Introduction
Leiomyosarcoma (LMS) of the inferior vena cava (IVC)
is a rare type of neoplasm, accounting for ~0.5% of adult soft
tissue sarcoma, affecting <1/100,000 of all adult malignancies
(1–3).
The prognosis is poor, as patients present with intra or
extra-luminal growth often with invasion of adjacent structures.
According to a recent pooled data analysis, <400 cases of IVC
LMS have been reported, with the majority of studies limited to
single case reports or compilations of a case series (4). The 5-year survival rate ranges between
31 and 66.7% for patients with IVC LMS following complete
macroscopic resection (1,5–9).
LMS of the IVC are usually presented as large tumors
at the time of diagnosis. In several studies, tumors were >10 cm
(1–3,8,10). Occasionally, LMS of the IVC occurs in
young patients, with few or no comorbidities, as localized disease.
They are predominant in females aged 54 years at the time of
diagnosis (11), IVC reconstruction
may be considered once long-term survival can be accomplished in
patients submitted to R0 resection (3,9).
Surgery is currently the only potentially curative
therapy. The paramount aim when approaching IVC LMS include
achieving local control, maintaining the patency of major venous
flow and identifying the most effective adjuvant therapeutic
strategies to reduce the recurrence rate. However, the technical
challenges presented by anatomical characteristics of this disease
raise important issues, such as the role of multivisceral resection
and vascular options of reconstructions. The clinical expertise on
radical resection and venous reconstruction remains limited and
data regarding multimodal therapies, such as chemotherapy (CT),
radiation therapy (RT), or both (CRT) in combination with surgical
resection are scarce, with the optimal treatment strategy remaining
unclear.
In the present study, a series of seven patients
submitted to operative treatment of primary LMS of the IVC was
reviewed, and the effect of multivisceral resection on survival
rate and the options of venous reconstruction were analyzed.
Materials and methods
A retrospective review was performed on the medical
records of all the patients treated with upfront resection of
primary IVC LMS over a five-year period between June/2007 and
October/2013. Variables collected from the medical records included
demographic and clinical data, tumor location along the IVC,
adjuvant therapies received, surgical technique employed, and the
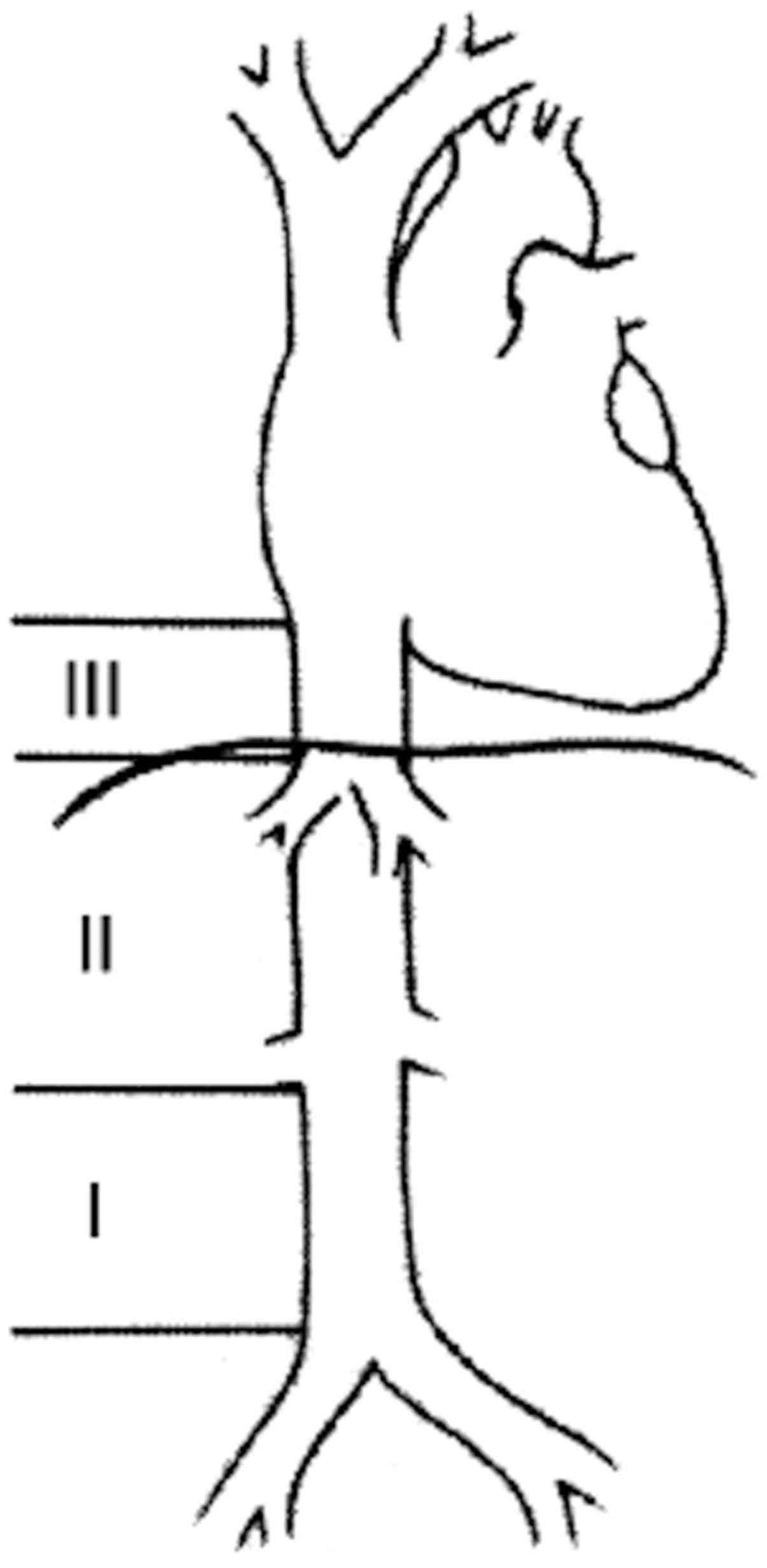
surgical pathology report. Segments of IVC affected by the tumor
were classified according to Kulaylat et al (12) as shown in Fig. 1, and tumor histology grade (I, II,
III) was reported according to the FNCLCC (Fédération Nationale des
Centres de Lutte Contre le Cancer or French Federation of Cancer
Centers Sarcoma Group) (13).
Multi-visceral resection was defined according to the en
bloc model (14,15) of tumor resection with adjacent tissue
graft reconstruction. Furthermore, data on the 30-day mortality,
30-day complication, disease-free survival (DFS) and overall
survival (OS) rates, and the site of recurrence were collected.
The primary endpoints of the study were
postoperative mortality and morbidity, and OS rate. Other
variables, such as status of resection secondary to radical
resection (R0, R1 or R2 resections) and the patency of the graft
utilized in the vascular reconstruction were reported as secondary
endpoints.
The classification by Kulaylat et al
(12) for an IVC tumor was classified
according to the level in the IVC: segment I, infrarenal; segment
II, inter- and supra-renal up to but not including the main
suprahepatic veins; and segment III, suprahepatic with possible
intracardiac extension.
OS rates were calculated using the time between the
date of surgery and the date of mortality or last contact. DFS was
defined as the time to local or distant tumor recurrence following
initial treatment. Kaplan-Meier estimator survival curves for OS
and DFS were determined (16). The
patients were followed up until they revealed the outcome of
interest or censoring by the date of the last follow-up.
Results
Patient 1
A 78-year-old male with a 4-month history of
abdominal pain was admitted to the pancreas and biliary tract
surgery service at the Clinics Hospital affiliated to the
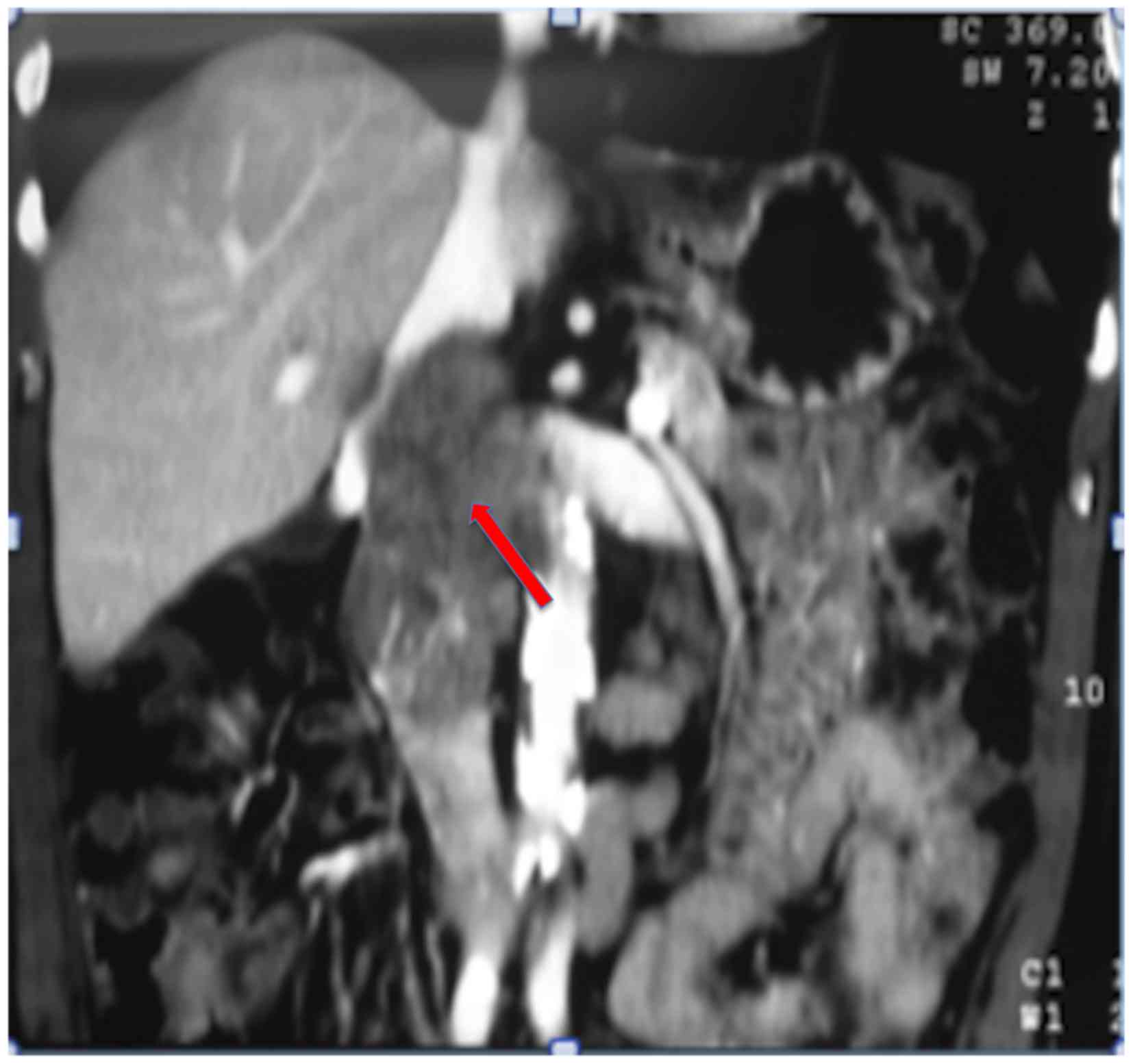
University of São Paulo (São Paulo, Brazil). A computed tomography
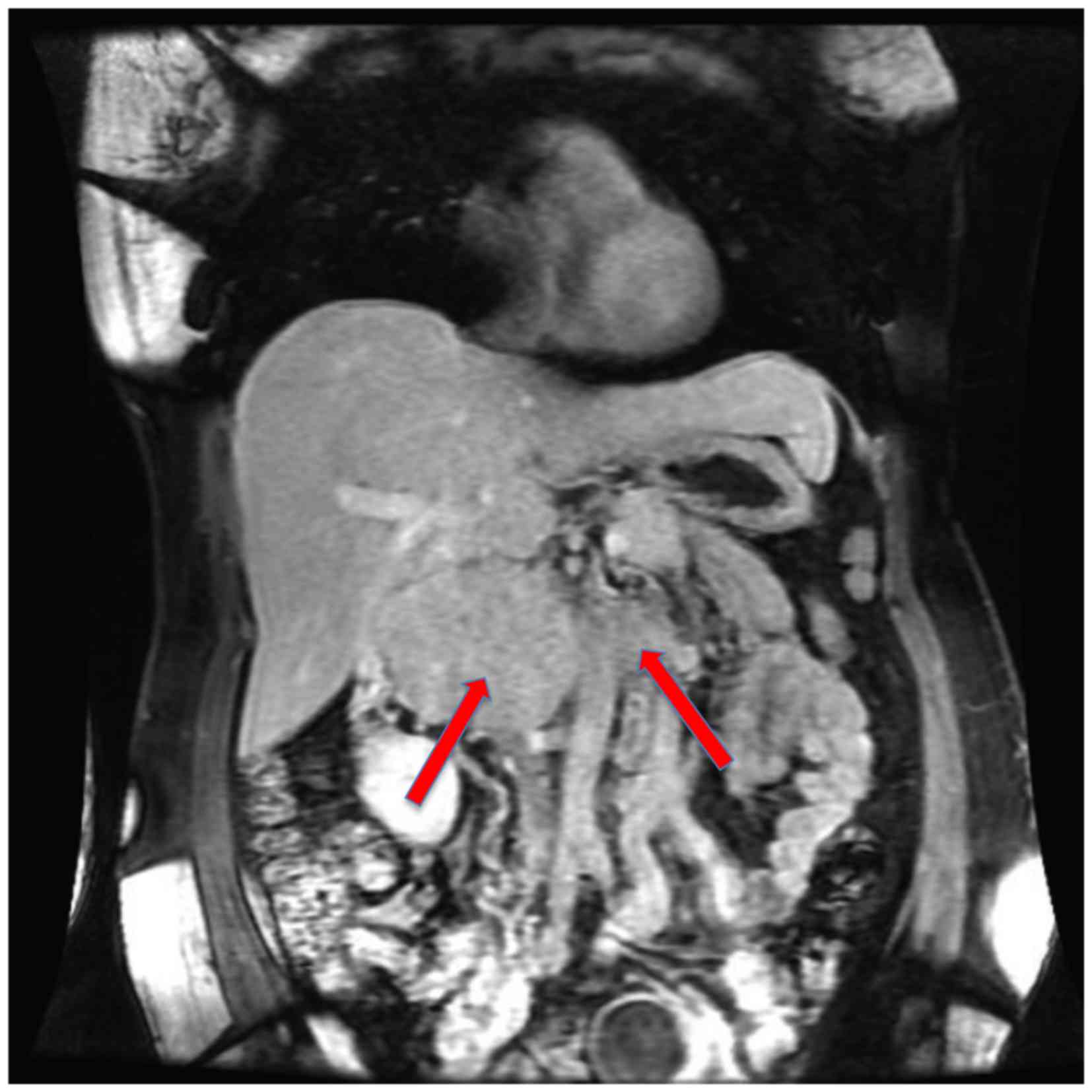
(CT) scan (Fig. 2) of the abdomen
revealed a 7.0 cm mass between the IVC and duodenum, which was
suspected as a primary IVC tumor. The patient was referred to the
surgical oncology group at the São Paulo Cancer Institute (São
Paulo, Brazil) for further evaluation. The vascular surgery and
surgical oncology teams planned the operative resection together.
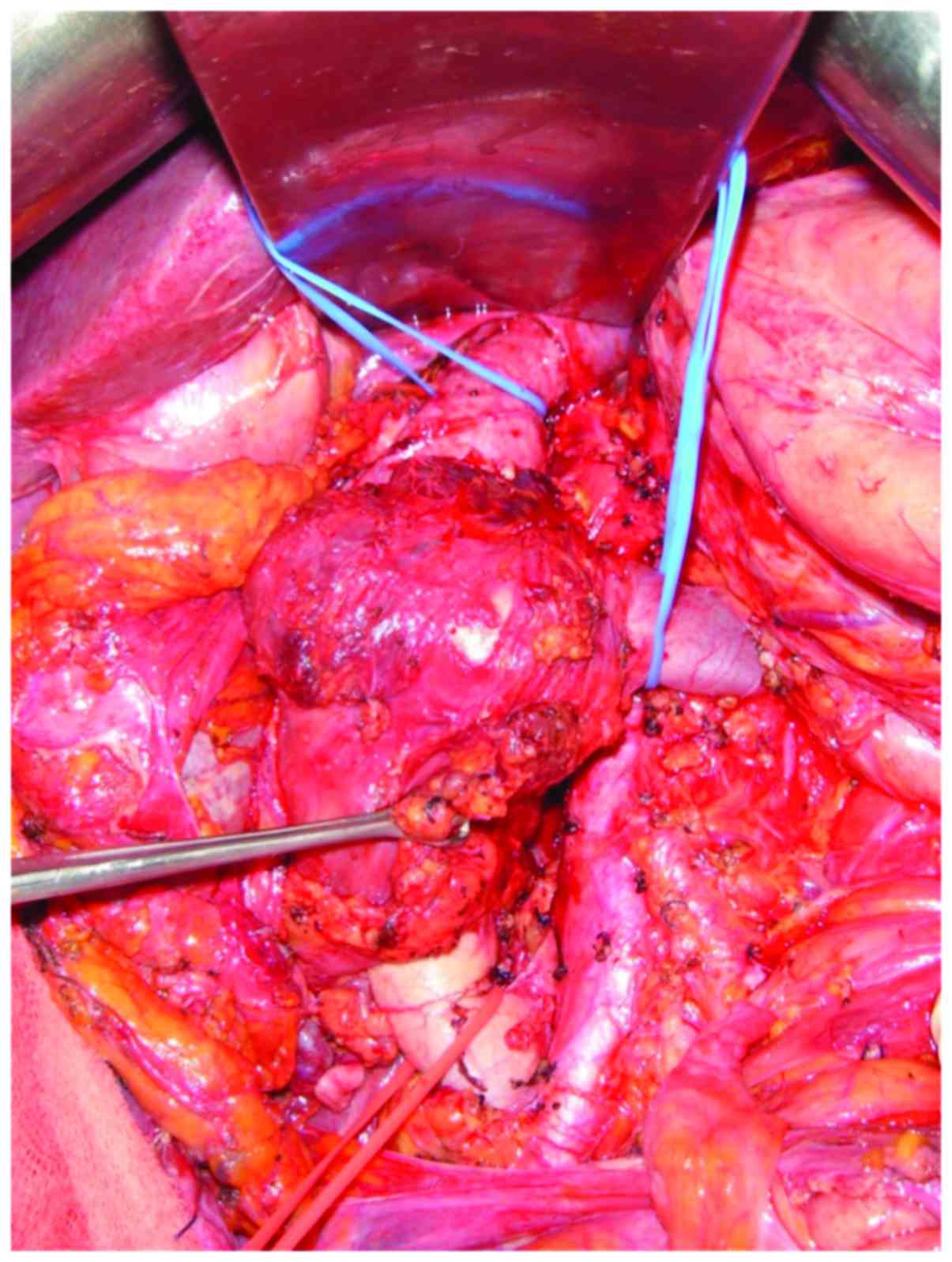
Exploration was performed via a midline incision. The mass was
identified arising from the IVC and involved the lower and middle
segment, and the right renal vein. Proximal and distal controls
from the IVC were obtained (Fig. 3),
and the mass and the right kidney were dissected free. The tumor
was excised en bloc with 9.5 cm of the IVC and the right
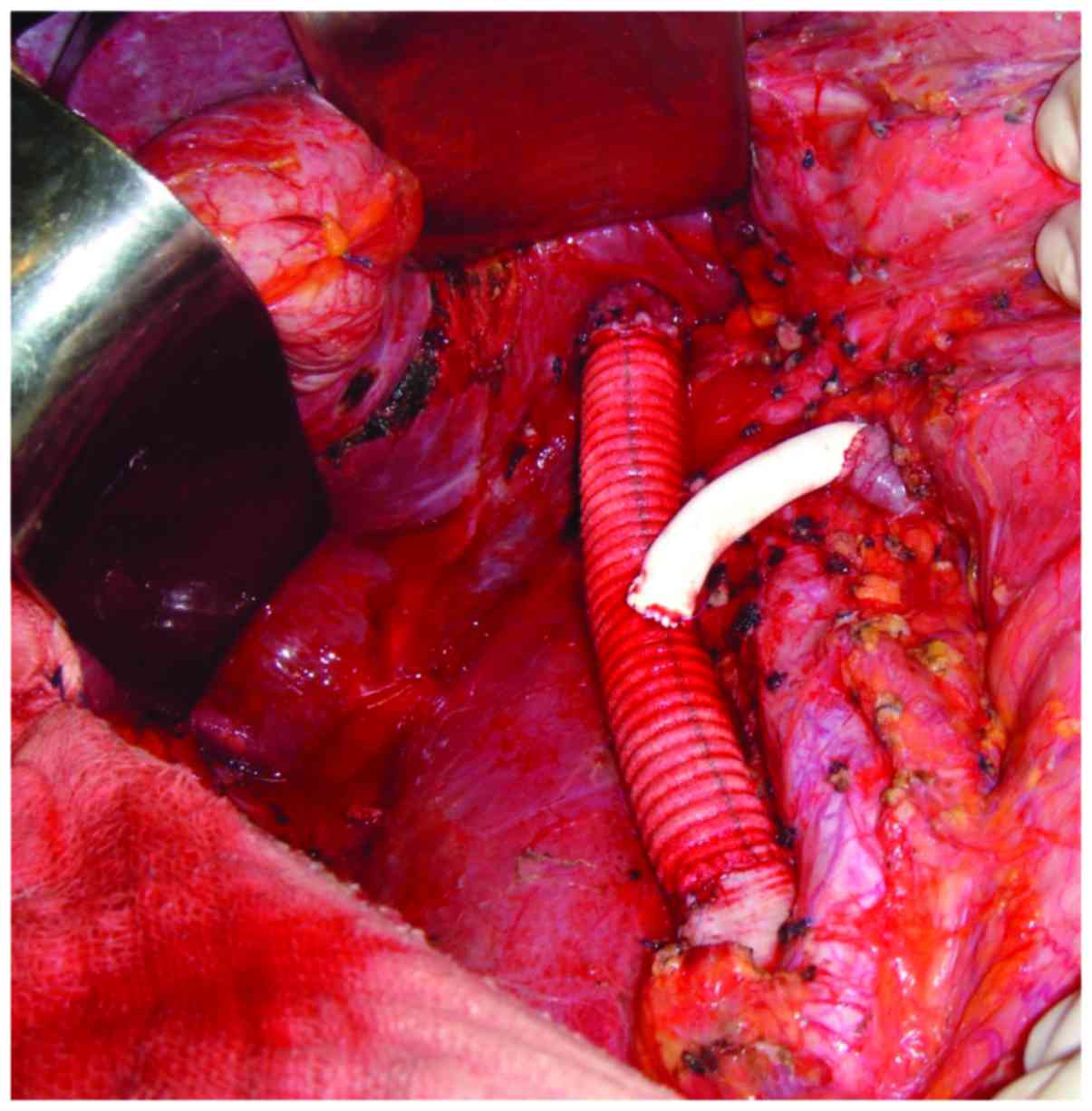
kidney with a 10 mm margin. IVC reconstruction was performed using
an 18-mm Dacron prosthetic graft. Left renal vein reconstruction
using a polytetrafluoroethylene (PTFE) prosthetic graft was
performed (Fig. 4). The patient was
heparinized. While on systemic anticoagulation, the patient was
submitted to reoperation due to a postoperative retroperitoneal
hematoma. The hematoma was evacuated and no active bleeding was
identified. The patient was discharged home on warfarin. Surgical
pathological examination demonstrated a multilobulated high-grade
8.0 cm LMS of the IVC with negative surgical margins. The patient
remained well for 28 months; however, multiple pulmonary nodules
were identified in the routine surveillance CT scan. The patient
subsequently underwent multiple metastasectomies with subsequent
recurrences. The patient succumbed to the disease 57 months
following the original resection.
Patient 2
A 68-year-old female with a six-month history of
right upper quadrant pain was found to have a 20 cm sub-hepatic
mass at the level of the right renal hilum. The patient was
admitted to the Heart Institute at the University of São Paulo and
was referred to the general surgical oncology group for further
evaluation. A CT scan of the abdomen revealed the mass arising from
the vena cava and extending into the lower segment of the IVC, with
compression of the right renal vein. Operative resection was
similar to the first case. Exploration was performed via a Mercedes
incision, and the tumor was identified along the IVC just below the
liver and the right renal vein. Proximal and distal controls from
the cava were obtained, and the mass and the right kidney were
dissected free. The IVC was clamped inferiorly 4 cm above the
bifurcation of the common iliac veins and superiorly in the
infra-hepatic segment of the IVC. The left renal vein was sectioned
near the IVC. A large mass was removed with 20 cm of the IVC en
bloc with the right kidney. Reconstruction of the IVC was
performed using a 20-mm Dacron graft and reconstruction of the left
renal vein was performed with an end-to-side anastomosis using a
6-mm PTFE graft. The postoperative course of the patient was
unremarkable. Surgical pathological examination demonstrated a
grade I (FNCLCC) 18×15×13 cm LMS of the IVC with negative surgical
margins. The patient was discharged on therapeutic enoxaparin.
There was no evidence of disease at the time of this report 39
months following the initial surgery.
Patient 3
A 34-year-old female with a six-month history of
right upper quadrant pain was found to have an 11 cm sub-hepatic
mass, which was suspected as a primary IVC tumor. A CT scan of the
abdomen revealed a mass arising from the vena cava and extending
into the lower segment of the IVC. Intraluminal filling defects
were detected, indicating the invasion of the tumor into the IVC
and bilateral renal veins (Fig. 5).
Vascular surgery and surgical oncology groups planned the operative
resection together. Following confirmation of the clinical staging
of the localized disease, the patient underwent surgery and a
bilateral subcostal incision combined with a median extension was
performed. The tumor was dissected free intra-abdominally and the
liver was mobilized. Thus, the IVC and bilateral renal veins were
clamped and the tumor was subsequently removed with a partial
resection of the subsegment IVb of the liver. The distal end of the
IVC was ligated and excluded. The proximal segment of the IVC was
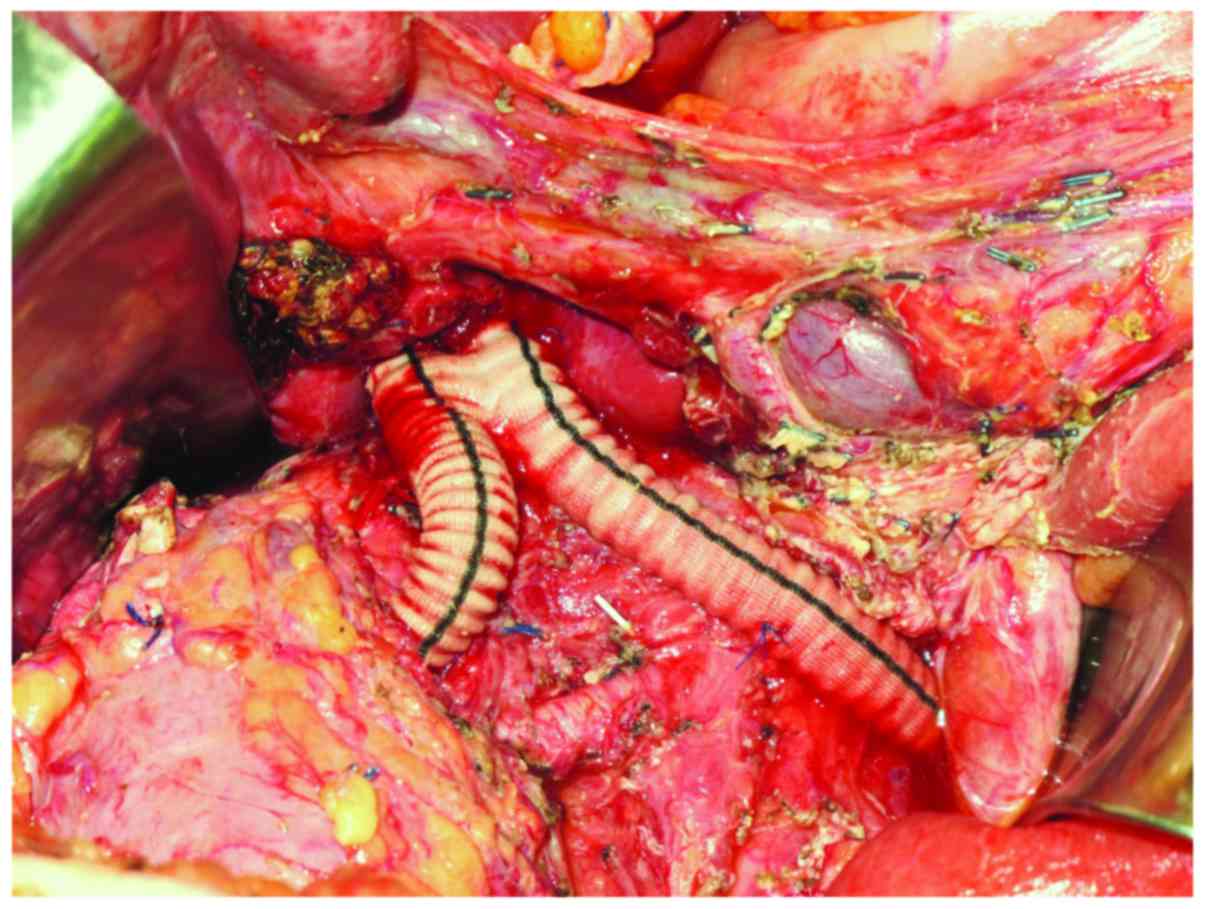
anastomosed to an 18-mm Dacron graft with 8-mm bilateral arms,
which were anastomosed to the bilateral renal veins (Fig. 6). Surgical pathological examination
demonstrated a grade III (FNCLCC) 12 cm LMS of the IVC with
negative surgical margins. The patient had an uneventful
postoperative recovery and was discharged home on the 22nd
postoperative day in good condition. Oral warfarin therapy was
administered for 6 months following discharge. After a follow-up
thoracic and abdominal CT scan 14 months postoperatively, the
patient was diagnosed with multiple pulmonary and hepatic
metastases. Systemic treatment with chemotherapy began and the
patient is alive with disease as of the last visit.
Patient 4
An 81-year-old female patient, who had atrial
fibrillation and was anticoagulated, was present with vague
abdominal pain for 3 months. The CT scan demonstrated an ~5 cm mass
arising from the IVC and inferior to the right renal vein with no
other associated abnormalities. The patient underwent surgical
resection of the mass, including complete resection of the IVC
below the level of the renal veins. No reconstruction was
performed. The patient had an uneventful postoperative recovery.
Pathological analysis revealed a high-grade 5.3 cm LMS of the IVC
with negative surgical margins. The patient has no evidence of
disease at the time of this report, 69 months following the initial
surgery. However, at 42 months the patient appeared in the
emergency department with vaginal bleeding. The pelvic CT scan
revealed an exuberant collateral circulation and multiple pelvic
varices. The bleeding stopped spontaneously. At present, the
patient is doing well with no more episodes of bleeding.
Patient 5
A 53-year-old female patient presented with right
lumbar pain for 2 months. An abdominal ultrasonography revealed a
4.5×4.5 cm mass, which was suspected as a primary tumor of the
right kidney. The patient was admitted to the urology service at
the Clinics Hospital. The CT scan demonstrated a 6.9×6.3 cm tumor
between the pancreas head, right kidney and liver, which was most
probably a primary IVC tumor. Preoperative endoscopic eco-guided
biopsy revealed fusiform cells with no atypia. The urology and
vascular surgery teams planned the operative procedure together.
Exploration was performed via a right subcostal incision. Complete
resection of the IVC below the level of the renal veins was
performed. Surgical margins were negative. IVC reconstruction was
performed using a 20-mm Dacron prosthetic graft. Pathological
analysis revealed an 8.0 cm grade II (FNCLCC) LMS of the IVC. The
postoperative course was unremarkable. Therapeutic anticoagulation
with enoxaparin was performed within 6 months. Thrombosis of the
graft was identified in an abdominal CT 3 months following
resection. A thoracic CT scan 4 months following surgery
demonstrated multiple small pulmonary nodules, which was suspected
as pulmonary metastasis. The last follow-up was 38 months following
diagnosis. The patient succumbed to the disease.
Patient 6
A 49-year-old female patient presented with right
lumbar pain for 1 month. The CT scan demonstrated a 9.2 cm
retroperitoneal mass suspected to be a right primary adrenal tumor
with invasion of the IVC. Biopsy guided by imaging revealed an LMS.
Surgical exploration was performed via a bilateral subcostal
incision. IVC ligation below the tumor was performed, as well as
ligation of the left renal vein. The retrohepatic IVC was sutured
with vascular reconstruction. The tumor was removed en bloc
with the right kidney and adrenal gland. Pathological analysis
revealed a 10.0×9.9×6.5 cm grade III (FNCLCC) LMS of the IVC
invading the renal hilum and parenchyma, and the adrenal gland. One
microscopic positive surgical margin was identified. Progression to
pulmonary metastasis was detected 8 months postoperatively. The
patient succumbed to the disease 48 months following resection of
the primary tumor.
Patient 7
A 53-year-old female patient presented with
abdominal pain. The CT scan revealed a heterogeneous 10.0×9.0×7.0
cm mass adjacent to the right kidney and invading the posterior
wall of the IVC. Following clinical staging, a complete macroscopic
resection was performed by the surgical urology team with ligation
of the IVC just below the right renal vein. No vascular
reconstruction was attempted. Pathological analysis revealed an
11.5×7.2×6.5 grade II (FNCLCC) LMS of the IVC with narrow margins.
The CT scan 4 months postoperatively revealed atrophy of the right
kidney with thrombosis of the right renal vein. Following 49 months
from the resection of the primary tumor, the patient is doing well
with no evidence of disease.
Summary of patient
characteristics
Out of the seven patients evaluated in the present
study, only one was male (Table I).
At hospital admission, the average age of patients was 59 years
(standard deviation, 15.6 years). All the patients were initially
treated with surgical resection first. Clinicopathological
characteristics of patients are described in Table I. Four patients were submitted to
en bloc resection (right kidney, 3; right adrenal gland, 1;
segmental hepatic resection, 1).
 | Table I.Surgical oncological features and
vascular reconstructions of patients with leiomyosarcoma of the
IVC. |
Table I.
Surgical oncological features and
vascular reconstructions of patients with leiomyosarcoma of the
IVC.
| Patient | Gender | IVC segment | R status | Organs
resected | IVC/LRV
reconstruction | RFS, months | Recurrence
site | Follow-up,
months | Current status |
|---|
| 1 | M | II | R0 | Right kidney | VC 18 mm Dacron
graft + PTFE on LRV | 28 | Lung | 57 | Succumbed |
| 2 | F | II | R0 | Right kidney | VC 20 mm Dacron
graft + 6 mm PTFE on LRV | – | None | 39 | Alive with NED |
| 3 | F | II | R0 | IVb liver
segment | Distal VC ligation
+ 18 mm Dacron with bilateral 8 mm RVA | 14 | Lung/liver | 46 | Alive with
disease |
| 4 | F | I | R0 | None | Both IVC segments
ligated with no vascular reconstruction | – | None | 69 | Alive with NED |
| 5 | F | I | R0 | None | VC 20 mm Dacron
graft | 38 | Lung | 38 | Succumbed |
| 6 | F | II | R1 | Right kidney and
adrenal | Both IVC segments
ligated with no reconstruction | 8 | Lung | 48 | Succumbed |
| 7 | F | I | R0 | None | Both IVC segments
ligated with no reconstruction | – | None | 49 | Alive with NED |
Reconstruction of the IVC was performed in 4
patients using Dacron grafts. Reconstruction of the left renal vein
was performed in 3 patients using PTFE grafts. No patients
underwent resection on cardiopulmonary or venovenous bypass. All
the patients underwent complete resection of the tumor and
microscopic-free surgical margins were accomplished in 6
patients.
The median tumor size was 10 cm [interquartile range
(IQR) 25–75%, 8–12 cm]. The tumor grade and follow-up for all the
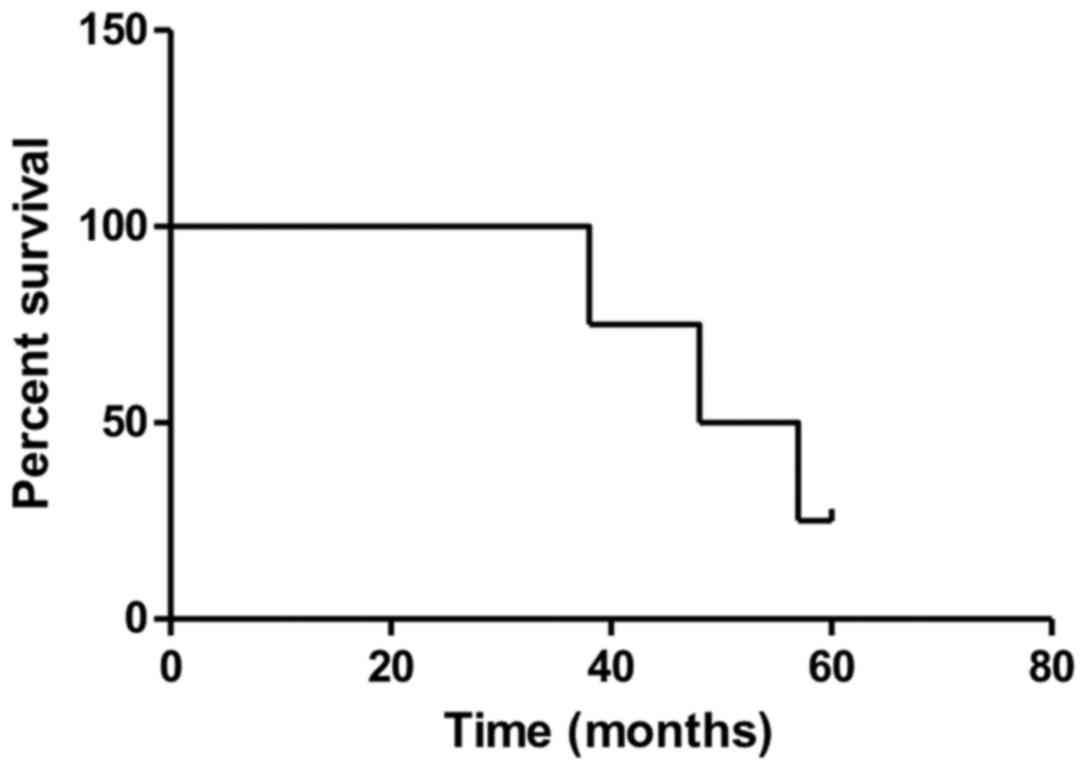
patients was reported. The OS rates were 100, 60 and 25% at 3, 4,
and 5 years (Fig. 7). The median OS
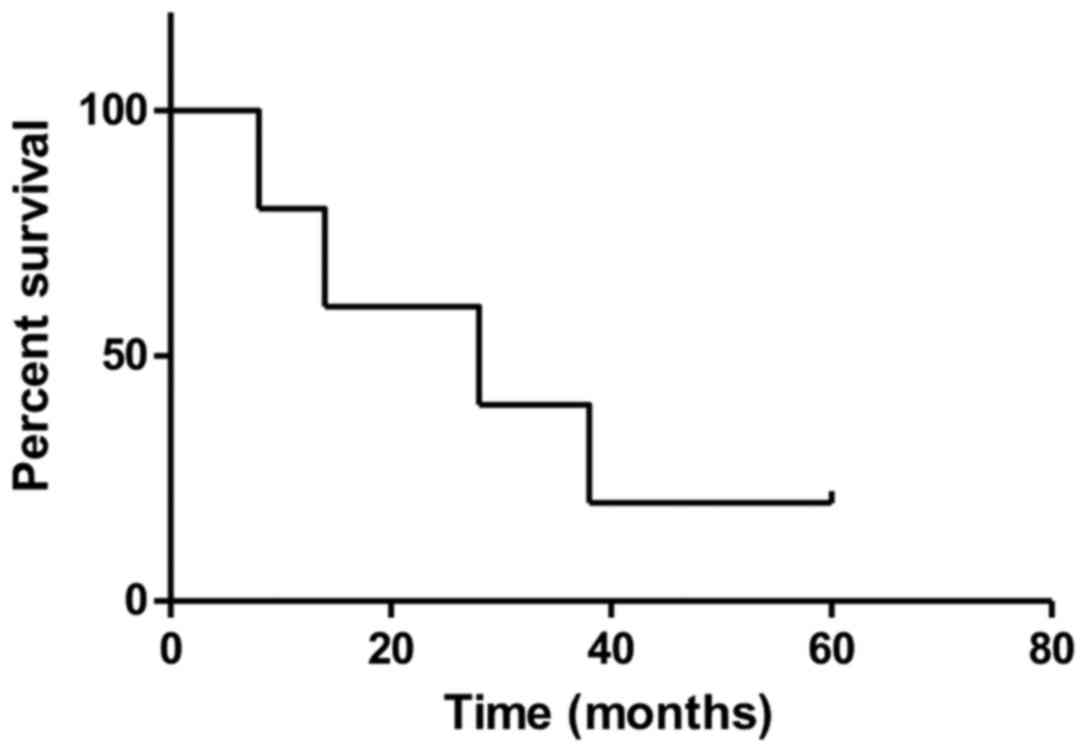
time was 53 months (IQR, 46–58 months). The DFS rates were 57, 33
and 20% at 3, 4, and 5 years (Fig.
8). The median DFS was 36 months (IQR, 21–46 months).
Discussion
LMS is one of the most frequent types of
retroperitoneal soft tissue sarcoma. For all newly diagnosed soft
tissue sarcoma, the estimated incidence of LMS ranges between 10
and 20% (17). However, vascular LMS
constitutes 1–2% of all soft tissue sarcoma and is associated with
poor prognosis (18). In a postmortem
examination in 1871, Perl et al (19) described the first LMS of the IVC.
Since then, <400 cases have been reported in the English
literature (3,4). This rare type of tumor originates from
the smooth muscle of the venous wall. Histological description
revealed that they are composed of fascicles of spindle cells. The
nuclei are hyperchromatic and there is abundant eosinophilic
cytoplasm. The pleomorphism is exuberant and occasionally resembles
an undifferentiated soft tissue sarcoma (20). LMS of the IVC predominates in females.
Its incidence peak is in the fifth decade of life (>75% of the
cases reported). The female/male ratio is ~3:1 (4).
Macroscopic surgical resection is the primary
curative treatment for patients with localized disease. This is the
only potentially curative therapy since the first resection of the
LMS of the IVC at Lexington Memorial Hospital in Chicago, in 1951
(21). However, complete surgical
removal of LMS of the IVC does not confer long-term survival for
all patients. Furthermore, the role of the multivisceral resections
remains controversial. Adjuvant therapeutic strategies, such as
systemic CT, RT (preoperative, postoperative or intraoperative) or
CRT are considered; however, the clinical benefits of these
treatments have not been clearly determined (5). The role of vascular reconstruction of
the IVC remains controversial. There is no consensus regarding
which option should be elected (primary repair, ligation or
reconstruction). All venous surgical treatment options have been
previously utilized (1,3,7,22–24).
The majority of patients described in the present
study presented with abdominal pain, which is the first indication
for upfront resection when feasible. Approximately 60% of the
patients presented with non-specific abdominal pain (4). Survival rates are more improved when
patients are treated with extended resection compared with those
who are managed with medical therapy alone (1,3). For
patients treated with non-surgical therapy, prognosis is poor and
survival time is measured in months (3,25). It
appears that complete macroscopic resection confers the longest
survival rates. Hollenbeck et al (1) reviewed a series of 25 patient cases that
underwent excisional therapy. A 3-year OS rate of 76% was observed
for patients with LMS of the IVC that had been completed removed
and 0% OS was observed when incomplete resections were performed
(1). In a previous case report on 14
patients, Hines et al (23)
reported a 5-year survival rate of 68% for patients with negative
margins identified upon pathological analysis compared with 0% for
patients with positive margins. Generally, a tumor free margin of 1
cm is necessary for soft tissue sarcoma. The proximity with various
organs may demand an en bloc multi-organ resection involving
anatomical structures, such as the aorta, kidneys, adrenal, liver
and colon (26).
In the present study, ~50% of patients underwent
en bloc resection of the tumor with ≥1 adjacent organs in
order to obtain free surgical margins. Right nephrectomy (60%),
right adrenalectomy (27%) and partial hepatectomy (20%) are the
most of the resected organs. En bloc resection was
demonstrated to be associated with a decrease in OS rate in a
recent pooled data analysis of 377 patients (4). The effect of clear margins on OS rate
remains unclear, according to studies conducted by Wachtel et
al (4) and Hines et al
(23), the effect of R0 or R1
resections OS and DFS rates are indifferent. However, macroscopic
positive margins have been identified to be associated with worse
prognosis, ~0% in 5 years, addressing the role of multivisceral
resection in appropriate cases.
In the present case series, the median survival of
21 months confirmed that radical resections of the LMS of the IVC
with the intention of obtaining a complete macroscopic resection
with negative margins should be the aim for those with localized
disease and acceptable clinical performance. All the patients
achieved complete macroscopic resections and only one patient had
microscopic positive surgical margins. This patient succumbed to
the disease 48 months following resection of the primary tumor. The
results of previous studies have revealed that microscopic positive
surgical margins have no effect on DFS and OS rates (1,8,23).
One issue following radical resection is the
reconstruction of the IVC and occasionally the left renal vein.
Several options exist when vascular reconstruction is considered
(5,27–31). Small
caval defects can be closed with primary sutures or with a
saphenous patch. However, in numerous patients circumferential
resection of the IVC is required. When a complete IVC thrombosis
has been revealed and the tumor of the IVC is localized to level I,
vascular reconstruction may not be necessary. Collateral
circulation is usually present and when patients develop leg edema,
in general, it is well tolerated. Some advantages of this approach
are the reduced operative time, no risk of synthetic graft
infection and no need for prolonged anticoagulation, reducing the
risk of reoperation due to bleeding. One of the patients discussed
in the present report developed a retroperitoneal hematoma
secondary to systemic anticoagulation requiring re-laparotomy. This
has also been reported in other studies (4).
In a long-term follow-up, no lower extremity edema
was observed in patients who underwent IVC reconstructions in the
current series. In a previous study, lower extremity edema was
considered significant in 50% of patients when no IVC
reconstruction is undertaken (26).
Late patency of the synthetic tube graft was observed in 75% of
patients and no significant lower extremity edema was observed when
thrombosis of the graft occurred (29). A previous study revealed patency rates
of 95% 5 years following IVC reconstruction (32). In the present study, ligation of the
IVC was performed in one patient and following several months the
patient developed pelvic varices, and presented in the emergency
department with vaginal bleeding secondary to varices. It was an
isolated event without hemodynamic instability and the bleeding
stopped spontaneously. Whether or not IVC reconstruction may
prevent this type of complication in long-term survival patients
remains unclear, but the long-term complications secondary to IVC
ligation are of concern (23).
When the patients presented with a patent IVC,
vascular reconstructions were performed, as identified by previous
results (8,27,29,30). We
suggest maintaining the venous return using synthetic tube grafts
whenever the patency of the IVC is confirmed preoperatively through
diagnostic imaging tests. For patients with level II LMS of the IVC
the option to perform IVC reconstruction considers vicariation of
the collateral circulation to guarantee the venous return of the
left kidney. The IVC and bilateral renal veins can be excluded
through ligation when preoperative imaging tests revealed exuberant
vicariation of collateral vessels (33). However, the risk of acute renal
failure following right nephrectomy and ligation of the left renal
vein is a concern. Vascular prosthesis to reconstruct the left
renal vein is recommended to maintain the venous outflow and avoid
renal dysfunction (34–36).
In the present study, two patients with level II LMS
underwent an end-to-side anastomosis between the left renal vein
and an 18-mm Dacron prosthesis was used to reconstruct the IVC. A
6-mm PTFE between the left renal vein and Dacron were chosen to
accomplish the left renal outflow. In another patient with level II
LMS of the IVC it was possible to remove the tumor and maintain
both kidneys. The distal end of the IVC was ligated and excluded,
and the bilateral veins were clamped. Vascular reconstruction was
performed creating an anastomosis between the cranial stump of the
IVC and an 18-mm Dacron graft was used with bilateral 8-mm arms
that were anastomosed to the bilateral renal veins. This type of
reconstruction has previously been reported in other studies
(34). All 3 patients remained
asymptomatic and did well throughout follow-up without renal
dysfunction.
In conclusion, LMS of the IVC is a rare
retroperitoneal sarcoma, and radical resection is the only
therapeutic option capable of conferring long-term survival. To
obtain complete macroscopic resection, removal of adjacent organs
is usually necessary. Microscopic-free surgical margins are
necessary but its effect on long-term survival remains unclear.
Venous reconstruction is selectively indicated. There is no
consensus, but in general, when partial obstruction of the IVC
occurs the reconstruction of the IVC is encouraged.
References
|
1
|
Hollenbeck ST, Grobmyer SR, Kent KC and
Brennan MF: Surgical treatment and outcomes of patients with
primary inferior vena cava leiomyosarcoma. J Am Coll Surg.
197:575–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laskin WB, Fanburg-Smith JC, Burke AP,
Kraszewska E, Fetsch JF and Miettinen M: Leiomyosarcoma of the
inferior vena cava: Clinicopathologic study of 40 cases. Am J Surg
Pathol. 34:873–881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mingoli A, Cavallaro A, Sapienza P, Di
Marzo L, Feldhaus RJ and Cavallari N: International registry of
inferior vena cava leiomyosarcoma: Analysis of a world series on
218 patients. Anticancer Res. 16:3201–3205. 1996.PubMed/NCBI
|
|
4
|
Wachtel H, Gupta M, Bartlett EK, Jackson
BM, Kelz RR, Karakousis GC, Fraker DL and Roses RE: Outcomes after
resection of leiomyosarcomas of the inferior vena cava: A pooled
data analysis of 377 cases. Surg Oncol. 24:21–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito H, Hornick JL, Bertagnolli MM, George
S, Morgan JA, Baldini EH, Wagner AJ, Demetri GD and Raut CP:
Leiomyosarcoma of the inferior vena cava: Survival after aggressive
management. Ann Surg Oncol. 14:3534–3541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dew J, Hansen K, Hammon J, McCoy T, Levine
EA and Shen P: Leiomyosarcoma of the inferior vena cava: surgical
management and clinical results. Am Surg. 71:497–501.
2005.PubMed/NCBI
|
|
7
|
Kieffer E, Alaoui M, Piette JC, Cacoub P
and Chiche L: Leiomyosarcoma of the inferior vena cava: Experience
in 22 cases. Ann Surg. 244:289–295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann GN, Mann LV, Levine EA and Shen P:
Primary leiomyosarcoma of the inferior vena cava: A 2-institution
analysis of outcomes. Surgery. 151:261–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wachtel H, Jackson BM, Bartlett EK,
Karakousis GC, Roses RE, Bavaria JE and Fraker DL: Resection of
primary leiomyosarcoma of the inferior vena cava (IVC) with
reconstruction: A case series and review of the literature. J Surg
Oncol. 111:328–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hardwigsen J, Balandraud P, Ananian P,
Saïsse J and Le Treut YP: Leiomyosarcoma of the retrohepatic
portion of the inferior vena cava: Clinical presentation and
surgical management in five patients. J Am Coll Surg. 200:57–63.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramponi F, Kench JG, Simring DV, Crawford
M, Abadir E and Harris JP: Early diagnosis and resection of an
asymptomatic leiomyosarcoma of the inferior vena cava prior to
caval obstruction. J Vasc Surg. 55:525–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kulaylat MN, Karakousis CP, Doerr RJ,
Karamanoukian HL, O'Brien J and Peer R: Leiomyosarcoma of the
inferior vena cava: A clinicopathologic review and report of three
cases. J Surg Oncol. 65:205–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trojani M, Contesso G, Coindre JM, Rouesse
J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F and
Lagarde C: Soft-tissue sarcomas of adults; study of pathological
prognostic variables and definition of a histopathological grading
system. Int J Cancer. 33:37–42. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gronchi A, Miceli R, Colombo C,
Stacchiotti S, Collini P, Mariani L, Sangalli C, Radaelli S,
Sanfilippo R, Fiore M and Casali PG: Frontline extended surgery is
associated with improved survival in retroperitoneal low- to
intermediate-grade soft tissue sarcomas. Ann Oncol. 23:1067–1073.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toulmonde M, Bonvalot S, Méeus P, Stoeckle
E, Riou O, Isambert N, Bompas E, Jafari M, Delcambre-Lair C and
Saada E: Retroperitoneal sarcomas: Patterns of care at diagnosis,
prognostic factors and focus on main histological subtypes: A
multicenter analysis of the French Sarcoma Group. Ann Oncol.
25:735–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
17
|
Serrano C and George S: Leiomyosarcoma.
Hematol Oncol Clin North Am. 27:957–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon TW, Sung KB, Cho YP, Kim DK, Yang SM,
Ro JY and Kim GE: Pararenal leiomyosarcoma of the inferior vena
cava. J Korean Med Sci. 18:355–359. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perl L: Ein fall von sarkom der vena cava
inferior. Arch Path Anat. 53:378–383. 1871. View Article : Google Scholar
|
|
20
|
Chen E, O'Connell F and Fletcher CD:
Dedifferentiated leiomyosarcoma: Clinicopathological analysis of 18
cases. Histopathology. 59:1135–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cope JS and Hunt CJ: Leiomyosarcoma of
inferior vena cava. AMA Arch Surg. 68:752–756. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JT, Kwon T, Cho Y, Shin S, Lee S and
Moon D: Multidisciplinary treatment and long-term outcomes in six
patients with leiomyosarcoma of the inferior vena cava. J Korean
Surg Soc. 82:101–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hines OJ, Nelson S, Quinones-Baldrich WJ
and Eilber FR: Leiomyosarcoma of the inferior vena cava: Prognosis
and comparison with leiomyosarcoma of other anatomic sites. Cancer.
85:1077–1083. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mastoraki A, Leotsakos G, Mastoraki S,
Papanikolaou IS, Danias N, Smyrniotis V and Arkadopoulos N:
Challenging diagnostic and therapeutic modalities for
leiomyosarcoma of inferior vena cava. Int J Surg. 13:92–95. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cacoub P, Piette JC, Wechsler B, Ziza JM,
Blétry O, Bahnini A, Kieffer E and Godeau P: Leiomyosarcoma of the
inferior vena cava. Experience with 7 patients and literature
review. Medicine (Baltimore). 70:293–306. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daylami R, Amiri A, Goldsmith B, Troppmann
C, Schneider PD and Khatri VP: Inferior vena cava leiomyosarcoma:
Is reconstruction necessary after resection? J Am Coll Surg.
210:185–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bower TC, Nagorney DM, Cherry KJ Jr,
Toomey BJ, Hallett JW, Panneton JM and Gloviczki P: Replacement of
the inferior vena cava for malignancy: An update. J Vasc Surg.
31:270–281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kraybill WG, Callery MP, Heiken JP and
Flye MW: Radical resection of tumors of the inferior vena cava with
vascular reconstruction and kidney autotransplantation. Surgery.
121:31–36. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hardwigsen J, Baqué P, Crespy B,
Moutardier V, Delpero JR and Le Treut YP: Resection of the inferior
vena cava for neoplasms with or without prosthetic replacement: A
14-patient series. Ann Surg. 233:242–249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarkar R, Eilber FR, Gelabert HA and
Quinones-Baldrich WJ: Prosthetic replacement of the inferior vena
cava for malignancy. J Vasc Surg. 28:75–83. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Illuminati G, Calio' FG, D'Urso A,
Giacobbi D, Papaspyropoulos V and Ceccanei G: Prosthetic
replacement of the infrahepatic inferior vena cava for
leiomyosarcoma. Arch Surg. 141:919–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Quinones-Baldrich W, Alktaifi A and Eilber
F and Eilber F: Inferior vena cava resection and reconstruction for
retroperitoneal tumor excision. J Vasc Surg. 55:1386–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamaguchi R, Yamaguchi A, Isogai M, Hori A
and Kin Y: Leiomyosarcoma of the inferior vena cava. Resection and
reconstruction of the renal vein using the gonadal vein. Surg
Today. 28:359–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Jiang J, Wang C, Lian G, Jin MS
and Cao X: Leiomyosarcoma of the inferior vena cava level II
involvement: Curative resection and reconstruction of renal veins.
World J Surg Oncol. 10:1202012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Angiletta D, Fullone M, Greco L, Marinazzo
D, Frontino P and Regina G: Leiomyosarcoma of the inferior vena
cava: Resection and vascular reconstruction using a dacron graft
and an Adam DeWeese clip-three-year follow-up. Ann Vasc Surg.
25(557): e5–e9. 2011.
|
|
36
|
Arkadopoulos N, Karmaniolou I,
Ekonomopoulos N, Vassiliu P and Smyrniotis V: Combination of total
abdominal inferior vena cava resection with a novel technique of
left renal outflow restoration. Surgery. 152:142–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|