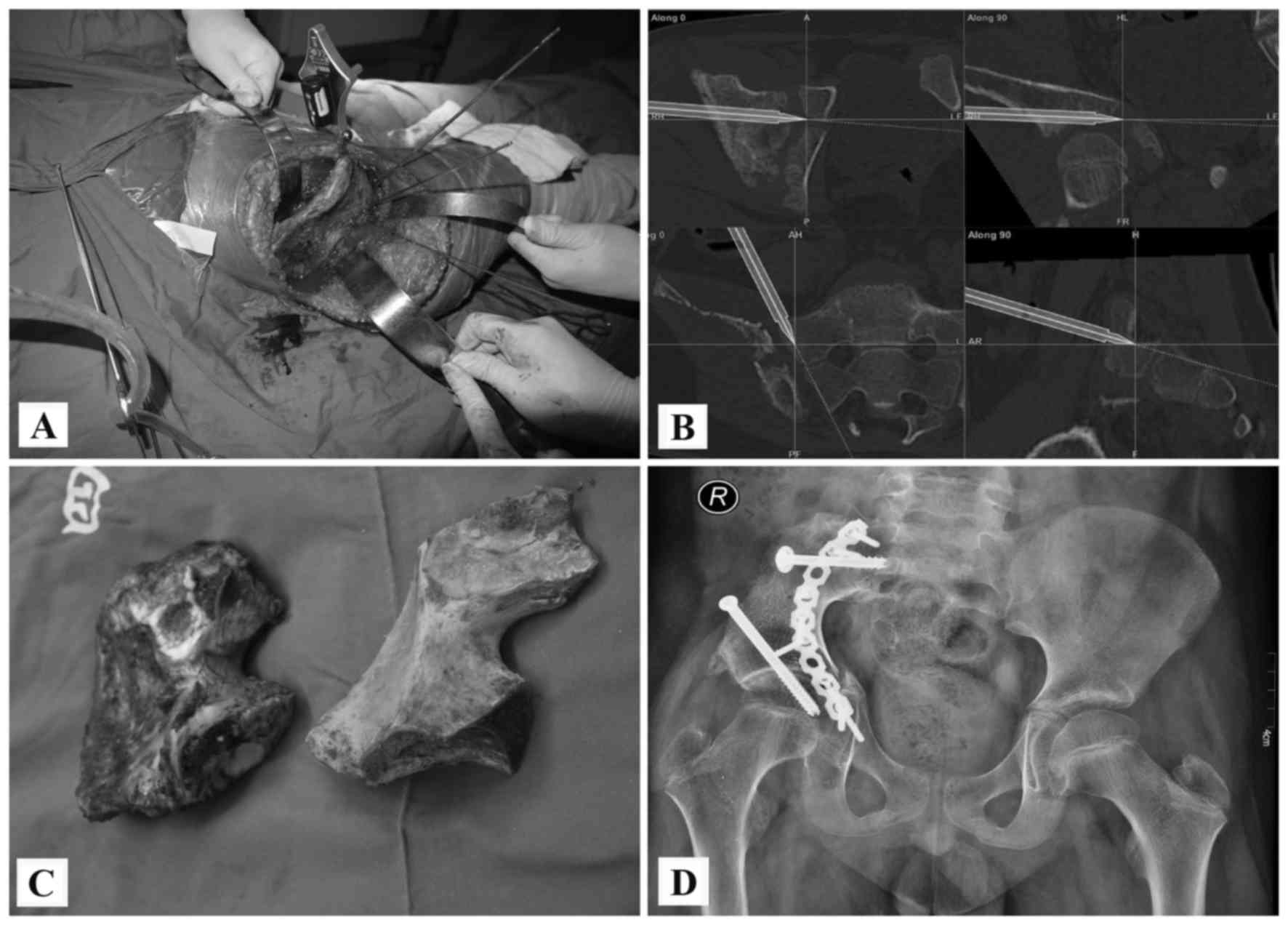
pedicle screw/rod/allograft composite reconstructions were
performed in 3 patients after a type I or type I+IV excision
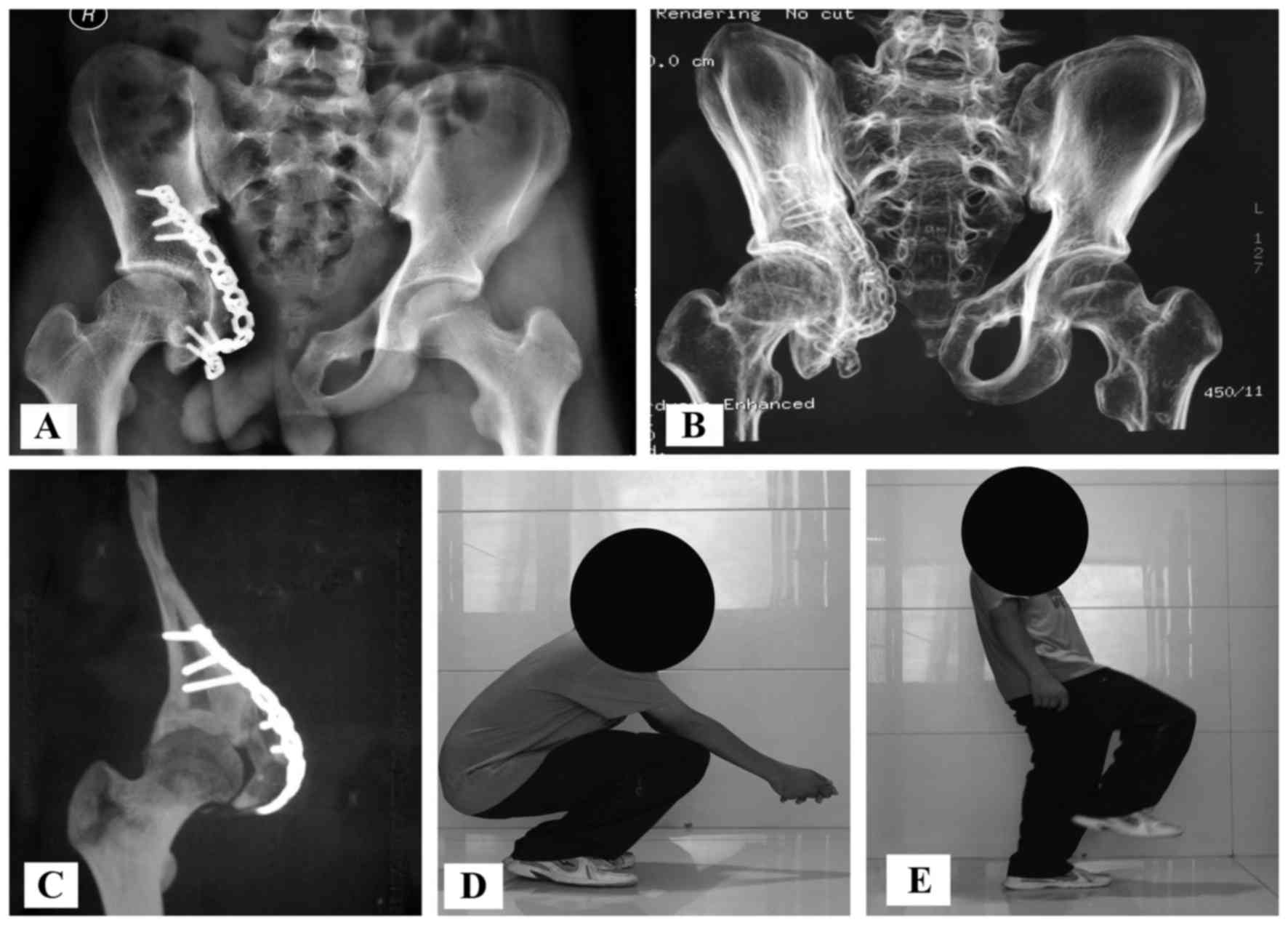
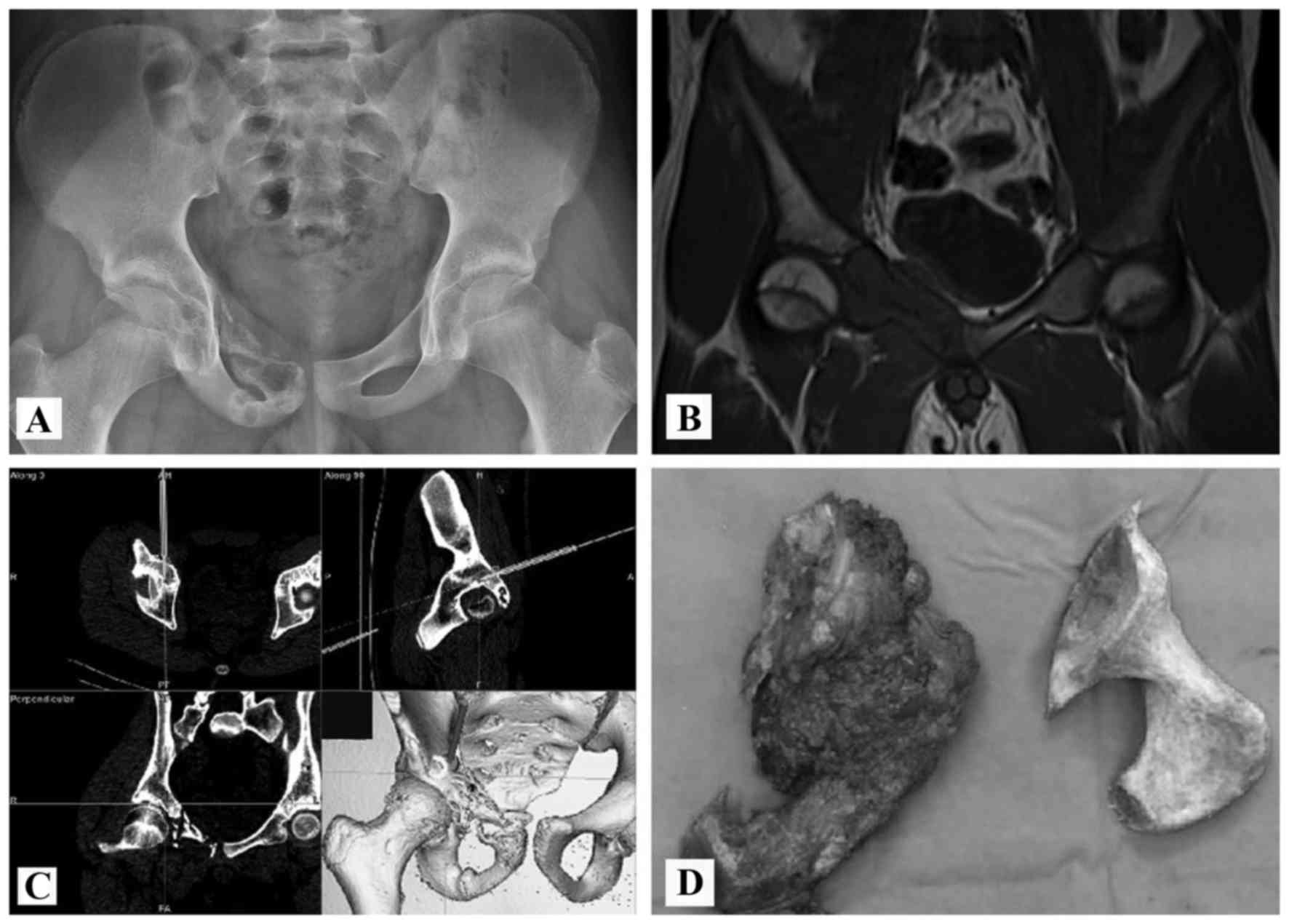
(Figs. 2 and 3). Reconstructions with allografts and
plates were performed in 8 patients with periacetabular lesions
(Figs. 4–9). No reconstruction was performed in 1
patient, who underwent a partial pubic resection (type III).
Selecting allografts that could closely match the excised bone was
important for periacetabular reconstruction. A 3D virtual bone bank
system has been developed in the Department of Orthopedic Surgery,
Xi-Jing Hospital. Using this system, 3D tumor models and allografts
were measured and compared to determine the most appropriate match.
Owing to the limited availability of pediatric allografts, the
majority of allografts in the bone bank were over-sized and
required cutting or trimming to match the defect as closely as
possible.
Antibiotic prophylaxis (cefazolin, 1 g/day) was
usually administered intravenously for the first week following
surgery. Prophylaxis could be administered for longer, depending on
multiple factors, including time of drain retention, medical
co-morbidities and potential wound healing problems. A total of 4
patients who had positive margins in final pathology reports
received postoperative irradiation (Table
I).
Patients were required to undergo non-weight-bearing
ambulation for the first 6–8 weeks after surgery. Partial
weight-bearing ambulation was required between weeks 9 and 12, and
full weight-bearing ambulation after 12 weeks. However, the full
weight-bearing time depended on radiographic healing time, which
varied between 3 and 5 months after surgery.
Follow-up and evaluation
The study was approved by the Institutional Review
Board of Xi-Jing Hospital. Informed consent was obtained from all
patients and procedures followed were in accordance with the
ethical standards of the Responsible Committee on Human
Experimentation of Xi-Jing Hospital and with The Helsinki
Declaration. All patients completed the follow-up. The median
follow-up time was 39 months (range, 24–72 months). Patients were
assessed at 2 weeks, 1 month and 3 months after surgery, and every
3 months thereafter until 2 years after surgery, and then every 6
months. Plain radiographs and physical examinations were performed
at each follow-up appointment. Chest CT was performed every 3
months until 2 years after surgery and then every 6 months to
assess for metastatic disease. Functional outcomes were determined
using the MSTS score 93 system (21).
In brief, this system assigned numerical values (0–5) for each of
the 6 categories: Pain, function, emotional acceptance, support,
walking and gait. The plain radiographs were evaluated according to
the International Society of Limb Salvage (ISOLS) grading system
(22), which had 5 categories,
including healing of osteotomies (union), contour of the graft
(graft shortening), graft fracture, density of the graft
(resorption) and stability of implant. The numerical values
(1–4)
were assigned for each category to indicate poor, fair, good and
excellent, respectively. The score was calculated by adding the
value for each criterion and dividing by the maximum attainable
score. This was then expressed as a percentage with a maximum score
of 100%. The MSTS and ISOLS scores were obtained and reported at
the time of the final follow-up appointment.
Results
Response to chemotherapy
The patients' responses to neoadjuvant chemotherapy
were assessed by CT/MRI scan and graded according to RECIST. The
results showed that 1 patient achieved a complete response, 9
achieved a partial response and 2 achieved stable disease (SD). No
patient exhibited progressive disease. In accordance with National
Comprehensive Cancer Network (NCCN) guidelines (23) for ES, excision of the tumors was
attempted en bloc in all patients.
The histological response was evaluated according to
a grading system developed by Ferrari et al (24). This three-grade system was defined as
following: A grade I response represented a tumor with
macroscopically viable tumor areas; a grade II response represented
a tumor with only microscopic foci of viable tumor; and a grade III
response indicated that there was no evidence of viable tumor. In
the present study, 1 patient was recorded as exhibiting a grade III
response (no viable tumor cells), 8 as exhibiting a grade II
response (microscopic foci) and 3 as exhibiting a grade I response
(macroscopic foci).
Survival
In total, 9 patients exhibited no evidence of
disease at a mean of 43 months after surgery (range, 24–72 months),
1 patient succumbed to disease at 24 months, and 2 patients
remained alive with disease but had slow progressive pulmonary
metastasis at 24 and 36 months, respectively. At the time of the
final follow-up appointment, 11 patients remained alive (Table I).
Local recurrence
The 5 patients who received precise excision using
CANS exhibited no local disease recurrence. In the 7 patients who
underwent conventional resections, 1 experienced local recurrence.
However, patient numbers are too small to suggest that CANS
provides superior local control. The patient with local recurrence
received a type I+IV resection in the first surgery. The response
to neoadjuvant chemotherapy was recorded as SD according to RECIST
and as grade I in Ferrari's three-grade system. At 12 months after
surgery, a soft-tissue mass was found. The biopsy indicated
recurrence and the patient underwent surgical intervention. This
patient also had concomitant pulmonary metastasis and was alive
with disease at the time of the final follow-up appointment
(Table I).
Metastasis
Distant metastases developed in 3/12 patients,
including 1 patient with concomitant local recurrence, at 12, 14
and 36 months after surgery, respectively. Of the patients, 1
succumbed to the disease 10 months after metastasis was detected.
The remaining 2 patients were alive with pulmonary metastases at
the time of the final follow-up appointment (Table I).
Function
At the final follow-up, the patients had a mean
functional score of 26 points (range, 18–28 points). The mean
radiographic score was 90.1% (range, 76–94%), which represented an
excellent radiographic result (Table
I).
Complications
No patients experienced hip dislocation following
acetabular reconstruction. In total, 3 patients experienced
surgical complications: 1 patient had a wound healing problem due
to fat liquefaction, which was superficial and healed after
surgical debridement; 1 patient had a leg-length discrepancy of 2
cm; and 1 patient had a loosening screw, for which no intervention
was required.
Discussion
The excision and reconstruction of pelvic ES in
children and adolesecents is challenging, particularly for
periacetabular lesions (7,8,25,26). Type I and type III excisions preserve
the acetabulum and allow for better function. In a previous study,
the defects of 4 patients were reconstructed with fibular-free
flaps after type I excisions. Functionality was excellent and all
patients were able to ambulate independently (27). Another study demonstrated that
movement and strength were rated as ‘excellent’ in 6 patients
following type I excisions (28).
However, when patients with type II excisions were included in
studies, the MSTS score decreased to 54–59% when using conventional
osteotomy. Complication rates were also varied, ranging between 71
and 100% (26,29). By contrast, the triradiate
cartilage-based trans-acetabular osteotomy in the present study
maximally preserved the unaffected acetabular components. The MSTS
score increased to 26 (87%), which correlated with the results of
other studies (Table II) (1).
 | Table II.Survival, function, and complications
after surgical treatment of pelvic Ewing's sarcoma. |
Table II.
Survival, function, and complications
after surgical treatment of pelvic Ewing's sarcoma.
| First author | Patients, n | Mean age,
years | Pelvic zone
involved (no. of patients) | Reconstruction
(patient no.) | Mean Follow-up,
months | Survival (patient
no.) | LR/Met, n | Complications | Function (no. of
patients) | (Refs.) |
|---|
| Sales et
al | 2 | 8 | II+III (2) | Non-reconstruction,
growth plate-based trans-acetabular osteotomy (2) | 90 | ANED (2) | No/no | No | MSTS score, 90 or
100% | (1) |
| Li et
al | 10 | 17 | I (7); I+II+III
(1); III (1); Sacrum (1) | Non-reconstruction
(10) | 57 | 36-month survival
rate of 80% | No/3 | No | Satisfactory with
occasional cane use | (25) |
| Rödl et
al | 36 | 18 | I (22); II (12);
III (2) | Non-reconstruction
(10); allograft (11); autograft (4); prostheses (2); rotated
proximal femur (1); transpositionplasty (8) | 48 | 5-year survival
rate of 45% | 2/2 | Infection (19);
non-union (2); skin slough/fistula (9) | Mean MSTS score of
59% | (26) |
| Hubert et
al | 4 | 13 | I (4) | Fibular free flap
(4) | 86 | ANED (2); AWD (1);
DOD (1) | 1/2 | Hip pain (2); foot
drop (1); mild scoliosis (2) | Excellent results
with independent ambulation | (27) |
| Porsch et
al | 7 | 14 | I (6); II (1); | Allograft (6);
arthrodesis (1) | 136 | ANED (6); DOD
(1) | 1/1 | Scar fibrosis (1)
good (3); fair (1); poor (1) | Excellent (2); | (28) |
| Campanacci et
al | 7 | Not stated | I+II+III (1); I+II
(5); II (1); | Allograft and
prosthesis (7) | 22 | ANED (2); AWD (2);
DOD (3) | 0/5 | Neurological
deficit (4); dislocation (2); deep infection (1) | Mean MSTS score of
54% | (29) |
| Present study | 12 | 13 | I (1); III (1);
I+IIA (3); I+IV (2); IIB+III (4); IIB+C+III (1) | Non-reconstruction
(1); allograft (11); (TR-based trans-acetabular osteotomy) | 39 (24–72) | ANED (9); AWD (2);
DOD (1); 2-year survival rate of 100% | 1/2 | Wound healing (1);
limb length discrepancy (1); screw loosening (1) | Mean MSTS score of
87% | − |
The acetabulum develops from the growth plates of
the ilium, ischium and pubis. All growth plates are centrally
confluent, with thick triradiate cartilage, which is a secondary
ossification center of the hip bones (30). In the skeletally immature pelvis,
these three bones are separated by triradiate cartilage. The
centers of ossification develop at ~8 years of age and the
triradiate cartilage usually closes at 16–18 years of age. The
physis has long been considered to act as a barrier, preventing the
spread of tumor to the epiphysis, although this barrier is not
impenetrable (31). This hypothesis
was supported by experimental research that identified the protein
substances within the physis that inhibited angiogenesis (32). In the current study, the prerequisite
for the trans-acetabular osteotomy strategy was an open triradiate
cartilage without tumor invasion.
An evaluation of the association between the tumor
and the triradiate cartilage is recommended when planning the
resection of a tumor that involves any part of the acetabulum. A
correlation study between the histological findings and MRI results
in limb malignancies showed that the accuracy of MRI was 90.3%
(33). The epiphysis could be safely
preserved in conditions in which the tumor did not contact or only
partially contacted the growth plate. The contraindication was that
the growth plate was penetrated or wholly affected by the tumor
(31). As the shape of the acetabulum
was quite different from that of a long bone, the sagittal and
coronal images captured by MRI were meticulously examined in the 8
patients with periacetabular lesions in the present study. The
non-involvement of triradiate cartilage was observed in 4 patients
and partial involvement was observed in another 4. The triradiate
cartilage was not penetrated or wholly affected by the tumor. After
2008, 5 patients received CANS-assisted surgeries. The
reconstructed 3D tumor model allowed for the clear delineation of
tumor and triradiate cartilage.
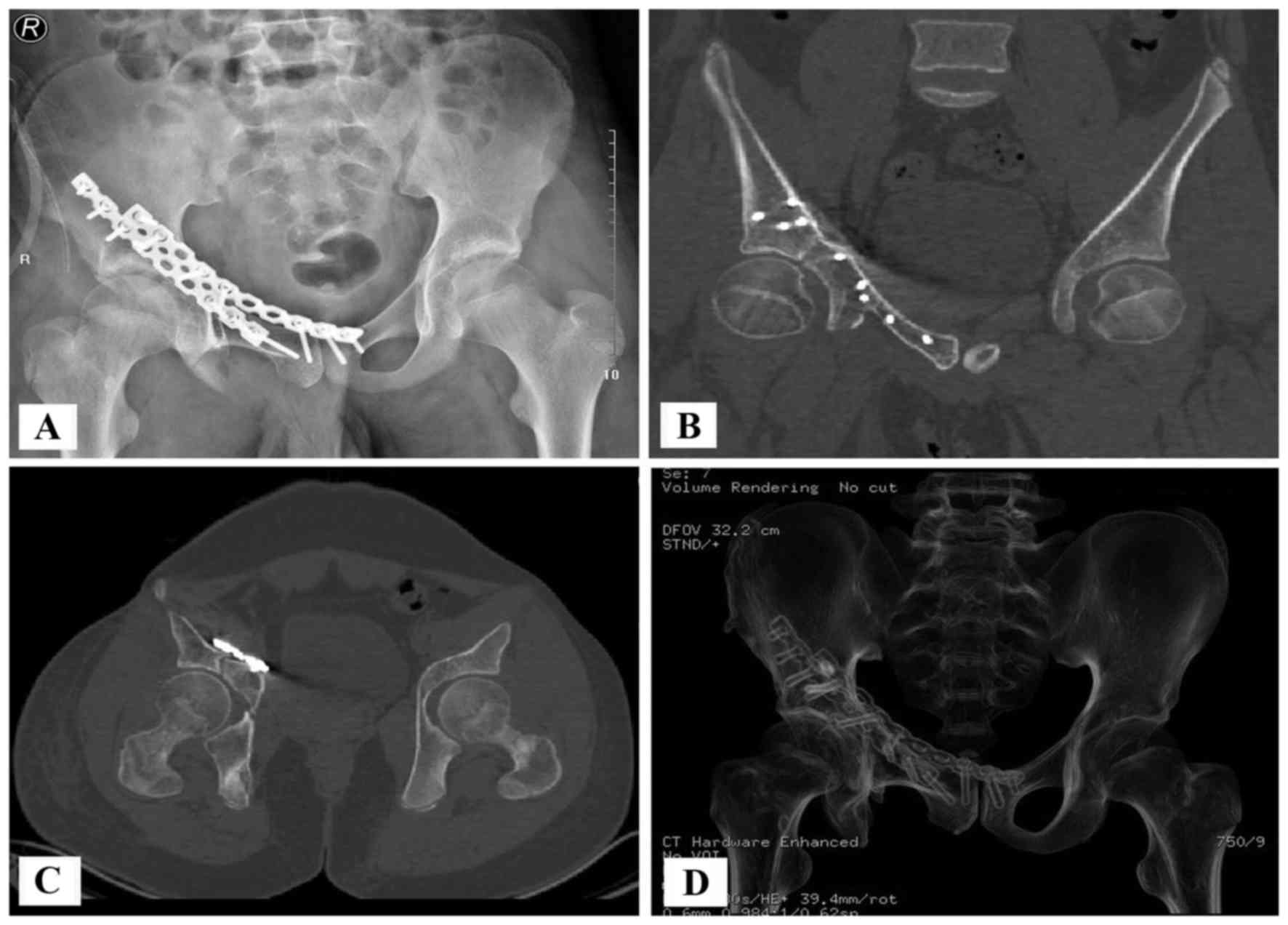
The majority of allografts in the bone bank were
over-sized and required cutting or trimming to fit the defects,
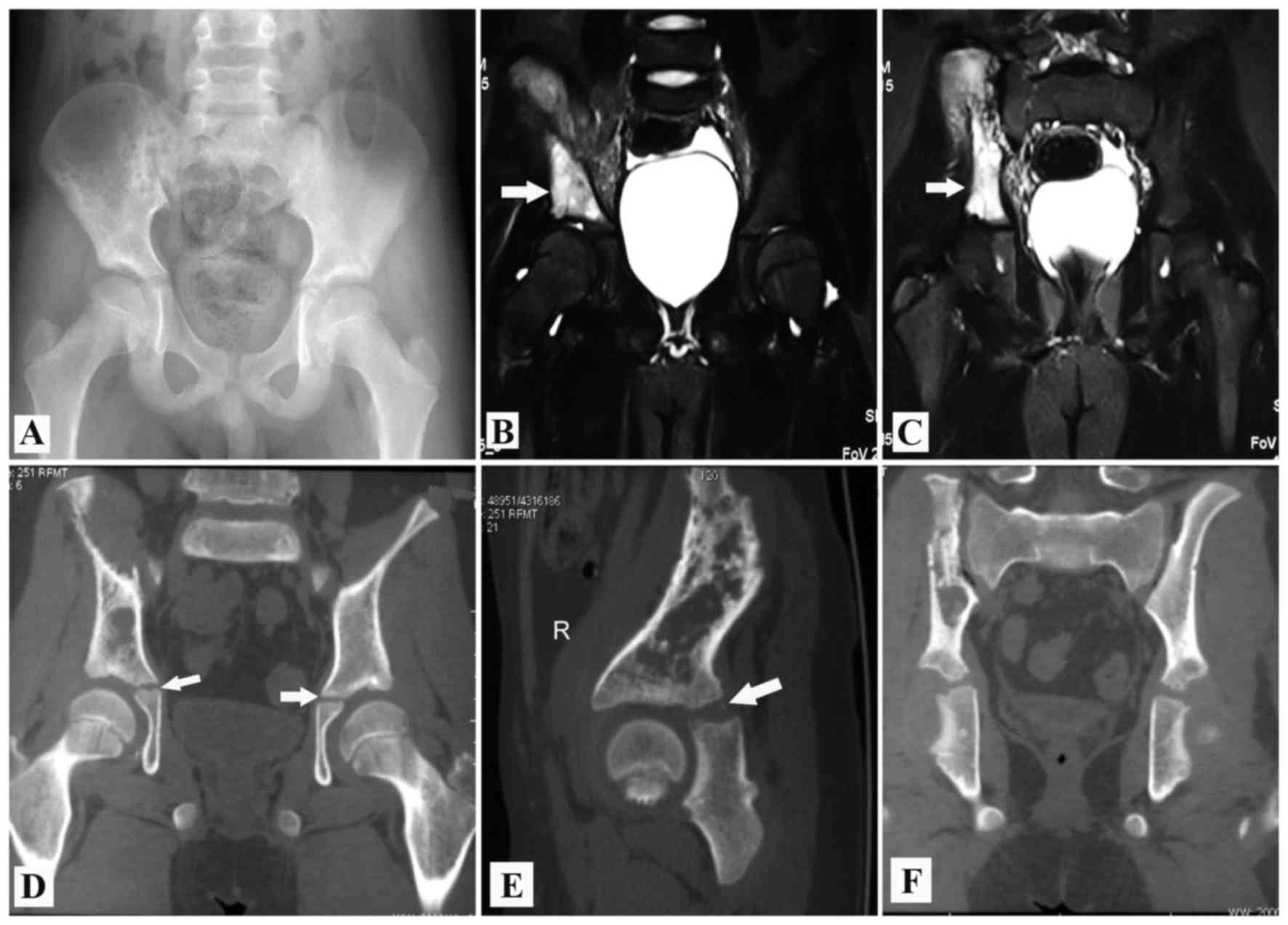
owing to the limited availability of pediatric allografts (Figs. 5C and 6D). This work was performed with the aid of
CANS after 2008. A model of the excised bone was printed using a
rapid prototyping technique. This model acted as a template for the
cutting and trimming of the allograft. A CT scan revealed that the
hip joint was congruent after acetabular reconstruction (Fig. 7). All of the aforementioned factors
contributed to a good functional score (Tables I and II). All patients with lower acetabular
involvement (types IIB, and IIB+IIC) received allograft
reconstructions in the present study. This could benefit the growth
and centralization of the femoral head. Plain radiographs indicated
that the femoral head was well centered and covered at 3 years
after surgery (Fig. 9). For patients
with upper acetabular involvement (type IIA), the physicians
strongly suggested reconstruction, as this area could bear the
majority of the load in the hip joint movement. The implanted
allograft did not grow like the other components of the acetabulum.
To match the host's femoral head, the surgeons usually implanted a
slightly larger osteoarticular allograft, which provided more space
for the growth of the femoral head. Patients undergoing type I
excision also required reconstruction to avoid pelvic instability
and leg length discrepancy (27),
whereas those undergoing type III excisions did not.
The present study had several limitations. Firstly,
the study used a small number of patients. As only the children and
adolescents with pelvic ES were included, it was difficult to
obtain a large number of patients from one institution. Although
the results cannot thoroughly prove the concept of trans-acetabular
osteotomy, the data support the rationale of this technique.
Secondly, this was a retrospective study with potentially
uncontrolled variables, including different locations and
variability in the required bone and soft tissue excisions.
Finally, as only 5 patients received navigation-guided osteotomies
after 2008, the present study does not have adequate power to
evaluate the beneficial effects of CANS-assisted surgery. In spite
of these drawbacks, the current study contributes further
information on surgical strategy to treat pelvic ES.
In conclusion, this study investigated a novel
surgical strategy for pelvic ES excisions in children and
adolescents. Navigation was used to perform osteotomy and allograft
trimming, which enhanced the accuracy of resection and
reconstruction. Although it remains a small series with limited
conclusions, the present study does introduce a notable surgical
strategy and aids the understanding of the management of patients
with rare diseases.
References
|
1
|
de Gauzy Sales J, Lafontan V, Urseï M and
Accadbled F: Ewing sarcoma of the acetabulum in children: A ‘growth
plate-based’ surgical strategy. J Pediatr Orthop. 34:326–330. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devaney K, Abbondanzo SL, Shekitka KM,
Wolov RB and Sweet DE: MIC2 detection in tumors of bone and
adjacent soft tissues. Clin Orthop Relat Res. 176–187.
1995.PubMed/NCBI
|
|
3
|
Abed R and Grimer R: Surgical modalities
in the treatment of bone sarcoma in children. Cancer Treat Rev.
36:342–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frassica FJ, Frassica DA, Pritchard DJ,
Schomberg PJ, Wold LE and Sim FH: Ewing sarcoma of the pelvis.
Clinicopathological features and treatment. J Bone Joint Surg Am.
75:1457–1465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ginsberg JP, Woo SY and Johnson ME:
Ewing's sarcoma family of tumors: Ewing's sarcoma of bone and soft
tissue and the peripheral primitive neuroectodermal tumorsPizzo PA
and Poplack DG: Principles and Practice of Pediatric Oncology. 4th.
Philidelphia, PA: Lippincott Williams & Wilkins; pp.
973–101614. 2002
|
|
6
|
Falk S and Alpert M: Five year survival of
patients with Ewing's sarcoma. Surg Gynecol Obstet. 124:319–324.
1967.PubMed/NCBI
|
|
7
|
Bacci G, Ferrari S, Bertoni F, Rimondini
S, Longhi A, Bacchini P, Forni C, Manfrini M, Donati D and Picci P:
Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated
with adjuvant chemotherapy: Analysis of 359 patients at the
Istituto Rizzoli. J Clin Oncol. 18:4–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhagat S, Sharma H, Pillai DS and Jane MJ:
Pelvic Ewing's sarcoma: A review from Scottish Bone Tumour
Registry. J Orthop Surg (Hong Kong). 16:333–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sucato DJ, Rougraff B, McGrath BE,
Sizinski J, Davis M, Papandonatos G, Green D, Szarzanowicz T and
Mindell ER: Ewing's sarcoma of the pelvis. Long-term survival and
functional outcome. Clin Orthop Relat Res. 193–201. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enneking WF and Dunham WK: Resection and
reconstruction for primary neo-plasms involving the innominate
bone. J Bone Joint Surg Am. 60:731–746. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connor MI and Sim FH: Salvage of the
limb in the treatment of malignant pelvic tumors. J Bone Joint Surg
Am. 71:481–494. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jansen JA, van de Sande MA and Dijkstra
PD: Poor long-term clinical results of saddle prosthesis after
resection of periacetabular tumors. Clin Orthop Relat Res.
471:324–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebert C, Wessling M, Hoffmann C, Roedl R,
Winkelmann W, Gosheger G and Hardes J: Hip transposition as a limb
salvage procedure following the resection of periacetabular tumors.
J Surg Oncol. 103:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liporace FA, Ong B, Mohaideen A, Ong A and
Koval KJ: Development and injury of the triradiate cartilage with
its effects on acetabular development: Review of the literature. J
Trauma. 54:1245–1249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponseti IV: Growth and development of the
acetabulum in the normal child. Anatomical, histological, and
roentgenographic studies. J Bone Joint Surg Am. 60:575–585. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheung WH, Lee KM, Fung KP and Leung KS:
Growth plate chondrocytes inhibit neo-angiogenesis: A possible
mechanism for tumor control. Cancer Lett. 163:25–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winkelmann W: A new surgical method in
malignant tumors of the ilium. Z Orthop Ihre Grenzgeb. 126:671–674.
1988.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New Guidelines to
Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
19
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 106–120. 1980.PubMed/NCBI
|
|
20
|
Fan H, Guo Z, Wang Z, Li J and Li X:
Surgical technique: Unicondylar osteoallograft prosthesis composite
in tumor limb salvage surgery. Clin Orthop Relat Res.
470:3577–3586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 241–246.
1993.PubMed/NCBI
|
|
22
|
Glasser D and Langlais F: The ISOLS
radiological implant evaluation systemLanglais F and Tomeno B: Limb
Salvage: Major Reconstructions in Oncologic and Nontumoral
Conditions. Springer-Verlag; Heidelberg, Germany: pp. 23–31.
1991
|
|
23
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari S, Bertoni F, Palmerini E, Errani
C, Bacchini P, Pignotti E, Mercuri M, Longhi A, Cesari M and Picci
P: Predictive factors of histologic response to primary
chemotherapy in patients with Ewing sarcoma. J Pediatr Hematol
Oncol. 29:364–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li WK, Lane JM, Rosen G, Marcove RC,
Caparros B, Huvos A and Groshen S: Pelvic Ewing's sarcoma. Advances
in treatment. J Bone Joint Surg Am. 65:738–747. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rödl RW, Hoffmann C, Gosheger G, Leidinger
B, Jürgens H and Winkelmann W: Ewing's sarcoma of the pelvis:
Combined surgery and radiotherapy treatment. J Surg Oncol.
83:154–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hubert DM, Low DW, Serletti JM, Chang B
and Dormans JP: Fibula free flap reconstruction of the pelvis in
children after limb-sparing internal hemipelvectomy for bone
sarcoma. Plast Reconstr Surg. 125:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porsch M, Kornhuber B and Hovy L:
Functional results after partial pelvic resection in Ewing's
sarcoma of the ilium. Arch Orthop Trauma Surg. 119:199–204. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Campanacci D, Chacon S, Mondanelli N,
Beltrami G, Scoccianti G, Caff G, Frenos F and Capanna R: Pelvic
massive allograft reconstruction after bone tumour resection. Int
Orthop. 36:2529–2536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Portinaro NM, Murray DW and Benson MK:
Microanatomy of the acetabular cavity and its relation to growth. J
Bone Joint Surg Br. 83:377–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Panuel M, Gentet JC, Scheiner C, Jouve JL,
Bollini G, Petit P, Bourliere-Najean B and Devred P: Physeal and
epiphyseal extent of primary malignant bone tumors in childhood.
Correlation of preoperative MRI and the pathologic examination.
Pediatr Radiol. 23:421–424. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langer R, Brem H, Falterman K, Klein M and
Folkman J: Isolations of a cartilage factor that inhibits tumor
neovascularization. Science. 193:70–72. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
San-Julian M, Aquerreta JD, Benito A and
Cañadell J: Indications for epiphyseal preservation in metaphyseal
malignant bone tumors of children: Relationship between image
methods and histological findings. J Pediatr Orthop. 19:543–548.
1999. View Article : Google Scholar : PubMed/NCBI
|