Introduction
Lung cancer is a leading cause of cancer-related
mortality worldwide. The most frequent type of lung cancer is
non-small cell lung cancer (NSCLC) (1). Despite improvements in the therapeutic
strategies of lung cancer, long-term survival is far from
satisfactory for lung cancer patients mainly due to recurrence and
metastasis (2). The molecular
mechanisms of lung carcinogenesis have yet to be completely
clarified (3–7).
ARHGAP10 belongs to the family of Rho GTPase
activating proteins (RhoGAP), which catalyze the hydrolysis of the
active Rho GTPases (GTP-bound) to the inactive form (GDP-bound),
and thus impedes Rho GTPases-regulated biological procedures,
including migration, cytoskeleton organization, cell proliferation
and gene transcription (8). It has
been reported that ARHGAP10 acts as a RhoGAP for several Rho
GTPases, including Cdc42 (9), RhoA
and RhoC (10). ARHGAP10 contains a
pleckstrin homology (PH) domain and a PSD95/DglA/ZO-1-like (PDZ)
domain. The two domains are essential for protein-protein
interaction. A number of proteins, including α-catenin (11), ARF1 (9),
FAK, PKC-ζ (12) and β-arrestin 1
(13), have been identified as
ARHGAP10 interaction proteins. By binding with these proteins,
ARHGAP10 actively participated in the regulation of the formation
of cell junction and stress fiber, vesicular transport of Golgi
membranes and influenza virus replication. Previous findings showed
that ARHGAP10 is associated with paediatric leukaemia (14), invasive breast cancer (15) and ovarian cancer (16). The present study aimed to investigate
the biological functions and expression of ARHGAP10 in lung
cancer.
In the present study, we compared ARHGAP10
expression level between normal lung tissues and lung cancer ones.
The effects of ARHGAP10 overexpression on cell migration, invasion
and proliferation of lung cancer cell lines were investigated.
Furthermore, gene set enrichment analysis (GSEA) and immunoblot
analyses suggested the metastasis and Wnt signaling pathways were
negatively correlated with ARHGAP10 expression. In conclusion, our
data indicated that ARHGAP10 may be a tumor suppressor protein for
lung cancer.
Materials and methods
Tissue samples
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University (Shanghai,
China). In total, 35 pathologically confirmed lung cancer patients
who had received surgery at the Second Affiliated Hospital of
Soochow University between January 2010 and December 2011 were
enrolled in the present study after obtaining of their written
informed consent. The same patients provided lung cancer tissues
along with adjacent normal ones. The paired tissues were stored at
−80°C after being snap-frozen.
Cell culture
We purchased the wild-type cancer cell lines of
human lung (A549, NCI-1975 and NCI-H1299) from the Cell Bank of the
Shanghai Institutes of Biological Sciences (Shanghai, China), which
were maintained with 5% CO2 at 37°C. RPMI-1640 medium
(HyClone, Logan, UT, USA) was used to grow the cell lines with
supplementation of 1% antibiotics and 10% fetal bovine serum (FBS;
Gibco, Carlsbad, CA, USA).
RNA extraction, cDNA synthesis and
reverse transcription-quantitative PCR
RNA isolated by TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) was treated with DNase and then subjected to
reverse transcription reaction employing reverse transcriptase
M-MLV and oligo(dT) (21-mer) (Takara, Dalian, China). RT-qPCR was
conducted to quantify the mRNA level of ARHGAP10 using a
SYBR® Green Master kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with an ABI 7300 instrument (Applied Biosystems,
Foster City, CA, USA) based on the manufacturer's protocols. The
PCR program used was: 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 45 sec. The primer sequences used in
the present study have been previously described (16). mRNA values of ARHGAP10 were normalized
to the internal control GAPDH. Each sample was run in triplicate
and confirmed by at least three replicates.
Immunoblotting assay
Total cell lysates isolated using RIPA buffer
containing protease inhibiting agent and phosphatase inhibitor
(Beyotime, Shanghai, China) were separated by 10% SDS-PAGE, and
then transferred onto nitrocellulose membranes (Millipore,
Bredford, MA, USA), which were blocked in skim milk at room
temperature for 1 h. The membranes were then probed with primary
antibodies against ARHGAP10 (1:150; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), matrix metalloproteinase (MMP)-2 (1:1,000),
MMP-9 (1:1,000), VEGF (1:1,000), β-catenin (1:1,000), c-Myc
(1:1,000), p21 (1:1,000) (all from Abcam, Cambridge, MA, USA) or
GAPDH (1:1,500; Cell Signaling Technology, Inc., Danvers, MA, USA)
overnight in gentle shaking at 4°C. The membranes were then
incubated for 1 h at room temperature with the corresponding
HRP-conjugated secondary antibodies (Beyotime). An enhanced
chemiluminescence kit (Millipore) was used to visualize signals
while ImageJ software quantified the band density, which was
normalized to GAPDH.
Cell proliferation analysis
The Cell Counting Kit-8 (CCK-8) assay kit (Beyotime)
was used to measure the cell proliferation rate. Cells were
cultured in 6-well plates under normal culture conditions. The
cells were infected with ARHGAP10 expression virus or control
vector virus. After 24 h, the cells were trypsinized and then
plated in 96-well plates (2×103 cells/well). After
incubation with CCK-8 solution at room temperature for 1 h at the
indicated time points (0, 24, 48 and 72 h), the optical density
(OD) was measured at a wavelength of 450 nm according to the
manufacturer's instructions. Each experiment was repeated three
times.
Transwell assay
Cell invasion and migration were evaluated using
Transwell assays performed in the Boyden chamber with and without
Matrigel coating (BD Biosciences, Franklin Lakes, NJ, USA),
respectively. Cells were cultured in 6-well plates, which were then
infected with ARHGAP10 expression virus or control vector virus.
The cells were trypsinized, suspended in RPMI-1640 medium
containing 0.1% FBS and plated on the upper chambers
(1.0×105 cells) after 24 h. RPMI-1640 medium with 10%
FBS was contained in the lower chambers. The cells adhering to the
upper chambers were carefully scrubbed off after incubation for 24
h. After fixation with 10% formalin and staining with 1% gentian
violet, the cells migrating onto the lower surface were counted
under an inverted microscope (AZ100; Nikon, Tokyo, Japan). Each
experiment was run in triplicate.
GSEA
We downloaded the dataset of TCGA lung cancer from
The Cancer Genome Atlas project (TCGA, https://tcga-data.nci.nih.gov/tcga/) and analyzed this
dataset using the software GSEA v2.2.2 (www.broadinstitute.org/gsea), as previously described
(17). In the present study, the
samples were classified as ARHGAP10 low and ARHGAP10 high to
annotate phenotype. The gene sets used to conduct GSEA were
obtained from the Molecular Signatures Database (http://www.broad.mit.edu/gsea/msigdb/index.jsp).
Lithium chloride (LiCl) treatment
A549 cells were plated in 6-well plates and infected
with ARHGAP10 expression virus or control vector virus. After 24 h,
the cells were treated with or without 10 mM LiCl for 24 h, and
then collected for immunoblotting analysis for β-catenin
expression.
Statistical analysis
GraphPad Prism 6.0 software (GraphPad, San Diego,
CA, USA) was employed to perform statistical analyses. The paired
t-test was used to analyze differences in ARHGAP10 expression of
lung cancer tissues from adjacent normal ones. Analysis was
conducted by one-way analysis of variance (ANOVA) for in
vitro cell experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
ARHGP10 is downregulated in lung
cancer tissues
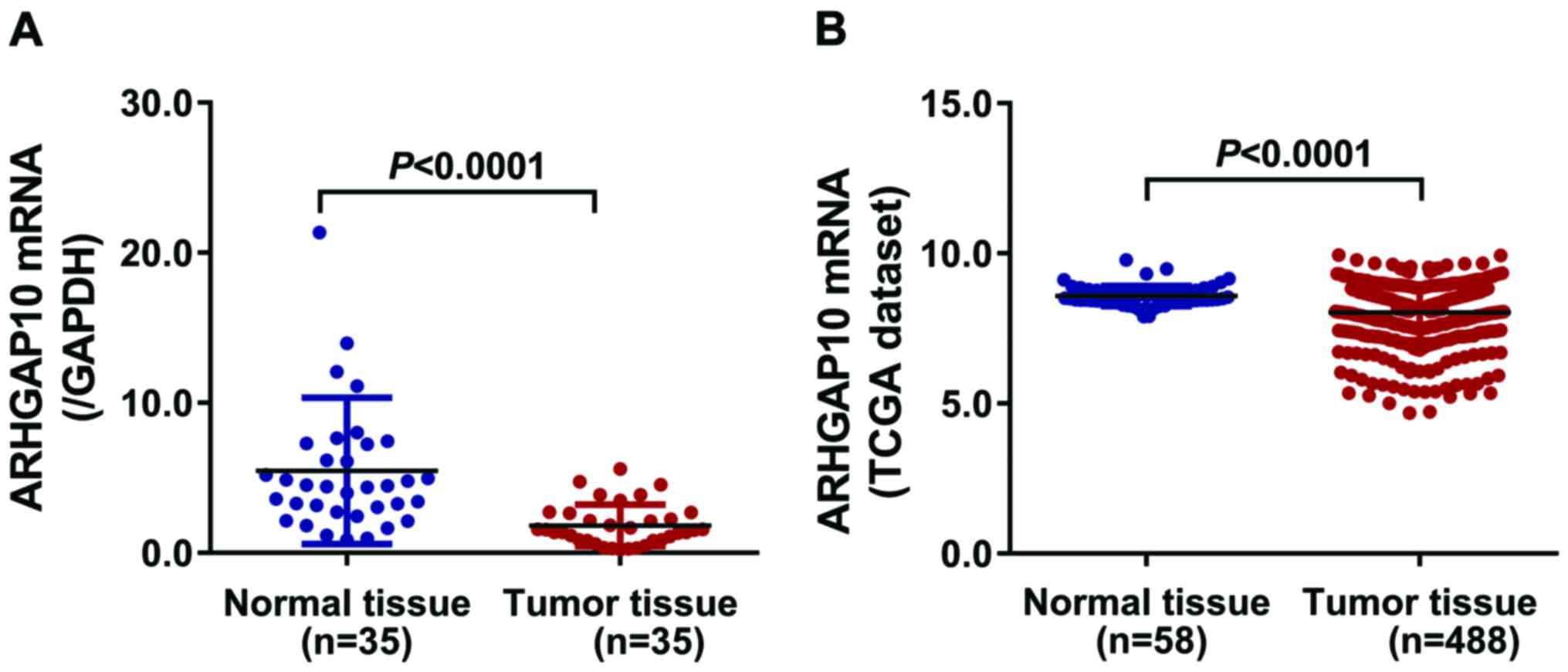
First, we evaluated ARHGAP10 mRNA levels in lung
cancer tissues as well as paired adjacent normal tissues of 35
patients by RT-qPCR. The mRNA levels of ARHGAP10 were more
downregulated in tumor tissues than that in adjacent normal tissues
(Fig. 1A). Moreover, a publically
available dataset from TCGA demonstrated that lung cancer tissues
had a lower ARHGAP10 expression level than that of normal tissues,
which was consistent with our findings (Fig. 1B).
Ectopic expression of ARHGAP10 in lung
cancer cells
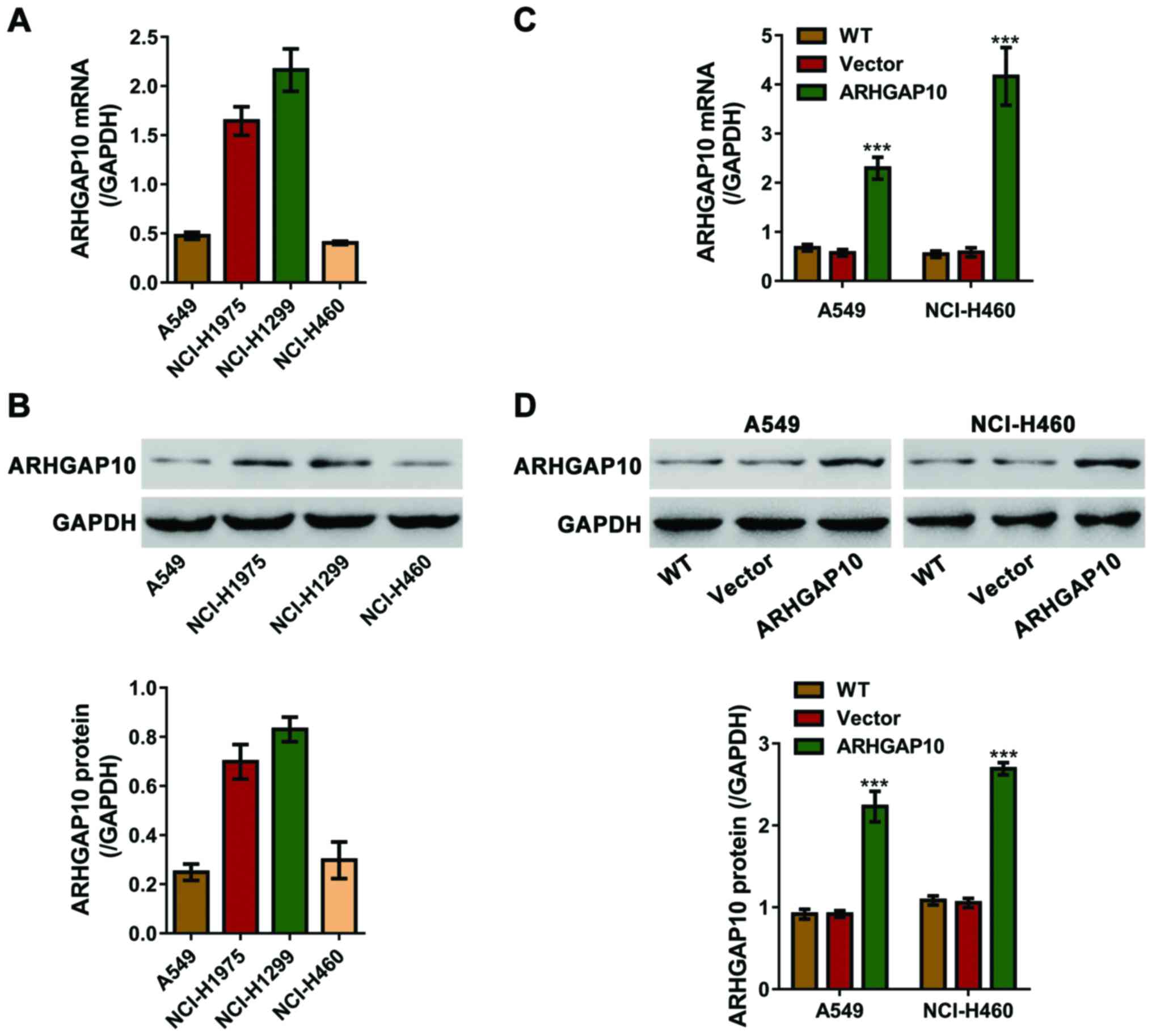
We analyzed the ARHGAP10 expression within the four
lung cancer cells. The protein and mRNA levels of ARHGAP10 in
NCI-H460 and A549 cells were relatively lower (Fig. 2A and B) and selected for further
overexpression experiments.
ARHGAP10-expressing lentiviral plasmid was
constructed and lentivirus was produced to infect A549 and NCI-H460
cells. ARHGAP10 expression in these cell lines was quantified using
RT-qPCR and immunoblotting. As shown in Fig. 2C and D, control vector lentiviral
infection (Vector) had no effects on ARHGAP10 expression as
compared to the wild-type (WT) cells. ARHGAP10-expressing
lentiviral infection significantly enhanced ARHGAP10 expression of
the protein and mRNA levels in contrast to WT cells and cells
infected with vector virus.
Ectopic ARHGAP10 expression inhibits
lung cancer cell proliferation
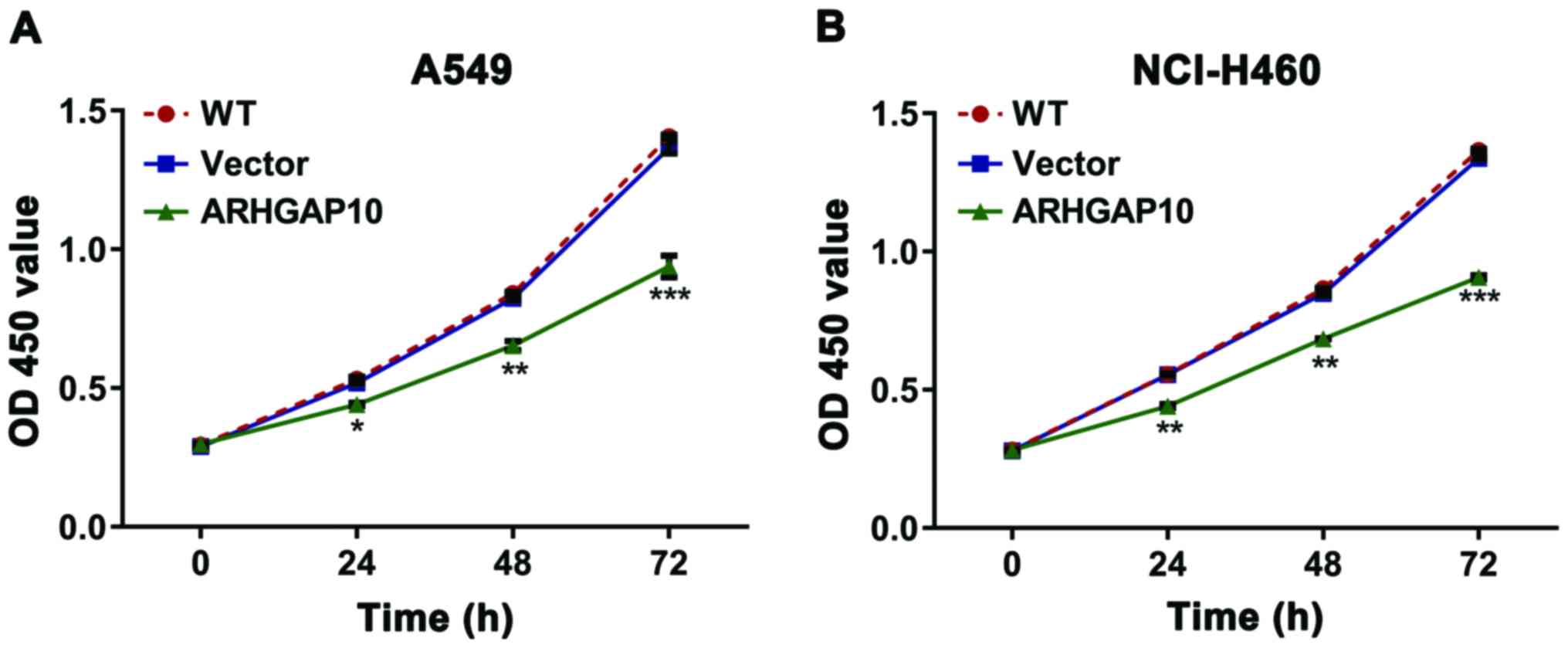
CCK-8 assay was employed to measure the effects of
ARHGAP10 on cell multiplication. As shown in Fig. 3, the cell multiplication rate of
NCI-H460 and A549 cells overexpressing ARHGAP10 was significantly
higher than the WT cells and cells infected with vector virus.
ARHGAP10 suppresses the invasion and
migration of lung cancer cells
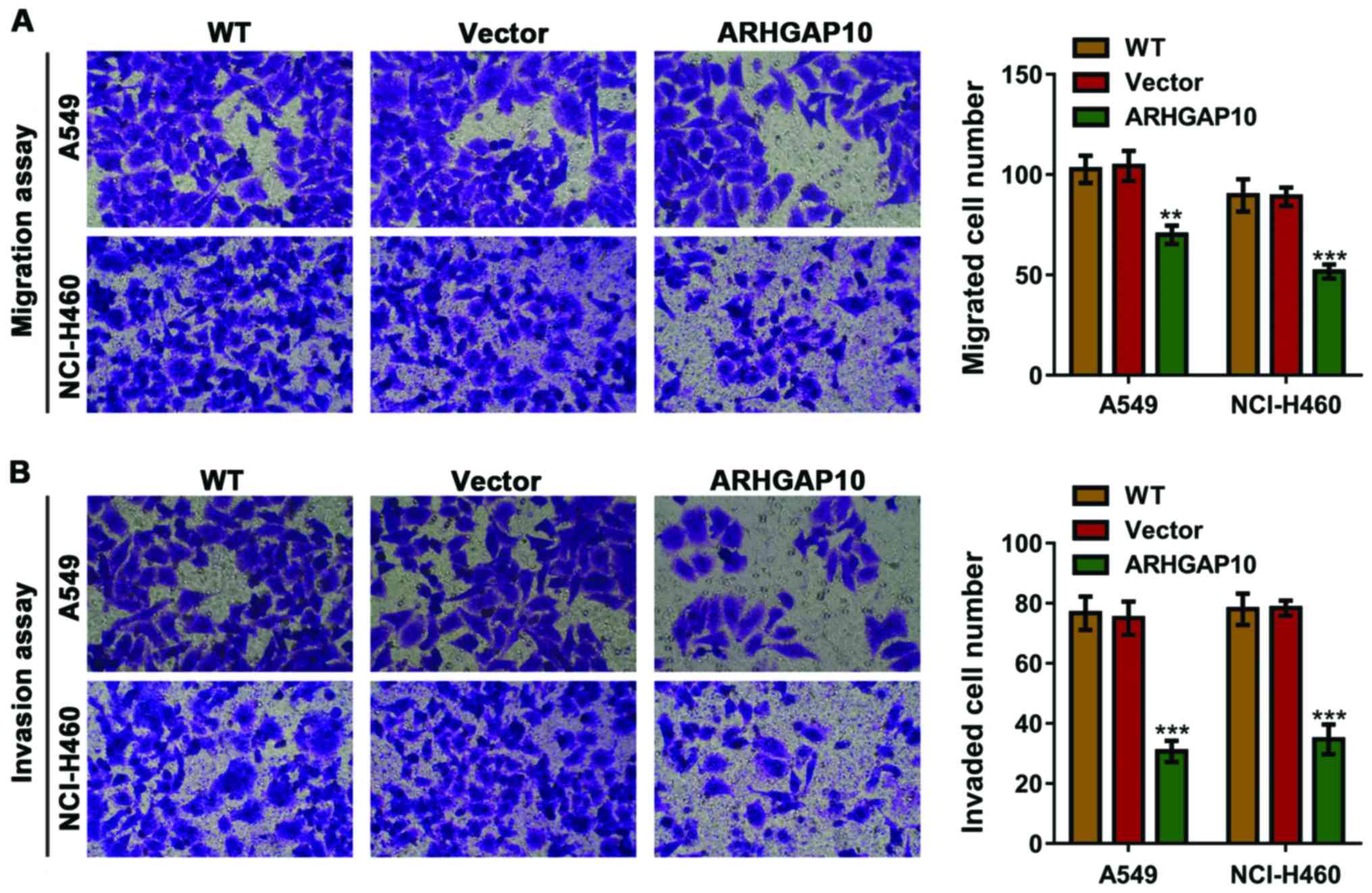
The effects that ARHGAP10 produced on the invasion
and migration of lung cancer cells were assessed using Transwell
assays (Fig. 4). ARHGAP10
overexpression in NCI-H460 and A549 cells significantly reduced the
invasion and migration capacity as compared to control cells (WT
and vector).
GSEA for ARHGAP10 expression in lung
cancer
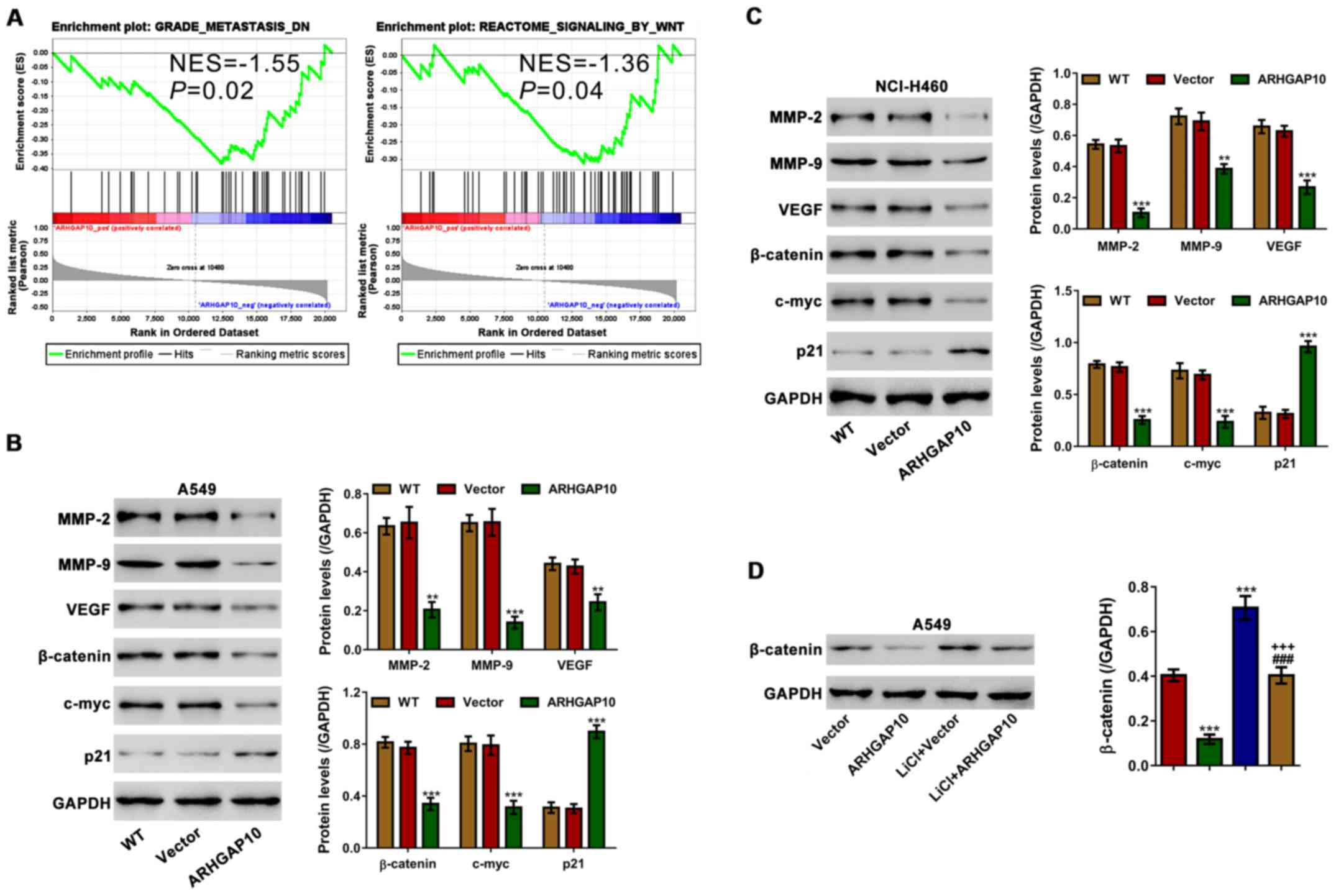
GSEA was performed to investigate pathways that were
associated with the different ARHGAP10 expression levels (Fig. 5A). Metastasis and Wnt signaling
pathways were negatively correlated with ARHGAP10 expression.
The effects of ARHGAP10 overexpression on the
expression of key proteins of metastasis (MMP-2, MMP-9 and VEGF)
and Wnt signaling pathways (β-catenin, c-Myc and p21) were then
assessed in A549 and NCI-H460 cells. In contrast to the control
cells, the mRNA and proteins levels of MMP-9, MMP-2, β-catenin as
well as c-Myc were significantly increased in lung cancer cells
with ARHGAP10 overexpression, while those of p21 were notably
decreased (Fig. 5B and C). These data
validated the results of GSEA.
LiCl, a GSK3β inhibitor, is able to enhance the
activation of Wnt canonical signaling (18). To further explore the effects of
ARHGAP10 on Wnt signaling, A549 cells were infected with
ARHGAP10-expressing lentivirus or vector lentivirus, and then
stimulated with 10 mM LiCl (Fig. 5D).
LiCl stimulation led to the accumulation of β-catenin, which was
significantly rescued by ARHGAP10 overexpression. These results
suggested that ARHGAP10 significantly inhibited Wnt/β-catenin
signaling.
Discussion
Recent studies have suggested the association
between ARHGAP10 and various types of cancer (10,14–16,19,20).
For example, Wong et al reported the methylation of ARHGAP10
in paediatric leukaemia (14). Azzato
et al performed a genome-wide study in breast cancer and
described single-nucleotide polymorphism located in ARHGAP10
(15). Luo et al identified a
decreased ARHGAP10 expression within ovarian cancer and suggested
that ARHGAP10 may serve as a biomarker for the prognosis of ovarian
cancer (16). The present study
demonstrated that ARHGAP10 expression was decreased within the
tumor tissue of patients with lung cancer as compared to normal
lung tissues from the same patients. These results were confirmed
by the analysis on the TCGA dataset. Our study suggests that
ARHGAP10 may serve as a useful biomarker of lung cancer.
The effects of ARHGAP10 on the biological behaviors
of lung cancer were examined by increasing its expression in the
two cell lines. We found that ARHGAP10 overexpression significantly
suppressed cell proliferation, and inhibited cell invasion and
migration. Similar functions of ARHGAP10 have been observed in
ovarian cancer cells (16). Our data
and this previous study demonstrated the tumor suppressor role of
ARHGAP10 in various types of cancer.
GSEA, a powerful computer-based program was applied
to identify the exact pathways that ARHGAP10 may regulate in lung
cancer. We found that metastasis and Wnt signaling pathways were
negatively associated with ARHGAP10 expression.
Cell metastasis and invasion are two main
characteristics of cancer. MMP-2 and MMP-9 belong to MMPs, which
degrade the extracellular matrix components and are essential for
tumor metastasis by promoting cell invasion (21). VEGF is a key target of tumor cell
invasion and metastasis (22).
Increased expression of MMP-2, MMP-9 and VEGF is frequently
observed in the most aggressive tumors (23,24).
ARHGAP10 overexpression significantly decreased the expression of
VEGF, MMP-9 and MMP-2, which may explain its inhibitory effects on
lung cancer cell invasion and migration.
The Wnt pathway plays diverse roles in the
progression, metastasis and initiation of various types of cancer
(25). c-Myc, a target gene of
Wnt/β-catenin signaling suppresses the accumulation of p21, a
cyclin-dependent kinase (CDK) inhibitor (26). In the present study, ARHGAP10
overexpression caused a significant decrease in β-catenin and
c-Myc, and an arresting reduction of p21. Moreover, the stimulation
effects of LiCl, a GSK3β inhibitor, on the accumulation of
β-catenin were notably suppressed by ARHGAP10 overexpression. Our
data suggest that ARHGAP10 has a critical role in regulating the
Wnt/β-catenin pathway.
To conclude, our study shows that ARHGAP10 is
downregulated in lung cancer specimens. Ectopic expression of
ARHGAP10 suppresses the migration, invasion and proliferation
produced by lung cancer cells. ARHGAP10 affects the metastasis and
Wnt signaling pathways in lung cancer. ARHGAP10 may therefore be a
novel target for the treatment of lung cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang T, Nelson RA, Bogardus A and Grannis
FW Jr: Five-year lung cancer survival: Which advanced stage
nonsmall cell lung cancer patients attain long-term survival?
Cancer. 116:1518–1525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Couraud S, Zalcman G, Milleron B, Morin F
and Souquet PJ: Lung cancer in never smokers - a review. Eur J
Cancer. 48:1299–1311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cornfield J, Haenszel W, Hammond EC,
Lilienfeld AM, Shimkin MB and Wynder EL: Smoking and lung cancer:
Recent evidence and a discussion of some questions. 1959. Int J
Epidemiol. 38:1175–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shtivelman E, Hensing T, Simon GR, Dennis
PA, Otterson GA, Bueno R and Salgia R: Molecular pathways and
therapeutic targets in lung cancer. Oncotarget. 5:1392–1433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bassères DS, Tizzei EV, Duarte AA, Costa
FF and Saad ST: ARHGAP10, a novel human gene coding for a
potentially cytoskeletal Rho-GTPase activating protein. Biochem
Biophys Res Commun. 294:579–585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubois T, Paléotti O, Mironov AA, Fraisier
V, Stradal TE, De Matteis MA, Franco M and Chavrier P:
Golgi-localized GAP for Cdc42 functions downstream of ARF1 to
control Arp2/3 complex and F-actin dynamics. Nat Cell Biol.
7:353–364. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lazarini M, Traina F, Machado-Neto JA,
Barcellos KS, Moreira YB, Brandão MM, Verjovski-Almeida S, Ridley
AJ and Saad ST: ARHGAP21 is a RhoGAP for RhoA and RhoC with a role
in proliferation and migration of prostate adenocarcinoma cells.
Biochim Biophys Acta. 1832:365–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sousa S, Cabanes D, Archambaud C, Colland
F, Lemichez E, Popoff M, Boisson-Dupuis S, Gouin E, Lecuit M,
Legrain P, et al: ARHGAP10 is necessary for alpha-catenin
recruitment at adherens junctions and for Listeria invasion. Nat
Cell Biol. 7:954–960. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borges L, Bigarella CL, Baratti MO,
Crosara-Alberto DP, Joazeiro PP, Franchini KG, Costa FF and Saad
ST: ARHGAP21 associates with FAK and PKCzeta and is redistributed
after cardiac pressure overload. Biochem Biophys Res Commun.
374:641–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anthony DF, Sin YY, Vadrevu S, Advant N,
Day JP, Byrne AM, Lynch MJ, Milligan G, Houslay MD and Baillie GS:
β-Arrestin 1 inhibits the GTPase-activating protein function of
ARHGAP21, promoting activation of RhoA following angiotensin II
type 1A receptor stimulation. Mol Cell Biol. 31:1066–1075. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong NC, Bhadri VA, Maksimovic J,
Parkinson-Bates M, Ng J, Craig JM, Saffery R and Lock RB: Stability
of gene expression and epigenetic profiles highlights the utility
of patient-derived paediatric acute lymphoblastic leukaemia
xenografts for investigating molecular mechanisms of drug
resistance. BMC Genomics. 15:4162014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azzato EM, Pharoah PD, Harrington P,
Easton DF, Greenberg D, Caporaso NE, Chanock SJ, Hoover RN, Thomas
G, Hunter DJ, et al: A genome-wide association study of prognosis
in breast cancer. Cancer Epidemiol Biomarkers Prev. 19:1140–1143.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo N, Guo J, Chen L, Yang W, Qu X and
Cheng Z: ARHGAP10, downregulated in ovarian cancer, suppresses
tumorigenicity of ovarian cancer cells. Cell Death Dis.
7:e21572016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/β-catenin signaling regulates cancer stem cells in lung cancer
A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bigarella CL, Borges L, Costa FF and Saad
ST: ARHGAP21 modulates FAK activity and impairs glioblastoma cell
migration. Biochim Biophys Acta. 1793:806–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carles A, Millon R, Cromer A, Ganguli G,
Lemaire F, Young J, Wasylyk C, Muller D, Schultz I, Rabouel Y, et
al: Head and neck squamous cell carcinoma transcriptome analysis by
comprehensive validated differential display. Oncogene.
25:1821–1831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su JL, Yang PC, Shih JY, Yang CY, Wei LH,
Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cockett MI, Murphy G, Birch ML, O'Connell
JP, Crabbe T, Millican AT, Hart IR and Docherty AJ: Matrix
metalloproteinases and metastatic cancer. Biochem Soc Symp.
63:295–313. 1998.PubMed/NCBI
|
|
24
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol.
4:a0080522012.doi:10.1101/cshperspect.a008052. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang Y, Simoneau AR, Liao WX, Yi G, Hope
C, Liu F, Li S, Xie J, Holcombe RF, Jurnak FA, et al: WIF1, a Wnt
pathway inhibitor, regulates SKP2 and c-myc expression leading to
G1 arrest and growth inhibition of human invasive urinary bladder
cancer cells. Mol Cancer Ther. 8:458–468. 2009. View Article : Google Scholar : PubMed/NCBI
|