Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a
subset of acute leukemia and an aggressive hematological
malignancy. T-ALL has the propensity to metastasize to other organs
of the body. Chemokines and their receptors have been established
to be important contributors to the metastatic potential of tumors
(1,2).
The interplay between chemokines, cells and the microenvironment is
complex and involves numerous other cellular molecules, including
ezrin/radixin/moesin and nuclear factor of activated T cells.
Sodium-hydrogen antiporter 1 (NHE1) is a molecule that may interact
with the cellular cytoskeleton and aid the formation of signaling
complexes, in addition to having an ion exchange function (3,4). NHE1 is a
transmembrane protein that consists of twelve segments and extrudes
a H+ in exchange for an extracellular Na+; it
is involved in the regulation of cellular pH, ion composition and
cellular volume (5), thereby serving
a role in diseases associated with the heart, blood vessels and
kidneys (6). NHE1 is also involved in
enzyme activation that degrades the extracellular matrix and is
therefore produced by invasive tissues, including breast cancer
cells (7,8). NHE1 is localized in the lamellipodia of
fibroblast cells, where it acts as an anchor at the cell membrane
for cellular cytoskeleton by directly binding to the
ezrin/radixin/moesin (ERM) family of actin-binding proteins
(9). The ERM family is established to
be important molecules in cytoskeletal activity as they link actin
to the plasma membrane proteins. ERMs are frequently identified to
be highly expressed in numerous cancer types and are important
mediators of cell survival signaling (10–12).
Previous studies have suggested that high ezrin expression may be
associated with cellular motility, therefore having a role in
metastasis and invasion (13–15). ERM proteins have previously been
identified to be important in T-ALL drug resistance with ezrin, an
important molecule in C-C-motif chemokine ligand 25 (CCL25)-induced
T-ALL metastasis (12,16).
The role of chemokines in cancer has been the focus
of a substantial amount of previous research. C-C chemokine
receptor type 9 (CCR9) is a G-protein-coupled receptor that is
primarily expressed on immature T cells, with restricted expression
in mature T cells (17,18). CCR9 has been identified to be
important in T-cell development and T-cell tissue-specific homing
once it is bound to its specific ligand CCL25 (12). A previous study indicated that CCR9 is
highly expressed in the T-ALL MOLT4 cell line and also that CCL25
induces chemotherapeutic drug resistance in T-ALL (19) and effectively polarizes MOLT4 cells
with ERM redistribution (12).
In addition, NHE1 activity has the ability to induce
chemotherapeutic resistance against drugs, including DOX and
imatinib, in renal and other cancer types (3,20–22). These results suggest that NHE1 has a
vital role in cell survival and proliferation.
Using the T-ALL MOLT4 cell line as a model, the
present study investigated whether NHE1 has a role in the
metastatic transformation of MOLT4 cells and whether ezrin
associates with NHE1 in metastatic MOLT4 cells.
Materials and methods
Cell culture
The human T-ALL MOLT4 cell line was obtained from
American Type Culture Collection (Manassas, VA, USA). Ev-ve MOLT4
cells (ezrin-silenced MOLT4 cells) were generated as previously
reported (16). The cells were
maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Hyclone; GE Healthcare, Logan, UT, USA) and cultured in a 5%
CO2 air incubator at 37°C.
NHE1 short interfering (si)RNA
transfection
The transfection was performed using Lipofectamine
2000 reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol in MOLT4 cells. In brief, the cells were
suspended in serum-free RPMI 1640 medium and plated in 6-well
plates at a density of 5×105 cells/ml, 2 ml per well. A
total of 2 µl siRNA (100 mmol/ml) was added to each well. Following
incubation for 8 h, 200 µl FBS was added to each well. The cells
were incubated at 37°C for 48 h and used for further experiments.
The siRNA sequences were as follows: NHE1 sense,
5′-GAUAGGUUUCCAUGUGAUCTT-3′ and anti-sense,
5′-GAUCACAUGGAAACCUAUCTT-3′; and negative control (scrambled)
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cariporide treatment
The MOLT4 cells were plated on 6-well plates at a
density of 5×105 cells/ml, 2 ml per well. The cells were
treated with 20 µM cariporide (Santa Cruz, Biotechnology, Inc.,
Dallas, TX, USA) and incubated for 24 h in RPMI 1640 medium with
10% FBS prior to use in the Transwell chamber assay.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from MOLT4 cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol and quantified using NanoDrop 2000
(Thermo Fisher Scientific, Inc.). A total of 2 µg RNA was
reverse-transcribed to cDNA with random primers using the Reverse
Transcription system (MyCycler™ thermal cycler, Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to manufacturer's
protocol. RT-PCR was performed with the Takara RNA PCR kit (Takara
Bio, Inc., Otsu, Japan). The primer sequences were as follows: NHE1
forward, 5′-GCCTTCTCTCTGGGCTACCT-3′ and reverse,
5′-CTTGTCCTTCCAGTGGTGGT-3′. The PCR thermocycling protocol was as
follows: 95°C for 1 min and then 35 cycles of 95°C for 30 sec,
annealing at 64°C for 30 sec and elongation at 72°C for 30 sec
followed by 95°C for 5 min once at the end of protocol. The PCR
products were separated by 1.5% agarose gel electrophoresis and
visualized under ultraviolet light following staining with ethidium
bromide.
Protein extraction and western blot
analysis
The cells were washed with PBS and lysed with
radioimmunoprecipitation assay lysis and extraction buffer (Thermo
Fisher Scientific, Inc.) containing protease inhibitor (1 mM
phenylmethylsulfonyl fluoride; Thermo Fisher Scientific, Inc.). The
total protein concentration was measured using the Pierce
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.) on the PerkinElmer 2030 VICTOR X Multilabel Plate Reader
(PerkinElmer, Inc., Waltham, MA, USA). Whole-cell lysates were
boiled at 100°C for 5 min in equal volumes of loading buffer
(Beyotime Institute of Biotechnology, Haimen, China). All samples
(15 µl per lane) were subjected to 10% SDS-PAGE and transferred to
PVDF membranes. Subsequent to blocking for 2 h at room temperature
in TBS-Tween 20 (TBST) containing 5% skimmed milk to block
membranes, the membranes were incubated with anti-NHE1 (dilution,
1:1,000; catalog no., ab67314; Abcam, Cambridge, MA, USA) and
anti-ezrin (dilution, 1:500; catalog no., ab41672; Abcam) primary
antibodies diluted in TBST containing 5% non-fat milk with gentle
shaking at 4°C overnight. Subsequent to washing three times, the
membranes were incubated with the 1:200 horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:200;
catalog no., GB23303; Wuhan Biological Technology Co., Ltd., Wuhan,
China) for 2 h at room temperature. The signals were detected using
an Enhanced Chemiluminescence Detection kit (Thermo Fisher
Scientific, Inc.).
Scanning electron microscopy
assay
The MOLT4 cells were overlaid on coated coverslips
and fixed with 2.5% glutaraldehyde in PBS overnight at 4°C.
Following a PBS wash, the cells were fixed in 1% osmium tetroxide
at 4°C for 2 h and washed thoroughly with distilled water.
Subsequently, the post-fixed specimens were dehydrated using a
graded series of ethanol and then transferred to a graded series of
t-butyl alcohols (freezing point 25.4°C), the t-butyl alcohol
substituted specimens were freeze-dried at 15°C and at high vacuum
(23). The experimental specimens
were sputter-coated with platinum and then observed using a
scanning electron microscope (S-750; Hitachi, Ltd., Tokyo, Japan)
operating at 20 kV (24).
Laser confocal microscopy assay
The MOLT4 cells were overlaid on glass slides, fixed
at 4°C in 4% paraformaldehyde, permeabilized at 4°C with 0.1%
Triton X-100 and then blocked for 1 h with 3% bovine serum albumin
(Gibco; Thermo Fisher Scientific, Inc.) at room temperature. The
cells were incubated with 1:1,000 anti-NHE1 (catalog no., ab67314;
Abcam) and 1:500 anti-ezrin (catalog no., ab41672; Abcam)
antibodies at 4°C overnight. Later, Alexa Fluo® 488
donkey anti-rabbit IgG (H+L) (dilution, 1:200; catalog no., ANT024;
AntGene Biotechnology Co., Ltd., Wuhan, China) and Alexa
Fluo® 594 donkey anti-rabbit IgG (H+L) (dilution, 1:200;
catalog no., ANT030; AntGene Biotech Co., Ltd.) secondary antibody
was added to the cells and incubated for 2 h at room temperature.
Experimental specimens were observed using a LCS-SP2-MP-AOB laser
confocal scanning microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Transwell assay
The chemotaxis assay was performed in a 24-Transwell
chamber (Corning Costar, USA). In brief, MOLT4 cells (100 µl,
1×106 cells/ml) were suspended in RPMI 1640 medium
containing 0.1% FBS and added to the upper chamber of the
Transwell, which was separated from the lower well by a 8-µm
pore-size polycarbonate membrane. CCL25 (100 ng/ml; Cedarlane
Laboratories, Burlington, Canada) in RPMI 1640 medium containing
0.1% FBS was placed in the lower wells, at a quantity of 600 µl per
well. The cells were allowed to migrate for 24 h at 37°C with 5%
CO2 atmosphere. After 24 h, cells in the lower chamber
were counted under a light microscope with a blood counting chamber
at ×400 magnification, and five fields were counted for each group
of cells.
Flow cytometry
MOLT4 cells (5×105 cells/ml) were treated
with 0.5 µg/ml DOX, incubated at 37°C for 48 h and then treated
with 100 ng/ml CCL25 for 10 min at 37°C. Untreated MOLT4 cells were
used as controls. The cells were washed twice with ice-cold PBS and
resuspended in 300 µl PBS for flow cytometric analysis (CytoFLEX;
Beckman Coulter, Inc., Brea, CA, USA). The excitation wavelength
for the cells was 488 nm and the emission wavelength was 590
nm.
Cell proliferation assay
MOLT4 cells (1×105 cells/ml) were plated
on 96-well plates in triplicate alongside a control group. The
cells were incubated with DOX (0.5 mg/ml or 1 mg/ml) at 37°C for 48
h and then 10 µl Cell Counting Kit-8 solution (catalog no., CK04;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
per sample for 3 h. Readings were taken at a wavelength of 450 nm
in a spectrometer and cellular survival was calculated accordingly.
All the steps were performed according to the manufacturer's
protocol.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism v5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values are
presented as the mean ± standard deviation of >3 independently
conducted experiments. Statistical significance was assessed by
t-test followed by paired comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
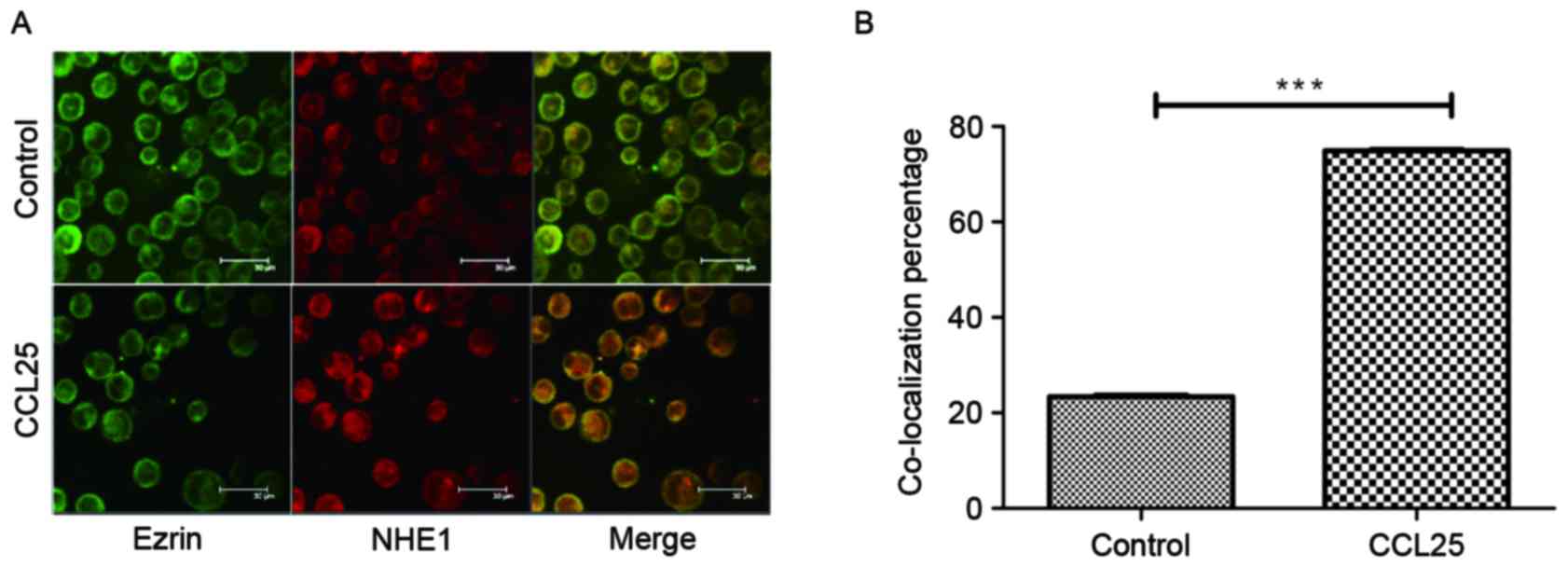
Co-localization of NHE1 and ezrin
The co-localization of NHE1 and ezrin was observed
in MOLT4 cells following stimulation with 100 ng/ml CCL25 for 10
min, but this was not observed in the control group (Fig. 1A). Statistical analysis (Fig. 1B) identified that the degree of cell
co-localization was significantly increased in the CCL25-treated
group compared with that in the control group. This observation
highlights the role of CCL25 in initiating cellular migration and
the association between NHE1 and ezrin indicated by their
co-localization.
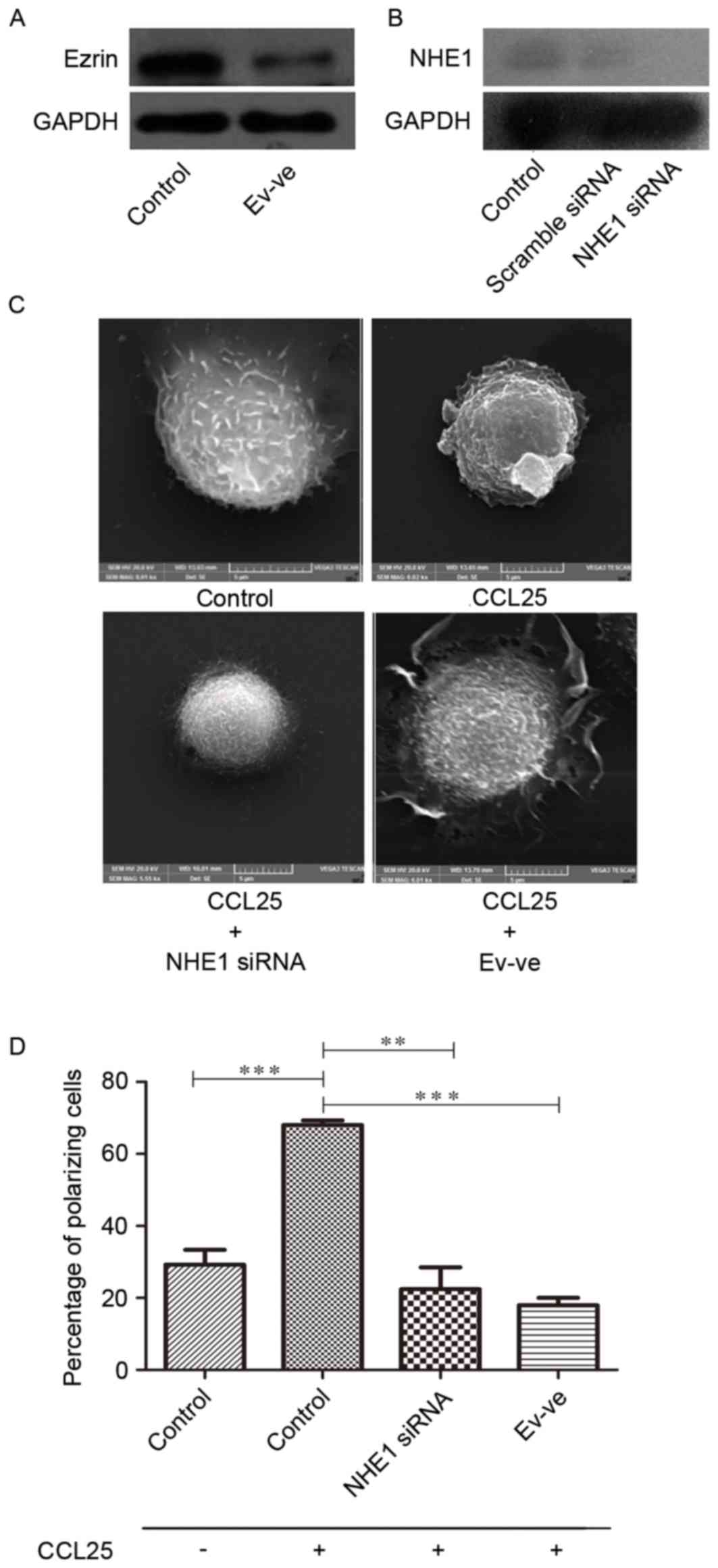
NHE1 and ezrin silencing
The western blot analysis results revealed that the
expression level of ezrin in Ev-negative MOLT4 cells was lower than
wild-type MOLT4 cells (Fig. 2A). At
the same time, NHE1 siRNA was used to reduce the expression of NHE1
in MOLT4 cells (Fig. 2B).
MOLT4 cell polarization is introduced
by CCL25
CCL25 introduces microvillus resorption and
polarization in MOLT4 cells (Fig.
2C). The formation of pseudopodia or lamellipodia is associated
with cellular invasive or migratory capability (25). The present study assessed this ability
in MOLT4 cells through the introduction of CCL25 to assess whether
there were any significant morphological alterations. A scanning
electron microscopy was used for this experiment. A total of 100
ng/ml CCL25 was introduced to MOLT4 cells for 10 min. Without
stimulation, the MOLT4 cells primarily exhibit numerous microvilli
of differing lengths, extending from the membrane, and a spherical
body shape (Fig. 2C). After 10 min of
CCL25 stimulation, substantial morphological alterations were
observed in wild-type MOLT4 cells (Fig.
2C). The majority of these cells had microvilli, tail-like
membrane extensions, ruffled edges and/or pseudopodia-like
structure formation in a certain direction due to polarization.
NHE1 silencing reduces CCL25-induced
polarization in MOLT4 cells
When compared to the wild-type MOLT4 cells treated
with CCL25 (100 ng/ml for 10 min), the NHE1-silenced cells treated
with CCL25 with the same conditions exhibited less polarization.
The majority of cells in NHE1 siRNA group exhibited an oval shape
with no or minimal microvilli and lamellipodia (Fig. 2C).
Ezrin silencing reduces CCL25-induced
polarization in MOLT4 cells
Ezrin, a member of the ERM protein family that links
the actin cytoskeleton to the plasma membrane, was also identified
to be important in cellular migration. Absence or deficiency of
ezrin in the Ev-ve MOLT4 group demonstrated decreased polarizing
capacity. The ezrin-negative MOLT4 cells treated with CCL25
exhibited a spherical profile with microvilli, compared with
wild-type MOLT4 cells treated with CCL25 which exhibited a state of
polarization (Fig. 2C). The general
absence of polarization morphology in this group of cells
emphasizes the involvement of the ezrin protein in migratory
ability of MOLT4 cells. Fig. 2D
demonstrates that NHE1 and ezrin expression are statistically
associated with increased degrees of MOLT4 cell polarization.
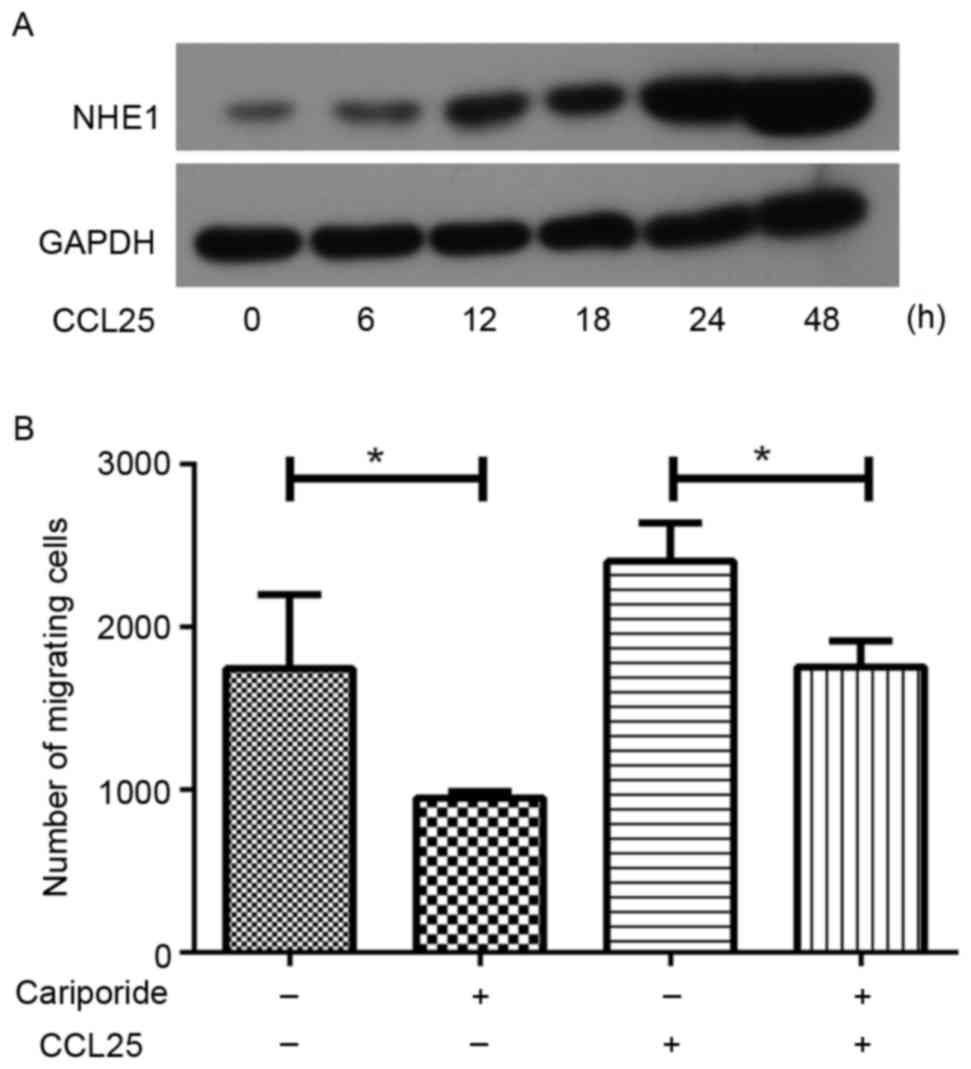
CCL25 stimulation induces the
increased expression of NHE1 in MOLT4 cells
Fig. 3A presents the
NHE1 expression levels following 100 ng/ml CCL25 treatment in
wild-type MOLT4 cells. The protein was isolated at 6, 12, 18, 24
and 48 h following CCL25 treatment. These results indicated that
NHE1 expression increased following exposure to CCL25 in a
time-dependent manner (Fig. 3A).
NHE1 silencing decreases CCL25-induced
migration in MOLT4 cells
CCL25 (100 ng/ml) induced an increase in migration
of MOLT4 cells compared with the control group; however, when NHE1
expression was inhibited with cariporide in MOLT4 cells, the
CCL25-triggered migration reduced significantly (Fig. 3B). This result demonstrated that NHE1
serves an important role in CCL25-induced migration in MOLT4
cells.
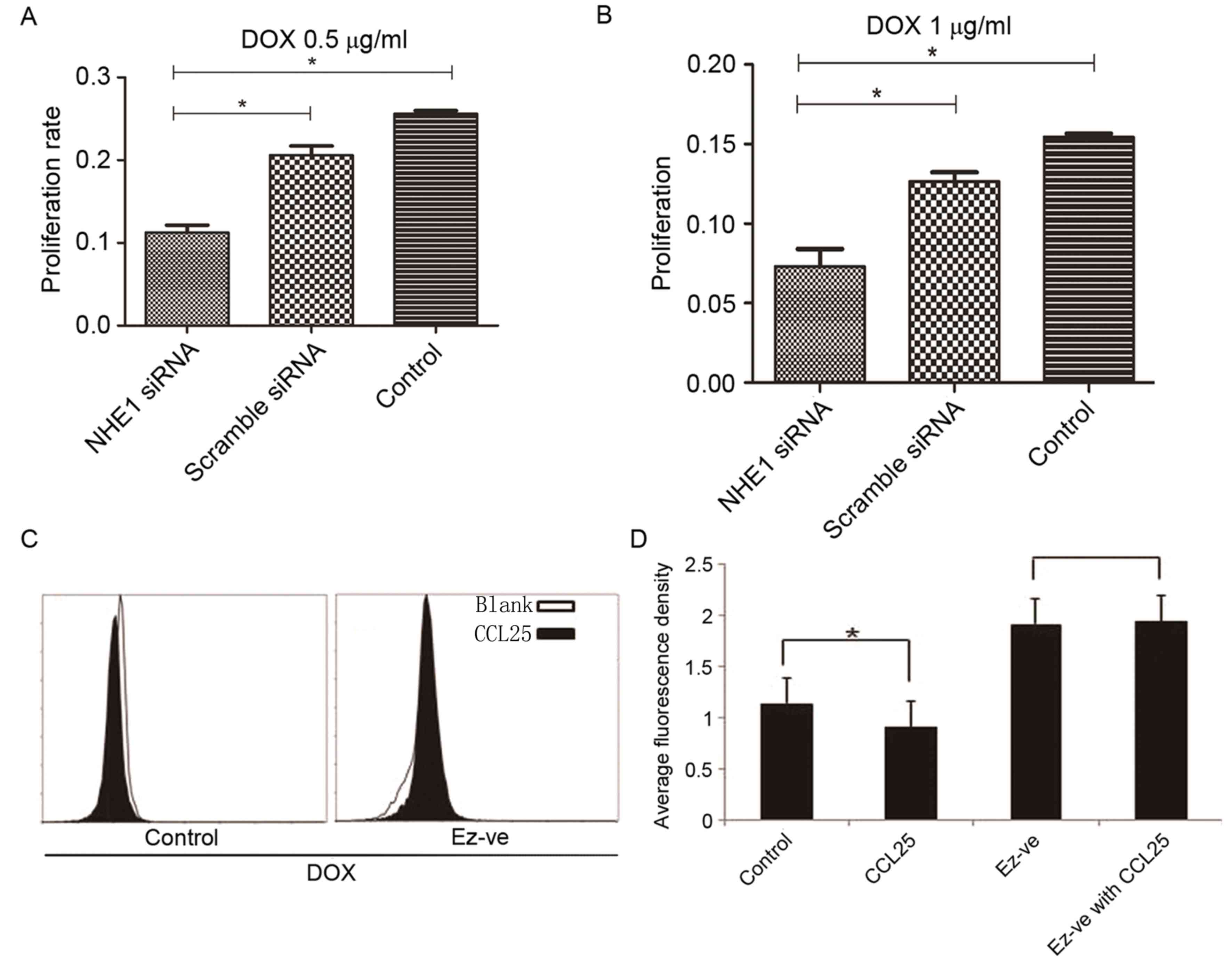
NHE1 knockdown increases the
sensitivity of MOLT4 cells to DOX
Treatment of MOLT4 cells with 0.5 mg/ml and 1 mg/ml
DOX demonstrated a significant increase in proliferation in the
wild-type and scrambled siRNA groups compared with the NHE1
siRNA-silenced group (Fig. 4A and B).
This observation suggested that NHE1 inhibition increased the
sensitivity of MOLT4 cells to DOX. Blocking the effect of NHE1, may
promote to an increased intracellular drug concentration and
therefore a higher apoptosis rates in tumor cells.
Ezrin knockdown increases the
accumulation of DOX in MOLT4 cells
The DOX accumulation in wild-type MOLT4 cells,
represented by average fluorescence density, was decreased
following CCL25 treatment. Conversely, CCL25 had no effect on DOX
accumulation in Ev-ve MOLT4 cells (Fig.
4C and D). This result shows that ezrin is important in
CCL25-induced DOX resistance.
Discussion
The etiology of T-ALL remains to be elucidated and
it is hypothesized that multiple factors contribute towards its
prevalence in childhood or in young adults (26). HTLV-1 infection is an established
etiological factor, but the incidence rate of leukemia following
this infection is low (27). Although
it is a rare neoplasm, T-ALL is an aggressive disease that is
characterized by a mature T-cell phenotype. Patients with this
neoplasm, of whom a substantial number are children, have a poor
prognosis following diagnosis (20–22,28). Due
to this, it is therefore important that effective treatment options
are available to clinicians to optimally manage this disease.
NHE1 has been implicated as serving notable roles in
invasive and metastatic neoplastic tissue. In addition to
increasing intracellular pH (6), NHE1
also binds to the cytoskeleton via ERM proteins and functions as a
platform for signaling (19). The
diverse functions of NHE1 make it a potential target for cancer
treatment.
Ezrin is a member of the ERM family of linker
proteins that link the cell membrane to the actin cytoskeleton. ERM
proteins are negatively regulated by an intramolecular interaction
between their N- and C-terminal domains, which masks the actin- and
membrane-binding sites (29). The
sequential binding of phosphatidylinositol-4,5-bisphosphate and the
phosphorylation of a conserved threonine residue (T567) are
required for the activation of ezrin to enable its
membrane-cytoskeleton linker function (30).
NHE1 is involved in cancer cell metastasis and
invasion (7,8,31,32). Therefore, the present study focused on
NHE1 and its association with ezrin in MOLT4 cells. Firstly, the
expression levels of NHE1 in wild-type and ezrin-silenced MOLT4
cells were assessed and a similar expression of NHE1 was observed
in the two groups (data not shown). The effect of CCL25 on NHE1 and
ezrin co-localization was observed and it was identified that the
degree of co-localization was increased in the CCL25-treated group
compared with the control group (Fig.
1). This may indicate that the complex formed by NHE1 and ezrin
serves an important role in CCL25-induced MOLT4 transformation. The
migratory and invasive ability of MOLT4 cells was assessed using
scanning electron microscopy, in which it was observed the
inhibition of NHE1 or ezrin led to a decrease in the polarization
of MOLT4 cells. It was previously observed that the response of
MOLT4 cells to CCL25 has a significant role in the metastasis and
invasion of MOLT4 cells as they undergo polarization (12). The co-localization of NHE1 and ezrin,
and the increased cell polarization observed supports the
hypothesis that NHE1 functions in association with ezrin to
transform MOLT4 cells into a metastatic phenotype. The results of
the present study indicate that NHE1 may be providing structural
support for the formation of a signaling complex involved in the
movement of the cell. ERM proteins function by promoting NHE1 to
connect actin with the cell membrane and form the structures that
contributes to the alteration of the shape of the cell and its
mobilization (3). Notably, ezrin has
been demonstrated to act with NHE1 to link the actin cytoskeleton
to the cell membrane (7). Ezrin is
established to serve a notable role in leukemia and breast cancer,
amongst other malignancies (12,30). The
present study demonstrates that NHE1 and ezrin may be involved in
the transformation of MOLT4 cells and to the best of our knowledge
this has not yet been described in the literature. The present
study further emphasizes the role of NHE1 in tissue physiology and
tumor pathogenesis (4).
The present study also assessed whether the
treatment with CCL25 effected the expression of NHE1 and
demonstrated that CCL25 upregulates NHE1 expression (Fig. 3A). This finding emphasizes the role of
NHE1 as well as the role of chemokines in cancer cell mobilization.
Furthermore, the chemotaxis assay (Fig.
3B) identified that CCL25 induced MOLT4 cell migration in an
NHE1-dependent manner. In addition, the inhibition of NHE1 by the
specific inhibitor cariporide resulted in the decreased chemotaxis
of MOLT4 cells, despite CCL25 administration (Fig. 3B). These data clearly indicate the
notable role of NHE1 in tumor migration and invasion.
To investigate the role of NHE1 in chemotherapeutic
drug resistance of MOLT4 cells, the proliferation of cells treated
with different concentrations of DOX was assessed in wild-type and
NHE1-silenced MOLT4 cells. The findings of the present study
demonstrate that proliferation was decreased in NHE1-silenced MOLT4
cells when exposed to a chemotherapeutic drug compared with
control-treated MOLT4 cells (Fig. 4A and
B). Man et al (3)
established that NHE1 has a notable role in resistance to sorafenib
in colon cancer cells. Therefore, blocking the effect of NHE1 may
lead to a higher intracellular drug concentration and a higher
apoptotic rate in tumor cells.
The present study reveals the potential role of
ezrin in the development of chemotherapeutic drug resistance in
MOLT4 cells. Ezrin-silenced cells did not differ considerably in
DOX accumulation in the presence or absence of CCL25; however, the
DOX accumulation of wild-type MOLT4 cells differed significantly in
the presence and absence of CCL25 (Fig.
4C and D). The silencing of ezrin therefore appeared to
maintain a higher intracellular drug concentration, thus improving
the therapeutic effect.
In conclusion, the present study indicated that NHE1
and ezrin may, in combination, have notable roles in the metastasis
and drug resistance of MOLT4 cells. These results inform on novel
potential targets for T-ALL diagnosis and treatment. The treatment
of T-ALL by blocking the activity NHE1 or ezrin may therefore be a
viable treatment option for patients with this type of
neoplasm.
Glossary
Abbreviations
Abbreviations:
|
T-ALL
|
T-cell acute lymphoblastic
leukemia
|
|
NHE1
|
sodium hydrogen antiporter 1
|
|
DOX
|
doxorubicin
|
|
ERM
|
ezrin/radixin/moesin
|
References
|
1
|
Gómez AM, Martínez C, González M, Luque A,
Melen GJ, Martínez J, Hortelano S, Lassaletta Á, Madero L and
Ramírez M: Chemokines and relapses in childhood acute lymphoblastic
leukemia: A role in migration and in resistance to antileukemic
drugs. Blood Cells Mol Dis. 55:220–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pitt LA, Tikhonova AN, Hu H, Trimarchi T,
King B, Gong Y, Sanchez-Martin M, Tsirigos A, Littman DR, Ferrando
AA, et al: CXCL12-producing vascular endothelial niches control
acute T cell leukemia maintenance. Cancer Cell. 27:755–768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Man CH, Lam SS, Sun MK, Chow HC, Gill H,
Kwong YL and Leung AY: A novel tescalcin-sodium/hydrogen exchange
axis underlying sorafenib resistance in FLT3-ITD+ AML. Blood.
123:2530–2539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amith SR and Fliegel L: Regulation of the
Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res.
73:1259–1264. 2013.
Slepkov ER, Rainey JK, Li X, Liu Y, Cheng FJ,
Lindhout DA, Sykes BD and Fliegel L: Structural and functional
characterization of transmembrane segment IV of the NHE1 isoform of
the Na+/H+ exchanger. J Biol Chem 280: 17863–17872, 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orlowski J and Grinstein S: Diversity of
the mammalian sodium/proton exchanger SLC9 gene family. Pflugers
Arch. 447:549–565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antelmi E, Cardone RA, Greco MR, Rubino R,
Di Sole F, Martino NA, Casavola V, Carcangiu M, Moro L and Reshkin
SJ: ß1 integrin binding phosphorylates ezrin at T567 to activate a
lipid raft signalsome driving invadopodia activity and invasion.
PLoS One. 8:e751132013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin Y, Wang J, Jin W, Wang L, Li H, Ma L,
Li Q and Pang T: NHE1 mediates migration and invasion of HeLa cells
via regulating the expression and localization of MT1-MMP. Cell
Biochem Funct. 30:41–46. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denker SP, Huang DC, Orlowski J, Furthmayr
H and Barber DL: Direct binding of the Na-H exchanger NHE1 to ERM
proteins regulates the cortical cytoskeleton and cell shape
independently of H(+) translocation. Mol Cell. 6:1425–1436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiang Y, Chou CY, Hsu KF, Huang YF and
Shen MR: EGF upregulates Na+/H+ exchanger NHE1 by
post-translational regulation that is important for cervical cancer
cell invasiveness. J Cell Physiol. 214:810–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cong D, Zhu W, Shi Y, Pointer KB, Clark
PA, Shen H, Kuo JS, Hu S and Sun D: Upregulation of NHE1 protein
expression enables glioblastoma cells to escape TMZ-mediated
toxicity via increased H+ extrusion, cell migration and
survival. Carcinogenesis. 35:2014–2024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou B, Leng J, Hu M, Zhang L, Wang Z, Liu
D, Tong X, Yu B, Hu Y, Deng C, et al: Ezrin is a key molecule in
the metastasis of MOLT4 cells induced by CCL25/CCR9. Leuk Res.
34:769–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song X, Yang J, Hirbawi J, Ye S, Perera
HD, Goksoy E, Dwivedi P, Plow EF, Zhang R and Qin J: A novel
membrane-dependent on/off switch mechanism of talin FERM domain at
sites of cell adhesion. Cell Res. 22:1533–1545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Wang D, Guo Z, Zhao J, Wu B, Deng
H, Zhou T, Xiang H, Gao F, Yu X, et al: Rho kinase phosphorylation
promotes Ezrin-mediated metastasis in hepatocellular carcinoma.
Cancer Res. 71:1721–1729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brambilla D, Zamboni S, Federici C, Lugini
L, Lozupone F, De Milito A, Cecchetti S, Cianfriglia M and Fais S:
P-glycoprotein binds to ezrin at amino acid residues 149–242 in the
FERM domain and plays a key role in the multidrug resistance of
human osteosarcoma. Int J Cancer. 130:2824–2834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Xiao R, Xiong J, Leng J, Ehtisham
A, Hu Y, Ding Q, Xu H, Liu S, Wang J, et al: Activated ERM protein
plays a critical role in drug resistance of MOLT4 cells induced by
CCL25. PLoS One. 1:e523842013. View Article : Google Scholar
|
|
16
|
Ziętara N, Łyszkiewicz M, Puchałka J,
Witzlau K, Reinhardt A, Förster R, Pabst O, Prinz I and Krueger A:
Multicongenic fate mapping quantification of dynamics of thymus
colonization. J Exp Med. 212:1589–1601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zlotoff DA, Sambandam A, Logan TD, Bell
JJ, Schwarz BA and Bhandoola A: CCR7 and CCR9 together recruit
hematopoietic progenitors to the adult thymus. Blood.
115:1897–1905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiuping Z, Jei X, Youxin J, Wei J, Chun L,
Jin W, Qun W, Yan L, Chunsong H, Mingzhen Y, et al: CC chemokine
ligand 25 enhances resistance to apoptosis in CD4+ T cells from
patients with T-cell lineage acute and chronic lymphocytic leukemia
by means of livin activation. Cancer Res. 64:7579–7587. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin W, Li Q, Lin Y, Lu Y, Li H, Wang L, Hu
R, Ma L, Wang J and Pang T: Reversal of Imatinib resistance in
BCR-ABL-positive leukemia after inhibition of the Na+/H+ exchanger.
Cancer Lett. 308:81–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bourgeois S, Meer LV, Wootla B,
Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR and Houillier P:
NHE4 is critical for the renal handling of ammonia in rodents. J
Clin Invest. 120:1895–1904. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CH, Chen TH, Wu MY, Chen JR, Tsai HF,
Hong LY, Zheng CM, Chiu IJ, Lin YF and Hsu YH: Peroxisome
proliferator-activated receptor alpha protects renal tubular cells
from gentamicin-induced apoptosis via upregulating Na+/H+ exchanger
NHE1. Mol Med. Nov 23–2015.(Epub ahead of print). View Article : Google Scholar
|
|
22
|
Hojo T: Specimen preparation of the human
cerebellar cortex for scanning electron microscopy using a t-butyl
alcohol freeze-drying device. Scanning Microsc Suppl. 10:345–348.
1996.PubMed/NCBI
|
|
23
|
Deng X, Tu Z, Xiong M, Tembo K, Zhou L,
Liu P, Pan S, Xiong J, Yang X, Leng J, et al: Wnt5a and CCL25
promote adult T-cell acute lymphoblastic leukemia cell migration,
invasion and metastasis. Oncotarget. 8:39033–39047. 2017.PubMed/NCBI
|
|
24
|
Dang I, Gorelik R, Sousa-Blin C, Derivery
E, Guérin C, Linkner J, Nemethova M, Dumortier JG, Giger FA,
Chipysheva TA, et al: Inhibitory signalling to the Arp2/3 complex
steers cell migration. Nature. 503:281–284. 2013.PubMed/NCBI
|
|
25
|
Girardi T, Vicente C, Cools J and De
Keersmaecker K: The genetics and molecular biology of T-ALL. Blood.
129:1113–1123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukasaki K and Tobinai K: Human T-cell
lymphotropic virus type I-associated adult T-cell
leukemia-lymphoma: New directions in clinical research. Clin Cancer
Res. 20:5217–5225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beldjord K, Chevret S, Asnafi V, Huguet F,
Boulland ML, Leguay T, Thomas X, Cayuela JM, Grardel N, Chalandon
Y, et al: Oncogenetics and minimal residual disease are independent
outcome predictors in adult patients with acute lymphoblastic
leukemia. Blood. 123:3739–3749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fehon RG, McClatchey AI and Bretscher A:
Organizing the cell cortex: The role of ERM proteins. Nat Rev Mol
Cell Biol. 11:276–287. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Babich V and Di Sole F: The Na+/H+
exchanger-3 (NHE3) activity requires ezrin binding to
phosphoinositide and its phosphorylation. PLoS One.
10:e01293062015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Over-expression of Na+/H+ exchanger 1 and its clinicopathologic
significance in hepatocellular carcinoma. Med Oncol. 27:1109–1113.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl)
amiloride reduces hypoxia-induced hepatocellular carcinoma invasion
and motility. Cancer Lett. 295:198–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoskin V, Szeto A, Ghaffari A, Greer PA,
Côté GP and Elliott BE: Ezrin regulates focal adhesion and
invadopodia dynamics by altering calpain activity to promote breast
cancer cell invasion. Mol Biol Cell. 26:3464–3479. 2015. View Article : Google Scholar : PubMed/NCBI
|