Introduction
Pancreatic cancer is one of the most lethal
malignancies. Approximately 35,000 cases were reported in Japan in
2014, of whom 32,000 died, and the 5-year survival rate has been
estimated as 7% (1). Malignant
neoplasms of the pancreas are classified based on the cellular
direction of differentiation into ductal, acinar, or neuroendocrine
carcinomas (NEC), or pancreatoblastoma (2). Pancreatic ductal adenocarcinomas
comprise about 90% of cases, NEC comprise 5%, while acinar cell
carcinomas (ACC) are the rarest at 1–2% (3). Although most pancreatic tumors arise as
a single cell type, either from the endocrine or exocrine pancreas,
mixed neoplasms are listed under WHO classifications as carcinomas
with mixed differentiation including mixed acinar-neuroendocrine
carcinoma, mixed acinar-ductal carcinoma, and mixed
acinar-neuroendocrine-ductal carcinoma (4). These mixed carcinomas are extremely
rare, so their clinical and genomic features are poorly understood.
Furthermore, it is not clear whether mixed carcinomas derive from
the same or distinct tumor clones within a nodule.
Here, we report a case of mixed
acinar-neuroendocrine-ductal carcinoma for which we performed
genetic analysis. We describe the pathological,
immunohistochemical, and molecular characterizations of a
pancreatic mixed acinar-neuroendocrine-ductal carcinoma.
Subjects and methods
Patient and sample preparation
Written informed consent for the research study and
publication was obtained from this case, which was performed in
accordance with the protocols approved by the Institutional Review
Board at our hospital. The study complied with Declaration of
Helsinki principles and its later amendments ethical standards.
Peripheral blood samples were obtained and buffy coats were
isolated. Buffy coat DNA was extracted using the QIAamp DNA blood
mini QIAcube kit (Qiagen, Hilden, Germany) (5).
Immunohistochemical analysis and
laser-capture microdissection
The sections were deparaffinized before the antigens
were retrieved by heat treatment in an EDTA solution at pH 8.0.
Protein expression was evaluated using 3-µm-thick formalin-fixed
and paraffin-embedded (FFPE) sections with anti-chromogranin A
(1:50 dilution; clone 5H7, NCL-CHROM-430; Novocastra, Newcastle,
UK), anti-trypsin (1:400 dilution; Meridian Life Science, Memphis,
TN, USA), anti-mucin 1 (MUC1) (1:50, clone DF-6; Novocastra),
anti-Ki67 (ready-to-use, clone SP6, Nichirei, Tokyo, Japan),
anti-TP53 (1:200, clone DO7; Leica Biosystems, Wetzlar, Germany)
antibodies using using the Ventana BenchMark ULTRA system (Roche,
Tucson, AZ, USA). A serial section of 10-µm FFPE tissue was stained
with haematoxylin-eosin and then microdissected using an ArcturusXT
laser-capture microdissection system (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The ductal component and mixed NEC/ACC
component were microdissected from FFPE tumor tissue. FFPE DNA was
extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen) (6).
Next-generation sequencing
Tumor and buffy coat samples were subjected to
next-generation sequencing analysis using the Ion AmpliSeq™ Cancer
Hotspot Panel v2 (Thermo Fisher Scientific, Inc.), targeting the
hotspot regions of 50 oncogenes and tumor suppressor genes.
Sequencing library was prepared by multiplex PCR with Ion AmpliSeq
Library kit 2.0 (Thermo Fisher Scientific, Inc.) as previously
before (6). Variants calling and
annotations were performed using an Ion Reporter Server System
(Thermo Fisher Scientific, Inc.), and buffy coat DNA was used as a
control to detect variants in tumors by ‘AmpliSeq CHPv2
tumor-normal pair’ workflow.
Results
A 50-year-old male was referred to our hospital with
intermittent symptoms of epigastralgia and back pain. He had no
history of smoking or drinking, and underwent an appendectomy at 10
years of age. Physical examination revealed no anaemia or icteric
findings. No tumor was palpable, but slight tenderness was felt at
the epigastrium without rebound tenderness. Laboratory data showed
slight elevation of amylase at 452 U/ml and lipase at 403 U/l,
while serum tumor markers were not elevated: carcinoembryonic
antigen (CEA) level at 1.0 ng/ml, carbohydrate antigen 19-9
(CA19-9) levels at 20.3 U/ml, Dupan-2 at <25 U/ml, and Span-1 at
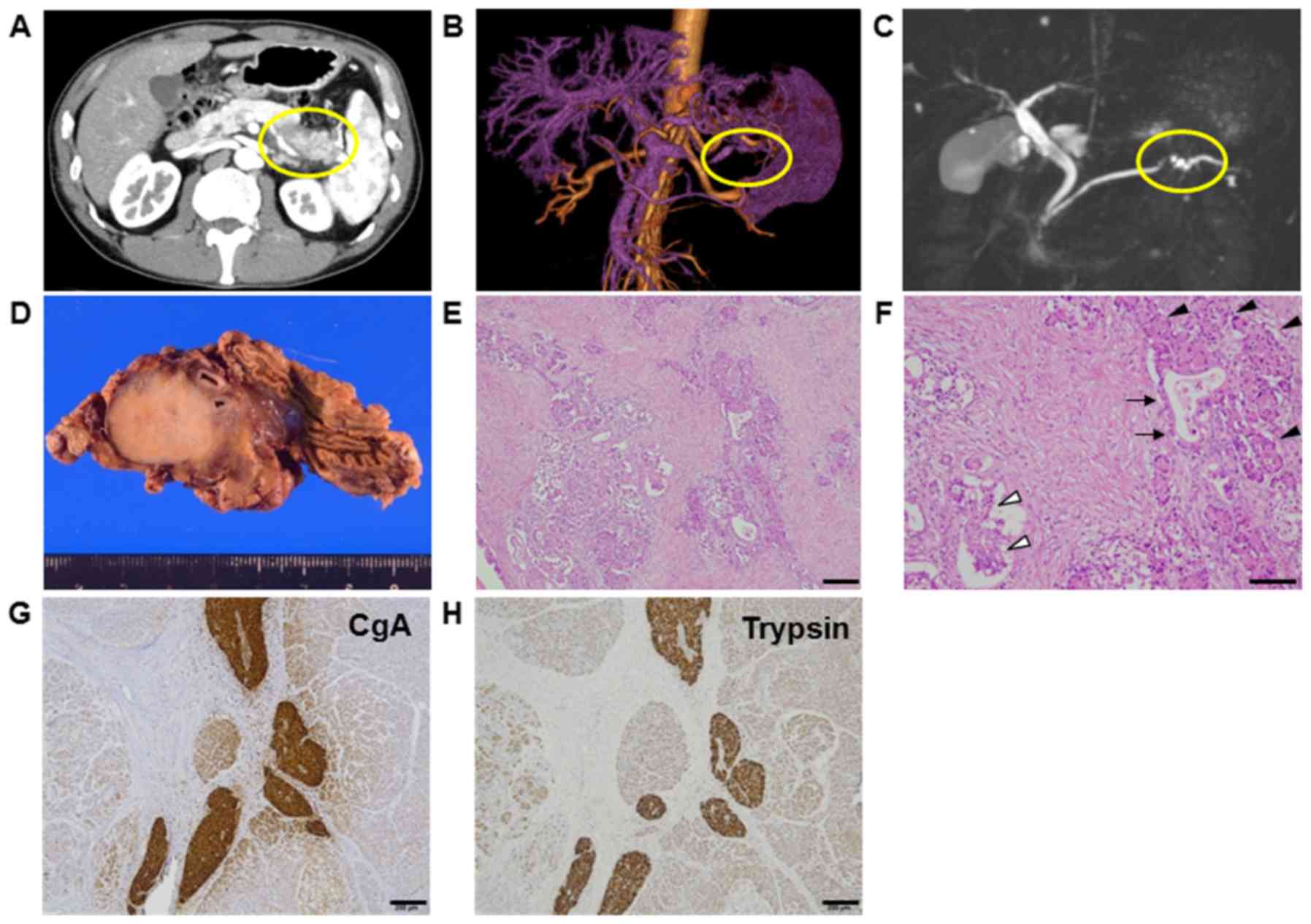
<11 U/ml. Abdominal computed tomography revealed a tumor
measuring 3 cm in diameter within the body of the pancreas
(Fig. 1A). Tumor enhancement from the
splenic artery was indicated by magnetic resonance angiography
(MRA) (Fig. 1B), and stenosis of the
pancreatic duct was detected by magnetic resonance
cholangiopancreatography (MRCP) (Fig.
1C). He was diagnosed with pancreatic cancer and underwent
distal pancreatectomy with splenectomy. Gross findings showed a
round, hard tumor with a serrated margin measuring 3 cm in diameter
in the body of the pancreas, (Fig.
1D). The postoperative course was uneventful for 1 year.
Pathological findings
Pathological examinations showed that the tumor was
histologically well to moderately differentiated adenocarcinoma.
Eosinophilic cytoplasm was also observed in solid neoplasm, mixed
with aforementioned ductal adenocarcinoma. This eosinophilic
cytoplasm pattern resembled acinar cell and/or islet cell.
Approximately 50% of each histological component was present in
tumor (Fig. 1E and F). In the solid
component, vascular invasion was observed. Lymph node metastasis
from the ductal adenocarcinoma was observed in one lymph node (data
not shown). Immunohistochemical analysis revealed positive
expression of chromogranin A and trypsin as markers of NEC and ACC,
respectively. These protein expressions were mostly overlapped in
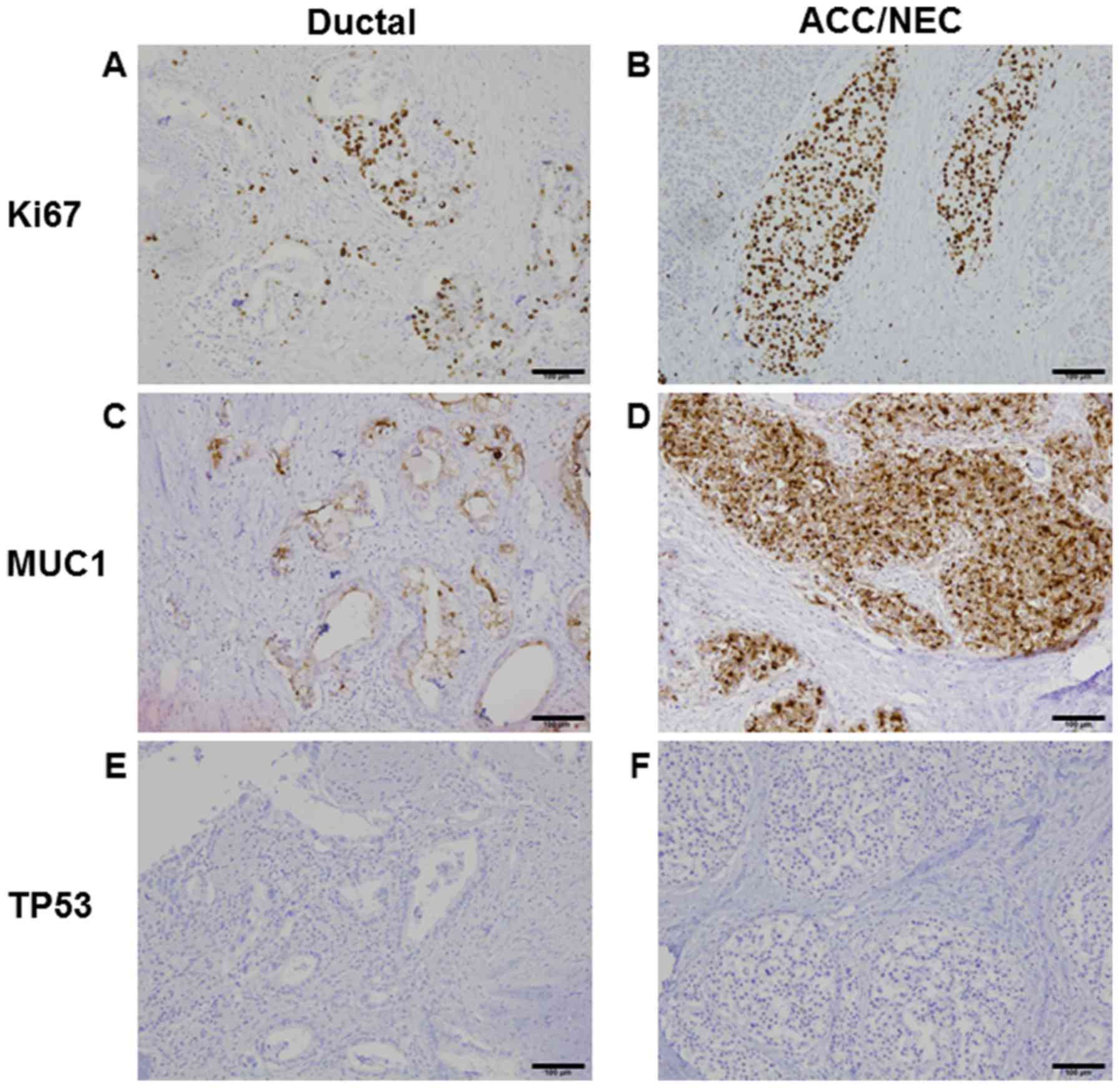
NEC and ACC, but not stained in ductal adenocarcinoma (Fig. 1G and H). Ki67 protein expression was
50% in ductal adenocarcinoma and 80% in NEC and ACC, suggesting
Ki67 index was high in both components (Fig. 2A and B). Although MUC production was
seen only in ductal adenocarcinoma (data not shown), MUC1 was
positive in all tumor components (Fig. 2C
and D). TP53 protein expression is negative in both ductal and
ACC/NEC components (Fig. 2E and F).
Mixed adenoneuroendocrine carcinoma (MANEC) was neglected because
of the presence of ACC.
Genomic analysis
To examine whether genetic profiles were similar or
distinct in ductal and ACC/NEC components, we performed targeted
sequencing using AmpliSeq Cancer Hotspot Panel v2, which covers the
hotspot regions of 50 cancer-related genes. We obtained the tumor
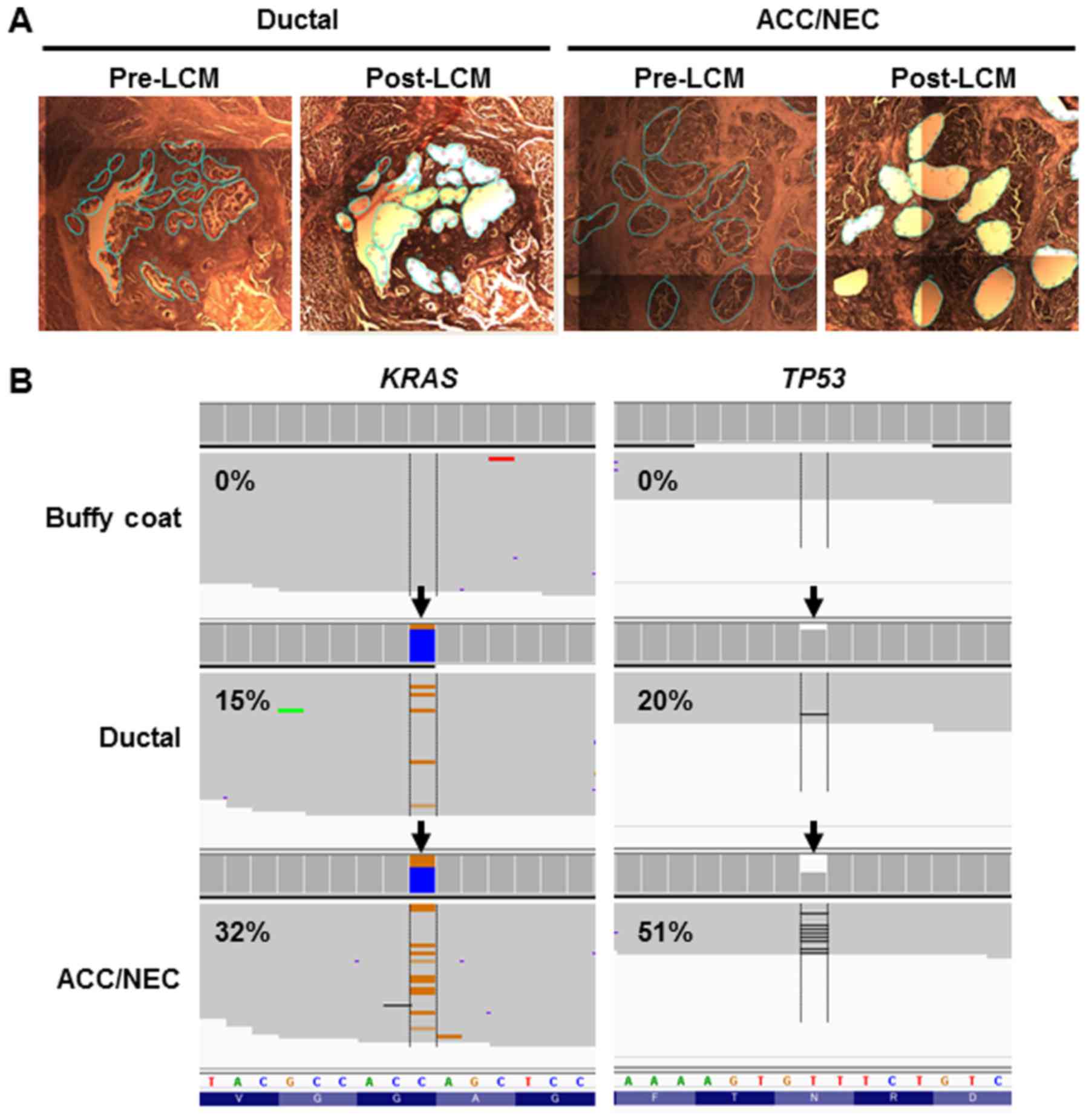
cells from ductal or ACC/NEC components by laser-capture
microdissection (Fig. 3A).
Next-generation sequencing analysis yielded a sufficient number of
mapped reads onto targeted regions, and the mean depth coverage was
2673x in the ductal tumor component and 4430x in the NEC/ACC mixed
component (Table I). We identified
the same two mutations (TP53 N210fs and KRAS G12R) in
both ductal and acinar/neuroendocrine lesions (Fig. 3B and Table
II). In accordance with this, TP53 protein expression was
diminished (Fig. 2E and F), because
the TP53 frameshift mutation was present in both neoplastic
components. These results indicated that these distinct
histological components derived from the same tumor clone and that
the pancreatic ductal and ACC/NEC carcinomas developed from the
same phylogeny in the present case.
 | Table I.Sequencing reads and coverage data
from next-generation sequencing. |
Table I.
Sequencing reads and coverage data
from next-generation sequencing.
| Sample name | Mapped reads | On target (%) | Mean depth | Uniformity (%) |
|---|
| Tumor ductal | 577,133 | 98.82 | 2,673 | 99.22 |
| Tumor ACC/NEC | 959,353 | 98.62 | 4,430 | 97.52 |
| Buffy coat | 888,135 | 96.13 | 4,045 | 98.31 |
 | Table II.Genetic alterations in ductal and
ACC/NEC. |
Table II.
Genetic alterations in ductal and
ACC/NEC.
| Histology | Position | Reference | Variant | Gene | Mutation | Allelic fraction
(%) |
|---|
| Ductal |
chr12:25398280 | C | G | KRAS | G12R | 15 |
| Ductal | chr17:7578219 | GT | G | TP53 |
N210fs | 20 |
| ACC/NEC |
chr12:25398280 | C | G | KRAS | G12R | 32 |
| ACC/NEC | chr17:7578219 | GT | G | TP53 |
N210fs | 51 |
Discussion
The pancreas is composed of both exocrine and
endocrine gland components: The exocrine part is formed from ductal
and acinar cells, whereas the endocrine component is made up of
endocrine cells. Most pancreatic tumors arise from a single cell
type; therefore, ductal carcinomas and ACCs are considered to
derive from the exocrine gland, and NECs from the endocrine gland.
However, the existence of carcinomas with mixed differentiation
complicates the situation. Additionally, few reports have defined
the biological behaviour of mixed carcinomas. For example,
pancreatic NEC and ACC appear to be distinct entities, but their
pathological and morphological appearances can be similar. ACC has
been reported to express neuroendocrine markers such as
synaptophysin and chromogranin A, while the coexistence of ACC and
NEC has also been reported. These observations suggest that the
origin of the three types of pancreatic cancer (ductal, ACC, and
NEC) is not clearly defined as either exocrine or endocrine
glands.
Genetic profiles provide the direct evidence of
tumor origins in the different histological components. While gene
sequencing has demonstrated shared mutations in the different
histologic components of mixed carcinomas (e.g., MANEC) of other
digestive organs (7,8), it has not been proven in mixed
acinar-ductal adenocarcinoma of the pancreas. Here, we present a
case that showed the coexistence of ACC and NEC which were clearly
seen together with ductal carcinoma. To investigate whether the
different histological types derived from the same or a distinct
tumor origin, we performed next-generation sequencing analysis and
revealed genetic alterations in these distinct tumor histologies.
We identified the same TP53 and KRAS mutations in
both tumor samples from ductal carcinoma and mixed ACC/NEC
carcinoma. Of note, there are the same genetic patterns between the
two components, which were the distinct differentiation patterns.
These results suggest that the same tumor clones are related to the
development of pancreatic mixed acinar-neuroendocrine-ductal
carcinoma. In line with this findings, previous studies also
suggested the mixed neoplasm derived from same tumor origin in
endocrine-exocrine tumors of the gut and MANEC of the
gastrointestinal tract (7,8).
Genetic alterations in pancreatic cancer are
different among the histological types. For instance, KRAS,
TP53, SMAD4, and CDKN2A genes are most commonly
mutated in pancreatic ductal cancer (9,10). In
particular, KRAS mutations are observed about 90% of
pancreatic ductal adenocarcinoma. Contrary, TP53 can be mutated in
a subset of ACCs and, more frequently, in NECs (11,12).
However, KRAS mutations are rarely observed in ACCs
(13,14). Therefore, it is particularly
noteworthy that a KRAS mutation was detected in the mixed
ACC/NEC tumor component of the present case. To understand this, it
is necessary to consider previously-reported step-wise pancreatic
tumorigenesis models. The most common model is that pancreatic
intraepithelial neoplasia is a neoplastic precursor of invasive
pancreatic ductal adenocarcinoma (15). Another is that pancreatic cancer
develops from acinar cells (16). In
the genetic pancreatic cancer mouse model, KRAS activation induces
the dedifferentiation of acinar cells, which transform and lose the
expression of typical marker proteins; thus, acinar to ductal
metaplasia (ADM) is a precancerous form of pancreatic ductal
carcinoma (17–19), and acinar cell hyperplasia has been
observed in the human pancreas (20).
In this study, we identified TP53 and KRAS mutations
that were shared by ductal and ACC/NEC tumors. This indicates that
KRAS activation in acinar cells, as well as other genetic or
epigenetic changes, triggers ADM and results in subsequent cell
differentiation into ductal adenocarcinoma. To our knowledge, this
is the first report of common mutational profiles in ductal and
acinar carcinomas in a human pancreatic tumor, which may provide
genetic evidence of pancreatic tumorigenesis involving ADM.
Acknowledgements
The authors would like to thank Hidetoshi Shigetomo,
Yumi Kubota, and Ritsuko Yokouchi for their help. This study was
supported by a Grant-in-Aid for Genome Research Project from
Yamanashi Prefecture (to Y.H. and M.O.) and a grant from The YASUDA
Medical Foundation (to Y.H.).
References
|
1
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H: Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the Monitoring of Cancer
Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longnecker DS: Pathologyof exocrine
pancreatic neoplasms. UpToDate. 2017 https://www.uptodate.com/contents/pathology-of-exocrine-pancreatic-neoplasmsAccessed.
Feb 06–2017.
|
|
3
|
Klimstra DS: Nonductal neoplasms of the
pancreas. Mod Pathol. 20 Suppl 1:S94–S112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukushima N, Hruban RH, Kato Y, et al:
Ductal adenocarcinoma variants and mixed neoplasms of the
pancreasWHO classification of Tumors of the Digestive System.
Bosman FT, Camerio F, Hruban RH and Thiise ND: Lyon: IARC; 2010
|
|
5
|
Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya
K, Mochizuki H and Omata M: Detection of BRCA1 and BRCA2 germline
mutations in Japanese population using next-generation sequencing.
Mol Genet Genomic Med. 3:121–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirotsu Y, Zheng TH, Amemiya K, Mochizuki
H, Guleng B and Omata M: Targeted and exome sequencing identified
somatic mutations in hepatocellular carcinoma. Hepatol Res.
5:1145–1151. 2016. View Article : Google Scholar
|
|
7
|
Furlan D, Cerutti R, Genasetti A, Pelosi
G, Uccella S, La Rosa S and Capella C: Microallelotyping defines
the monoclonal or the polyclonal origin of mixed and collision
endocrine-exocrine tumors of the gut. Lab Invest. 83:963–971. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scardoni M, Vittoria E, Volante M, Rusev
B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G,
Butturini G, et al: Mixed adenoneuroendocrine carcinomas of the
gastrointestinal tract: Targeted next-generation sequencing
suggests a monoclonal origin of the two components.
Neuroendocrinology. 100:310–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waddell N, Pajic M, Patch AM, Chang DK,
Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al:
Whole genomes redefine the mutational landscape of pancreatic
cancer. Nature. 518:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al: Pancreatic cancer genomes reveal aberrations in axon
guidance pathway genes. Nature. 491:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
La Rosa S, Bernasconi B, Frattini M,
Tibiletti MG, Molinari F, Furlan D, Sahnane N, Vanoli A, Albarello
L, Zhang L, et al: TP53 alterations in pancreatic acinar cell
carcinoma: New insights into the molecular pathology of this rare
cancer. Virchows Arch. 468:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yachida S, Vakiani E, White CM, Zhong Y,
Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM,
et al: Small cell and large cell neuroendocrine carcinomas of the
pancreas are genetically similar and distinct from
well-differentiated pancreatic neuroendocrine tumors. Am J Surg
Pathol. 36:173–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao Y, Yonescu R, Offerhaus GJ, Klimstra
DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang
CL, et al: Whole-exome sequencing of pancreatic neoplasms with
acinar differentiation. J Pathol. 232:428–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chmielecki J, Hutchinson KE, Frampton GM,
Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O,
et al: Comprehensive genomic profiling of pancreatic acinar cell
carcinomas identifies recurrent RAF fusions and frequent
inactivation of DNA repair genes. Cancer Discov. 4:1398–1405. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tuveson DA and Neoptolemos JP:
Understanding metastasis in pancreatic cancer: A call for new
clinical approaches. Cell. 148:21–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmid RM: Acinar-to-ductal metaplasia in
pancreatic cancer development. J Clin Invest. 109:1403–1404. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eser S, Reiff N, Messer M, Seidler B,
Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B, et
al: Selective requirement of PI3K/PDK1 signaling for Kras
oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell.
23:406–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liou GY, Döppler H, DelGiorno KE, Zhang L,
Leitges M, Crawford HC, Murphy MP and Storz P: Mutant KRas-induced
mitochondrial oxidative stress in acinar cells upregulates EGFR
signaling to drive formation of pancreatic precancerous lesions.
Cell Rep. 14:2325–2336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi C, Hong SM, Lim P, Kamiyama H, Khan M,
Anders RA, Goggins M, Hruban RH and Eshleman JR: KRAS2 mutations in
human pancreatic acinar-ductal metaplastic lesions are limited to
those with PanIN: Implications for the human pancreatic cancer cell
of origin. Mol Cancer Res. 7:230–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longnecker DS, Shinozuka H and Dekker A:
Focal acinar cell dysplasia in human pancreas. Cancer. 45:534–540.
1980. View Article : Google Scholar : PubMed/NCBI
|