Introduction
Mucinous breast carcinoma (MBC), also known as
colloid carcinoma, is a rare subtype of breast tumors that accounts
for 1–7% of all breast cancer cases. MBC is characterized by the
presence of extracellular mucin (MUC) (1). MBC includes mixed MBC, consisting of
other cancer types such as invasive ductal carcinoma, and pure MBC
(PMBC), in which the entire mass is almost occupied by mucinous
cancer cells and is without conventional invasive ductal carcinoma
cells (2). PMBC is represented by a
mass with a >90% mucinous component (3). MBC is linked with a more favorable
prognosis, a longer disease-free interval and a lower incidence of
axillary node metastasis compared with infiltrating ductal
carcinoma-not otherwise specified (IDC-NOS) (1,2,4). However, recurrence and metastasis of MBC
are frequently present in clinical practice.
Angiogenesis is a prerequisite for tumor
development; there is a close association between the formation of
blood vessels in the vicinity of tumor cells and the potential for
tumor formation, invasion and metastasis. Angiogenesis is induced
and developed in response to two sets of extracellular signals:
soluble angiogenic factors and the extracellular matrix (5). Breast carcinoma has been shown to be an
angiogenesis-dependent tumor through experimental and clinical
data. Vascular endothelial growth factor (VEGF) is the most potent
endothelial cell mitogen (6) and a
regulator of vascular permeability, therefore, VEGF has been
considered as a powerful novel prognostic tool (7). However, the associations between VEGF
expression in MBC and IDC-NOS and the morphology, behavior and
prognosis of tumors, and the differences between MBC and IDC-NOS,
are unclear.
The good prognosis of MBC is closely associated with
the formation of MUC around the cells (8). Previous studies have revealed the
expression of the MUC1, MUC2, MUC3, MUC4, MUC5A and MUC6 proteins
in PMBC, and this expression has been suggested to be a prognostic
factor (9). Gel-forming secretory
MUCs, including MUC2 and MUC6, exhibit a high expression rate in
mucinous carcinoma, indicating that high production of these types
of MUCs may act as a barrier to the extension of cancer, resulting
in less aggressive biological behavior. However, the expression of
MUC1, which is associated with a poor prognosis in gastric and
colorectal cancer types, is low in MBC (9). A study by Ahmed (10) highlighted that the MUC of MBC is
derived from cell breakdown. We hypothesize that significant cell
apoptosis may exist in the MBC tissues and produce a large amount
of mucus. The most well-known biochemical hallmark of early- and
late-stage apoptosis is cysteine protease activation.
Arginine-glycine-aspartate synthetic peptides induce apoptosis by
direct caspase-3 activation (11).
Caspase-3 is also required for the DNA fragmentation and
morphological changes associated with apoptosis (12). A high level of active caspase-3 in
cells and tissues is an important biomarker for apoptosis induced
by a wide variety of apoptotic signals (13). Thus, detection of caspase-3 expression
can reflect the apoptotic status of tumor cells, which may aid in
explaining the differences in prognosis and survival between MBC
and IDC-NOS.
In the present study, the expression of VEGF and
caspase-3 in MBC and IDC-NOS was investigated using
immunohistochemical staining, and the association between the
expression levels of VEGF and caspase-3 and clinicopathological
features were further investigated.
Materials and methods
Patients and tissues
A total of 54 patients with MBC and 60 randomly
selected patients with IDC-NOS who underwent surgery at the First
Affiliated Hospital of China Medical University (Shenyang,
Liaoning, China) between May 2009 and June 2011 were included in
the present study. MBC is a rare type of breast cancer, so the 54
patients with MBC included all the MBC in accordance with the set
of conditions between May 2009 and June 2011 who underwent surgery
at the First Affiliated Hospital of China Medical University. Cases
in which complete clinicopathological information and
formalin-fixed, paraffin-embedded breast tissues could not be
obtained were excluded. All patients had not received radiotherapy
or chemotherapy prior to surgery. The diagnosis of all cases was
confirmed according to the criteria of the World Health
Organization (14), as assessed by
the Department of Pathology (First Affiliated Hospital). Archival
formalin-fixed paraffin-embedded breast tissues were retrieved.
Patients were followed up for a median period of 47 months (range,
28–77 months) subsequent to the initial cancer surgery. Follow-up
consisted of regular clinic visits and ultrasound of the breast and
axillary node, supraclavicular area and infraclavicular region,
with or without mammography, lung computed tomography, liver
ultrasound, bone emission computed tomography and blood tests at
the discretion of the treating specialist. Relevant clinical and
pathological information are described in Table I.
 | Table I.Clinical and pathological features of
the patients (n=114). |
Table I.
Clinical and pathological features of
the patients (n=114).
| Characteristics | MBC (n=54) | IDC (n=60) | P-value |
|---|
| Age, years |
|
|
|
| Mean | 53.87 | 50.08 | 0.079 |
| ≤50, n
(%) | 26 (48.15) | 35 (58.33) | 0.276 |
| >50, n
(%) | 28 (51.85) | 25 (41.67) |
|
| T stage, n (%) |
|
| 0.203 |
| pT1 | 23 (42.59) | 19 (31.67) |
|
| pT2 | 28 (51.85) | 40 (66.67) |
|
|
pT3-4 | 3 (5.56) | 1 (1.67) |
|
| N stage, n (%) |
|
| 0.010a |
| N0 | 45 (83.33) | 33 (55.00) |
|
| N1 | 5 (9.26) | 15 (25.00) |
|
| N2 | 3 (5.56) | 7 (11.67) |
|
| N3 | 1 (1.85) | 5 (8.33) |
|
| TNM stage, n (%) |
|
| 0.023a |
| I | 23 (42.59) | 13 (21.67) |
|
| II | 27 (50.00) | 35 (58.33) |
|
| III | 4 (7.41) | 12 (20.00) |
|
| ER status, n (%) |
|
| 0.001a |
|
Negative | 6 (11.11) | 23 (38.33) |
|
|
Positive | 48 (88.89) | 37 (61.67) |
|
| PR status, n (%) |
|
| 0.147 |
|
Negative | 18 (33.33) | 28 (46.67) |
|
|
Positive | 36 (66.67) | 32 (53.33) |
|
| Hormone receptor
status, n (%) |
|
| 0.001a |
|
Negative | 5 (9.26) | 22 (36.67) |
|
|
Positive | 49 (90.74) | 38 (63.33) |
|
| HER-2 status, n
(%) |
|
| 0.085 |
|
Negative | 1 (1.85) | 35 (58.33) |
|
|
Positive | 43 (79.63) | 10 (16.67) |
|
|
Unknown | 10 (18.52) | 15 (25.00) |
|
| Ki-67, n (%) |
|
| 0.004a |
|
≤20% | 41 (75.93) | 30 (50.00) |
|
|
>20% | 13 (24.07) | 30 (50.00) |
|
| p53, n (%) |
|
| 0.883 |
|
Negative | 19 (35.19) | 22 (36.37) |
|
|
Positive | 28 (51.85) | 32 (53.33) |
|
|
Unknown | 7 (12.96) | 6 (10.00) |
|
| Surgery |
|
| 0.420 |
|
Mastectomy | 50 (92.59) | 58 (96.67) |
|
|
BCS | 4 (7.41) | 2 (3.33) |
|
| Axillary
operation |
|
| 0.045a |
|
Sentinel lymph node
biopsy | 8 (14.81) | 2 (3.33) |
|
|
Axillary clearance | 46 (85.19) | 58 (96.67) |
|
| Chemotherapy |
|
|
<0.001a |
| No | 12 (22.22) | 2 (3.33) |
|
|
Yes | 42 (77.78) | 58 (96.67) |
|
|
Anthracycline included | 31 (57.41) | 13 (21.67) |
|
| Taxane
included | 4 (7.41) | 3 (5.00) |
|
| Anthracycline and
taxane included | 6 (11.11) | 41 (68.33) |
|
|
Other | 1 (1.85) | 1 (1.67) |
|
| Radiotherapy |
|
| 0.12 |
| No | 54 (100.00) | 56 (93.33) |
|
|
Yes | 0 (0.00) | 4 (6.67) |
|
Immunohistochemical staining
Immunohistochemical examination was performed on
4-µm thick, formalin-fixed, paraffin-embedded sections using
UltraSensitive™ SP IHC kit (MXB Co., Ltd., Fuzhou, China). Briefly,
following deparaffinization (with xylene) and rehydration (with
alcohol), the endogenous peroxidase activity was blocked with 3%
H2O2. Antigen retrieval was performed with a
high-pressure cooker and normal serum (part of the UltraSensitive™
SP IHC kit) was applied to the sections at room temperature for 30
min to block non-specific antibody binding. The sections were then
incubated overnight at 4°C with the primary antibodies, including
monoclonal mouse-anti-human caspase-3 (1:50 dilution; catalog no.
ab2171; Abcam, Cambridge, MA, USA) and monoclonal mouse-anti-human
VEGF (1:300 dilution; catalog no. sc-7269; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Sections were further
incubated with the biotin-labeled IgG secondary antibody solution
from the UltraSensitive™ SP IHC kit at room temperature for 15 min,
followed by streptavidin-peroxidase incubation at room temperature
for 15 min. Finally, sections were stained with
3,3-diaminobenzidine, counterstained with hematoxylin for 5 min and
mounted. Negative controls were processed with PBS instead of the
primary antibody.
Immunohistochemical scoring
The immunostained sections were assessed with an
optical microscope at ×400 magnification, based on manual counting
of positive cells in each tissue by two observers blinded to
clinical outcomes. Cases of disagreement were reviewed jointly to
obtain a consensus score. The percentage of positive cells and
staining intensity of VEGF and caspase-3 were scored. Intensity was
graded as negative (score 0), weak (score 1), moderate (score 2) or
strong (score 3), and percentage of positive cells was graded as
<5% (score 0), 5–25% (score 1), 26–50% (score 2), 51–75% (score
3) and >75% (score 4). The final score of VEGF and caspase-3
expression was determined by multiplying the intensity score and
percentage score, with a range of 0–12. According to the scoring
results, all patients were divided to two groups: Low (score of
0–5) and high (score of 6–12) expression.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 statistical software (IBM Corp., Armonk, New York,
USA). A χ2 test and Fisher's exact test were used to
identify the differences between MBC and IDC-NOS with regard to
clinicopathological features, and for the associations between VEGF
or caspase-3 and clinicopathological variables. Disease-free
survival (DFS) was recorded from the date of surgery to the relapse
date or the last follow-up date, and was estimated using the
Kaplan-Meier analysis. The statistical significance of differential
survival was assessed using the log-rank test. Cox regression
analysis for DFS was used. P<0.05 was used to indicate a
statistically significant difference. Variables with a univariate
p-value of <0.1 were included in the multivariate model.
Results
Characteristics of the study
population
A total of 54 female patients with MBC (median age
53.87 years; range, 24–82 years) and 60 IDC-NOS (median age 50.08
years; range, 27–78 years) were included. The cohort consisted of
94.5% pathological tumor stage 1–2 (pT1-2) patients,
5.6% pT3-4 patients and 16.8% node-positive patients in
the MBC group, and 98.4% pT1-2 patients, 1.7%
pT3-4 patients and 45.0% node-positive patients in the
IDC-NOS group. No metastasis was observed in either group prior to
surgery. In the MBC group, 92.6% of patients chose to undergo a
mastectomy and 7.4% of patients chose breast-conserving surgery.
Furthermore, 14.8% of patients underwent sentinel lymph node biopsy
and 85.2% of patients received axillary clearance. In the MBC
group, 22.2% of patients did not accept adjuvant chemotherapy and
no patients accepted adjuvant radiotherapy following surgery. There
were no significant differences in terms of age, T stage, breast
surgery and adjuvant radiotherapy between the two groups. In
contrast to IDC-NOS, the MBC patients showed a higher rate of
positive ER and hormone receptor, and a larger population of which
expression Ki-67 was ≤20%. MBC patients tended to have
significantly less lymph node metastasis and a lower
tumor-node-metastasis (TNM) stage (15) compared with IDC-NOS patients (P=0.010
and P=0.023, respectively) (Table
I).
VEGF and caspase-3 expression in MBC
and IDC-NOS patients
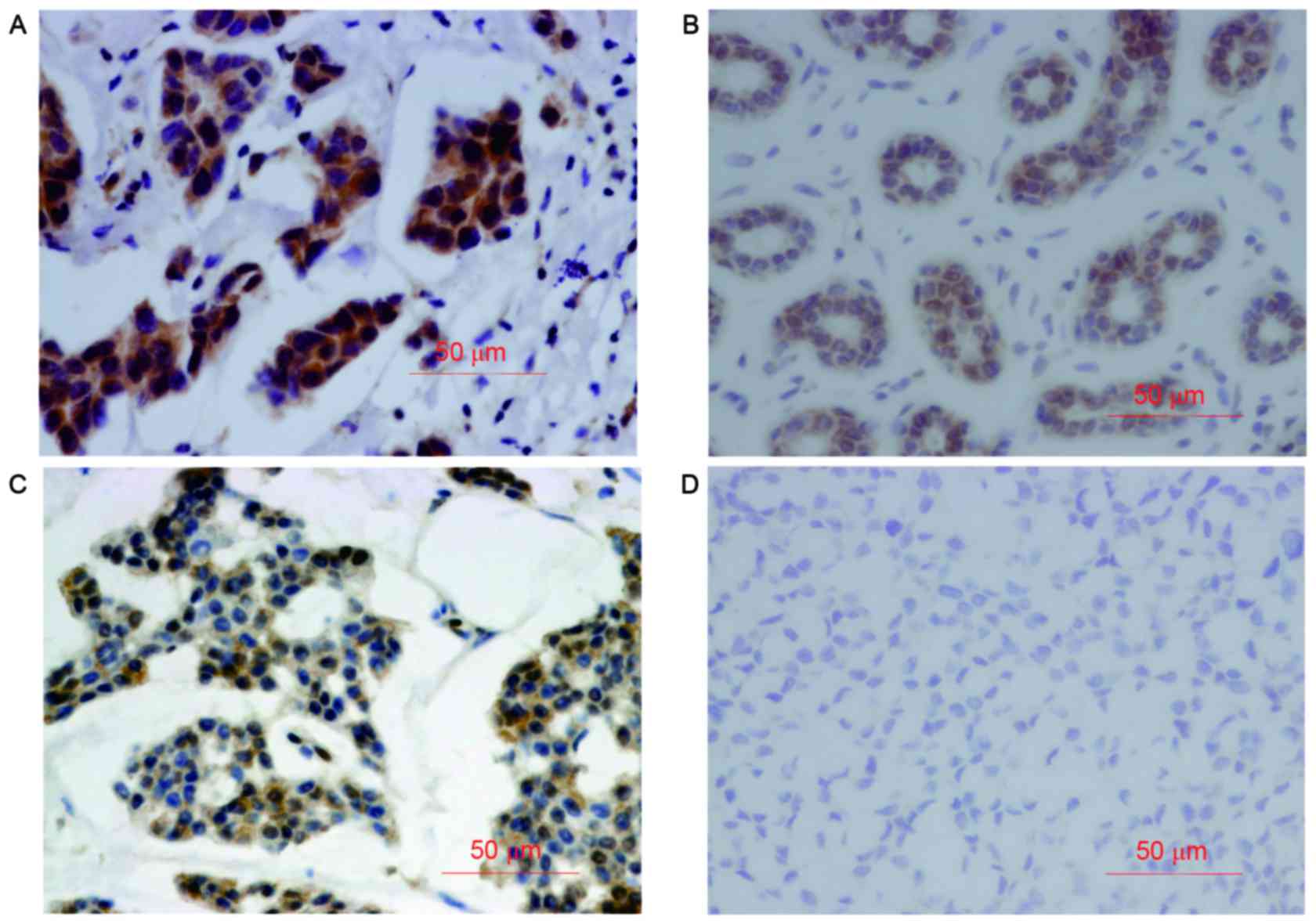
The positive staining of VEGF and caspase-3 was
mainly observed in the cytoplasm (Fig.
1). The expression of VEGF and caspase-3 was significantly
different between the MBC and IDC-NOS patients. In total, 42.59% of
MBC patients exhibited a high VEGF score (≥6), with this percentage
being 61.67% in the IDC-NOS group (P=0.042). Furthermore, 31 cases
(57.4%) of MBC patients exhibited high caspase-3 expression (≥6),
but only 20 cases (33.33%) in the IDC-NOS group exhibited high
caspase-3 expression (P=0.028) (Table
II).
 | Table II.VEGF and caspase-3 expression in MBC
and IDC-NOS. |
Table II.
VEGF and caspase-3 expression in MBC
and IDC-NOS.
| Expression | MBC, n (%) | IDC-NOS, n (%) | P-value |
|---|
| VEGF high | 23 (42.59) | 37 (61.67) | 0.042a |
| Caspase-3 high | 31 (57.41) | 20 (33.33) | 0.010a |
Association between VEGF and caspase-3
expression and DFS in MBC patients
Since the expression, function and mechanism of
IDC-NOS is already clear, the present study shows the associations
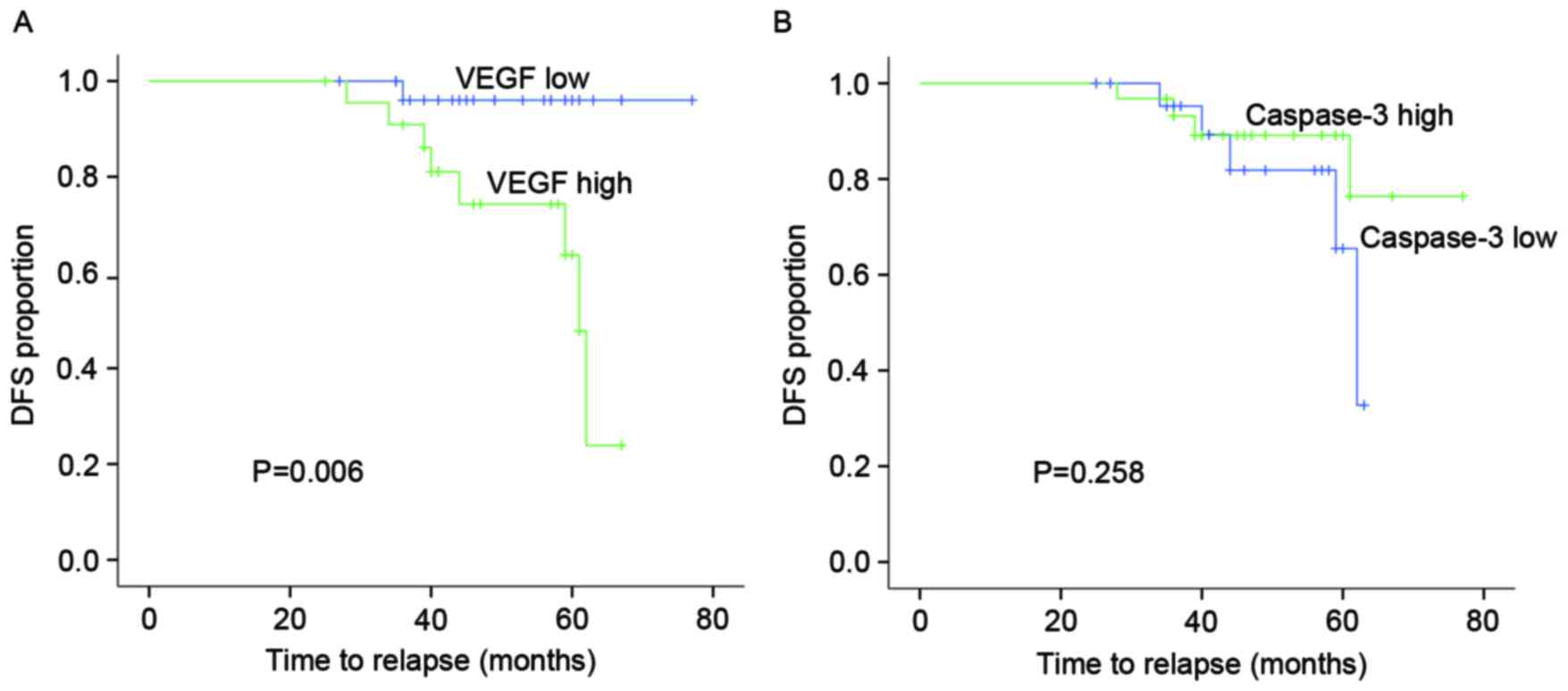
between them in MBC. Kaplan-Meier log-rank test showed that the
patients with high VEGF expression tended to experience shorter DFS
times (P=0.006) compared with those with low expression in MBC.
However, there was no association between caspase-3 expression and
DFS time in MBC patients (Fig.
2).
Association between VEGF and caspase-3
expression and clinicopathological variables in MBC patients
There was a significant association between VEGF
expression and age, nodal status and TNM stage in the MBC patients,
but there was no significant association between caspase-3
expression and age, tumor stage, nodal status, TNM stage, estrogen
receptor (ER) status, progesterone receptor (PR) status or Ki-67
expression (Table III).
 | Table III.Association between VEGF, caspase-3
and other variables in MBC. |
Table III.
Association between VEGF, caspase-3
and other variables in MBC.
| Variables | VEGF (−) | VEGF (+) | P-value | Caspase-3 (−) | Caspase-3 (+) | P-value |
|---|
| Age, years |
|
| 0.013a |
|
| 0.783 |
|
≤50 | 20 | 7 |
| 12 | 15 |
|
|
>50 | 11 | 16 |
| 11 | 16 |
|
| Tumor stage |
|
| 0.220 |
|
| 0.658 |
|
pT1 | 11 | 12 |
| 9 | 14 |
|
|
pT2-3 | 20 | 11 |
| 14 | 17 |
|
| Nodal status |
|
| 0.002a |
|
| 0.717 |
|
Negative | 30 | 15 |
| 20 | 25 |
|
|
Positive | 1 | 8 |
| 3 | 6 |
|
| TNM stage |
|
| 0.008a |
|
| 1.000 |
|
I–IIa | 30 | 16 |
| 20 | 26 |
|
|
IIb-IIIc | 1 | 7 |
| 3 | 5 |
|
| ER |
|
| 1.000 |
|
| 0.384 |
|
Negative | 3 | 3 |
| 4 | 2 |
|
|
Positive | 28 | 20 |
| 19 | 29 |
|
| PR |
|
| 0.107 |
|
| 0.554 |
|
Lowb | 12 | 14 |
| 10 | 16 |
|
|
Highc | 19 | 9 |
| 13 | 15 |
|
| Ki-67, % |
|
| 0.358 |
|
| 0.346 |
|
≤20 | 22 | 19 |
| 16 | 25 |
|
|
>20 | 9 | 4 |
| 7 | 6 |
|
Cox analysis in MBC
Univariate Cox regression analyses showed higher
VEGF score, positive nodal status and higher TNM stage were
significant predictors of worse DFS. Caspase-3 expression had no
significant predictive value in terms of DFS. Moreover,
multivariate analysis analyzed the correlation between DFS and VEGF
expression, nodal status and TNM stage, and found that TNM stage
was significantly associated with worse DFS (Table IV). Ki-67 expression was also
analyzed. The rate of Ki-67 (≤20%) expression was 75.93% in the MBC
patients compared with 50% in the IDC-NOS patients (P<0.05),
suggesting that MBC tumors may have lower proliferative ability
than IDC-NOS tumors. However, Cox survival analysis showed that
Ki-67 expression was not significantly associated with DFS in the
MBC patients.
 | Table IV.Cox univariate analysis and
multivariate analysis of clinicopathological variables, including
VEGF, for DFS in MBC. |
Table IV.
Cox univariate analysis and
multivariate analysis of clinicopathological variables, including
VEGF, for DFS in MBC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| VEGF |
|
|
|
|
|
|
|
High | 10.640 | (1.326–85.403) | 0.026a | 5.881 | (0.632–54.741) | 0.120 |
|
Low |
|
|
|
|
|
|
| Caspase-3 |
|
|
|
|
|
|
|
High | 0.473 | (0.125–1.785) | 0.269 |
|
|
|
|
Low |
| Age, years |
|
|
|
|
|
|
|
≤50 | 1.809 | (0.452–7.244) | 0.402 |
|
|
|
|
>50 |
|
|
|
|
|
|
| Tumor stage |
|
|
|
|
|
|
|
pT2/pT3 | 1.941 | (0.479–7.863) | 0.353 |
|
|
|
|
pT1 |
|
|
|
|
|
|
| Nodal status |
|
|
|
|
|
|
|
Positive | 5.844 | (1.452–23.529) | 0.013a | 0.477 | (0.060–3.809) | 0.485 |
|
Negative |
|
|
|
|
|
|
| TNM |
|
|
|
|
|
|
|
II/III | 11.689 | (2.762–49.465) | 0.001a | 10.386 | (1.230–87.703) | 0.032a |
| I |
|
|
|
|
|
|
| ER |
|
|
|
|
|
|
|
Positive | 0.872 | (0.172–4.409) | 0.868 |
|
|
|
| Negative |
|
|
|
|
|
|
| PR |
|
|
|
|
|
|
|
Highb | 0.542 | (0.135–2.618) | 0.386 |
|
|
|
|
Lowc |
|
|
|
|
|
|
| Ki-67, % |
|
|
|
|
|
|
|
>20 | 1.299 | (0.262–6.454) | 0.749 |
|
|
|
|
≤20 |
|
|
|
|
|
|
Discussion
The majority of previous MBC clinical studies found
that MBC showed higher ER- and PR-positive rates, less lymph node
metastasis, lower TNM stage and notably higher OS and DFS rates
(16–20). The present study analyzed the basic
information of MBC patients and compared it with that from IDC-NOS
patients treated in the same period. The results were consistent
with those of the previous studies, in which the patients with MBCs
had a better prognosis than those with IDC-NOS.
Jao et al (21)
studied 7 cases of MBC using electron microscopy and found that in
addition to the abundant production of mucosubstance, MBC also
featured the absence of myoepithelial differentiation and basal
lamina deposition, the presence of notably developed cytoplasmic
filamentous systems, a relatively scarcity of lysosomes, apparently
and frequently well-developed intercellular junctions and a marked
paucity of stromal vessels. These data suggest that the favorable
clinical prognosis of MBC may be the result of multiple complicated
factors (21).
Tumor growth requires constant vascular growth and
remodeling so that solid tumors can exceed 1–2 mm3 in
size. VEGF and its receptors are key regulators of angiogenesis,
meaning that they are attractive therapeutic targets (22). Microvessel density and tumor VEGF
expression in hepatocellular carcinoma have previously been
assessed, and the results indicated that upregulation of VEGF
promoted angiogenesis, tumor growth and intrahepatic metastasis
(23). VEGF-C-producing cancer cells
may induce lymphatic vessel proliferation and dilation, resulting
in cancer cell invasion into the lymphatic vessels and lymph node
metastasis (24). Cancer treatment
using a number of VEGF-targeted inhibitory agents is currently
being assessed. VEGF-targeted therapy has been approved for the
clinical treatment of metastatic triple-negative breast cancer
(25). The paucity of stromal vessels
in MBC may be an important adverse factor for angiogenesis
(21). In the present study, VEGF
expression was assessed in MBC and IDC-NOS samples, and VEGF
expression in MBC was found to be lower than that in IDC-NOS. It
was concluded that low VEGF expression in MBC may be associated
with the paucity of tumor vessels and result in a better prognosis.
In the MBC patients, high VEGF expression was significantly
associated with primary lymph node metastasis and high TNM stage.
Kaplan-Meier curves showed that VEGF was associated with DFS in the
MBC patients, while in the Cox multivariate analysis model, only
TNM stage was the independent prognostic factor.
A large amount of mucus is a typical feature of MBC
samples. Norris and Taylor (8) found
that the mucus in the tumor tissues plays an important role in the
prognosis of the patients and the whole clinical course of the
disease. Ahmed (10) noted that the
formation of mucus is caused by the breakdown of cancer cells,
followed by the degeneration of mitochondria, and that it leads to
a marked decrease in tumor invasion. In addition to necrosis,
apoptosis is a large part of cell disintegration. The caspases are
a family of genes that are important for the maintenance of
homeostasis via the regulation of cell death and inflammation
(26). Caspase-3 is the key molecular
factor in various apoptotic pathways (27,28). The
present study examined caspase-3 expression in each group. MBC
samples showed high caspase-3 expression, suggesting that
caspase-3-mediated apoptosis may hinder tumor progression in MBC
patients. Unexpectedly, caspase-3 expression was not associated
with DFS or other clinicopathological parameters in the MBC
patients.
The Ki-67 index has potential prognostic and
predictive value in breast cancer, and has become an important,
routinely used proliferation biomarker (29). The present study analyzed the
expression of Ki-67 and found that the rate of Ki-67 (≤20%)
expression in MBC was 75.93% compared with 50% in IDC-NOS. A
significant difference exists between MBC and IDC-NOS, which
suggests that tumor cells of MBC may have a lower proliferation
ability than those of IDC-NOS. However, Cox survival analysis
showed that Ki-67 expression was not directly associated with DFS
in MBC patients.
In conclusion, the present study revealed that high
rate of hormone receptor and caspase-3, low expression of VEGF and
Ki-67 and earlier TNM stage may contribute to improved prognosis of
MBC compared with IDC-NOS. VEGF and caspase-3 may serve a role in
mucus production, which is important in MBC progression. However,
neither high expression of VEGF nor caspase-3 had a significant
direct association with the DFS of patients with MBC. The mucinous
breast cancer cell lines may need to be cultured in the future to
explore the proliferation, invasion and migration ability of cancer
cells and the exact role of mucus in tumor progression.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81172199).
References
|
1
|
Dumitru A, Procop A, Iliesiu A, Tampa M,
Mitrache L, Costache M, Sajin M, Lazaroiu A and Cirstoiu M:
Mucinous breast cancer: A review study of 5 year experience from a
hospital-based series of cases. Maedica (Buchar). 10:14–18.
2015.PubMed/NCBI
|
|
2
|
Ranade A, Batra R, Sandhu G, Chitale RA
and Balderacchi J: Clinicopathological evaluation of 100 cases of
mucinous carcinoma of breast with emphasis on axillary staging and
special reference to a micropapillary pattern. J Clin Pathol.
63:1043–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Komaki K, Sakamoto G, Sugano H, Morimoto T
and Monden Y: Mucinous carcinoma of the breast in Japan. A
prognostic analysis based on morphologic features. Cancer.
61:989–996. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha KY, Deleon P and Deleon W: Invasive
mucinous carcinoma of the breast. Proc (Bayl Univ Med Cent).
26:295–297. 2013.PubMed/NCBI
|
|
5
|
Le Querrec A, Duval D and Tobelem G:
Tumour angiogenesis. Baillieres Clin Haematol. 6:711–730. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gasparini G: Prognostic value of vascular
endothelial growth factor in breast cancer. The oncologist 5 Suppl.
1:37–44. 2000. View Article : Google Scholar
|
|
8
|
Norris HJ and Taylor HB: Prognosis of
mucinous (Gelatinous) carcinoma of the breast. Cancer. 18:879–885.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsukita S, Nomoto M, Kitajima S, Tanaka
S, Goto M, Irimura T, Kim YS, Sato E and Yonezawa S: Expression of
mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the
breast: Comparison with invasive ductal carcinoma. Histopathology.
42:26–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed A: The myoepithelium in human breast
carcinoma. J Pathol. 113:129–135. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buckley CD, Pilling D, Henriquez NV,
Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN,
Lord JM and Salmon M: RGD peptides induce apoptosis by direct
caspase-3 activation. Nature. 397:534–539. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jänicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 414:13–21.
2008.PubMed/NCBI
|
|
14
|
Frank GA, Danilova NV, Andreeva IuIu and
Nefedova NA: WHO classification of tumors of the breast, 2012. Arkh
Patol. 75:53–63. 2013.(In Russian). PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR and Compton CC: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY: 2010
|
|
16
|
Kashiwagi S, Onoda N, Asano Y, Noda S,
Kawajiri H, Takashima T, Ohsawa M, Kitagawa S and Hirakawa K:
Clinical significance of the sub-classification of 71 cases
mucinous breast carcinoma. Springerplus. 2:4812013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zekioglu O, Erhan Y, Ciris M and
Bayramoglu H: Neuroendocrine differentiated carcinomas of the
breast: A distinct entity. Breast. 12:251–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ
and Yang JH: Mucinous carcinoma of the breast in comparison with
invasive ductal carcinoma: Clinicopathologic characteristics and
prognosis. J Breast Cancer. 14:308–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Koo J, Kim JH, Yang WI, Park BW
and Lee KS: Clinicopathological characteristics of mucinous
carcinoma of the breast in Korea: Comparison with invasive ductal
carcinoma-not otherwise specified. J Korean Med Sci. 25:361–368.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng HS, Lin C, Chan SE, Chien SY, Kuo
SJ, Chen ST, Chang TW and Chen DR: Pure mucinous carcinoma of the
breast: Clinicopathologic characteristics and long-term outcome
among Taiwanese women. World J Surg Oncol. 11:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jao W, Lao IO, Chowdhury LN and Gould VE:
Ultrastructural aspects of mucinous (colloid) breast carcinoma.
Diagn Gynecol Obstet. 2:83–92. 1980.PubMed/NCBI
|
|
22
|
Rosen LS: VEGF-targeted therapy:
Therapeutic potential and recent advances. Oncologist. 10:382–391.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ng IO, Poon RT, Lee JM, Fan ST, Ng M and
Tso WK: Microvessel density, vascular endothelial growth factor and
its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J
Clin Pathol. 116:838–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yonemura Y, Endo Y, Fujita H, Fushida S,
Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K
and Sasaki T: Role of vascular endothelial growth factor C
expression in the development of lymph node metastasis in gastric
cancer. Clin Cancer Res. 5:1823–1829. 1999.PubMed/NCBI
|
|
25
|
Hein A, Lambrechts D, von Minckwitz G,
Häberle L, Eidtmann H, Tesch H, Untch M, Hilfrich J, Schem C, Rezai
M, et al: Genetic variants in VEGF pathway genes in neoadjuvant
breast cancer patients receiving bevacizumab: Results from the
randomized phase III GeparQuinto study. Int J Cancer.
137:2981–2988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kontzoglou K, Palla V, Karaolanis G,
Karaiskos I, Alexiou I, Pateras I, Konstantoudakis K and Stamatakos
M: Correlation between Ki67 and breast cancer prognosis. Oncology.
84:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|