Introduction
Hepatocellular carcinoma (HCC) is the third-leading
cause of cancer-associated mortality worldwide, accounting for more
than 600,000 deaths per year (1). A
large proportion of these patients are not candidates for
potentially curative therapy, including surgical resection, liver
transplantation or local ablation therapy, as they are diagnosed
with advanced disease with vascular invasion and/or distant
metastasis (2,3). Sorafenib is a multi-kinase inhibitor,
which inhibits a number of kinase-dependent signaling pathways
associated with tumor progression and angiogenesis, including those
involving Raf serine/threonine kinase, vascular endothelial growth
factor receptor (VEGFR)-2, VEGFR-3, and platelet-derived growth
factor β-receptor (4,5). The efficacy of sorafenib was
demonstrated in two large phase III clinical trials including the
Sorafenib HCC Assessment Randomized Protocol (SHARP) trial and the
Asia-Pacific trial (conducted in the Asia-Pacific region) (6,7). Although
there was a survival benefit for patients with advanced HCC, only a
limited number of patients demonstrated a partial response, and
there were no cases of complete remission (CR). Furthermore, the
marginal observed survival gain of 2.8 months in the SHARP trial
and 2.3 months in the Asia-Pacific trial did not meet the
expectations of clinical practice. Following the approval of
sorafenib, several cases of CR subsequent to sorafenib therapy were
reported worldwide (8–11). However, there is little data regarding
the clinical course and safety of long-term sorafenib therapy in
cases of CR. Therefore, the present study reports a case of
long-term maintenance therapy with sorafenib following the
achievement of CR in a patient with advanced HCC.
Case report
A 72-year-old male patient was referred for the
evaluation of a liver mass identified on an abdominal ultrasound
performed for routine health screening purposes. The patient was
taking medications for diabetes, hypertension and an old cerebral
infarction. Initial laboratory results were as follows: White blood
cells 5,920/mm3 (normal range,
4–10×103/mm3), hemoglobin level 14.0 g/dl
(normal range, 12–16 g/dl), platelet count
237×103/mm3 (normal range,
100–300×103/mm3), alanine aminotransferase
(ALT) 29 IU/l (normal range, 0–40 IU/l), total bilirubin 1.0 mg/dl
(normal range, 0.1–1.1 mg/dl), albumin 3.9 mg/dl (normal range,
4.0–5.5 mg/dl) and prothrombin time 11.6 sec (normal range,
10.4–13.3 sec). With regard to virological markers, the patient was
positive for hepatitis C virus antibody and negative for hepatitis
B surface antigen. The serum α-fetoprotein (AFP) level and protein
induced by vitamin K absence or antagonist-II level were 60,500
ng/ml (normal range, 0–1 ng/ml) and 2,000 mAU/ml (normal range,
0–40 mAU/ml), respectively. Baseline electrocardiography (ECG) and
echocardiography revealed normal left ventricular systolic function
(left ventricular ejection fraction, 65%) with normal sinus rhythm.
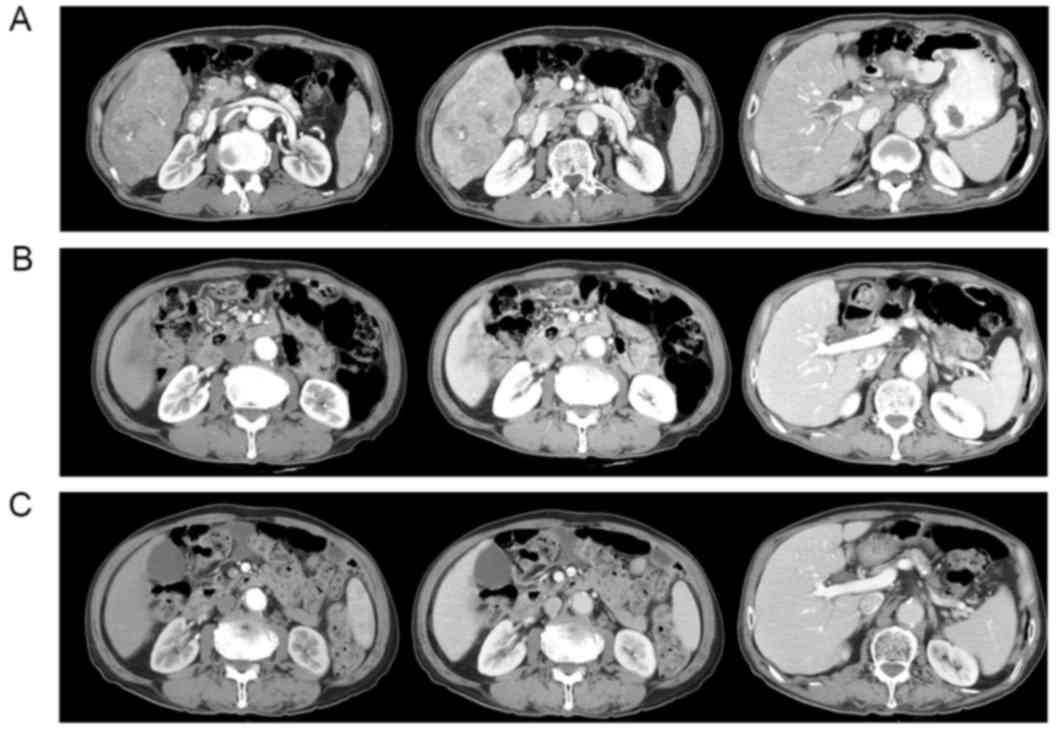
Multiphasic computed tomography (CT) scans of the abdomen
demonstrated a huge, 13 cm-sized mass replacing the right lobe of
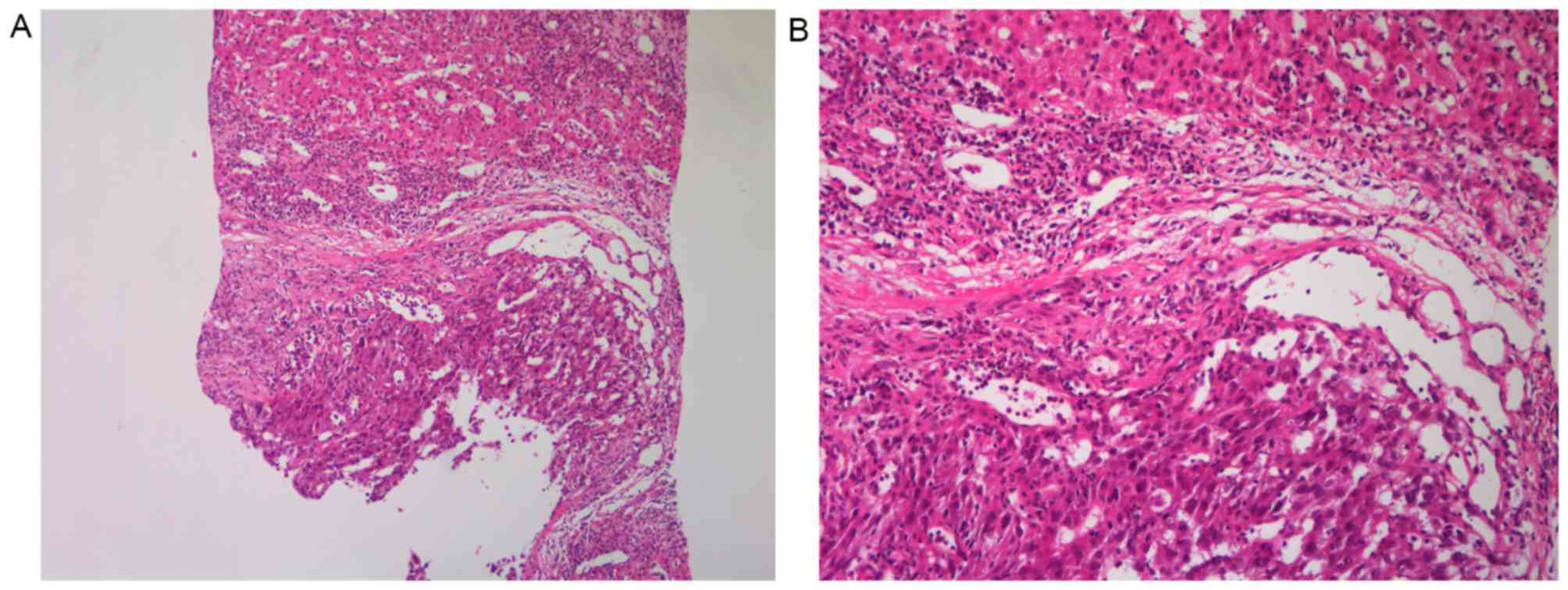
the liver, with portal vein thrombosis (Fig. 1A). HCC was confirmed by
ultrasound-guided percutaneous needle biopsy of the liver mass
(Fig. 2). The cancer was staged as
advanced HCC according to the Barcelona Clinic Liver Cancer staging
system (12) and the patient's
Eastern Cooperative Oncology Group performance status (13) was 1; the patient was treated with 400
mg sorafenib (Nexavar; Bayer AG, Leverkusen, Germany) twice daily.
Following the initiation of sorafenib therapy, the patient
experienced a grade 1 (14) hand-foot
skin reaction, which was well tolerated without requiring a dose
reduction.
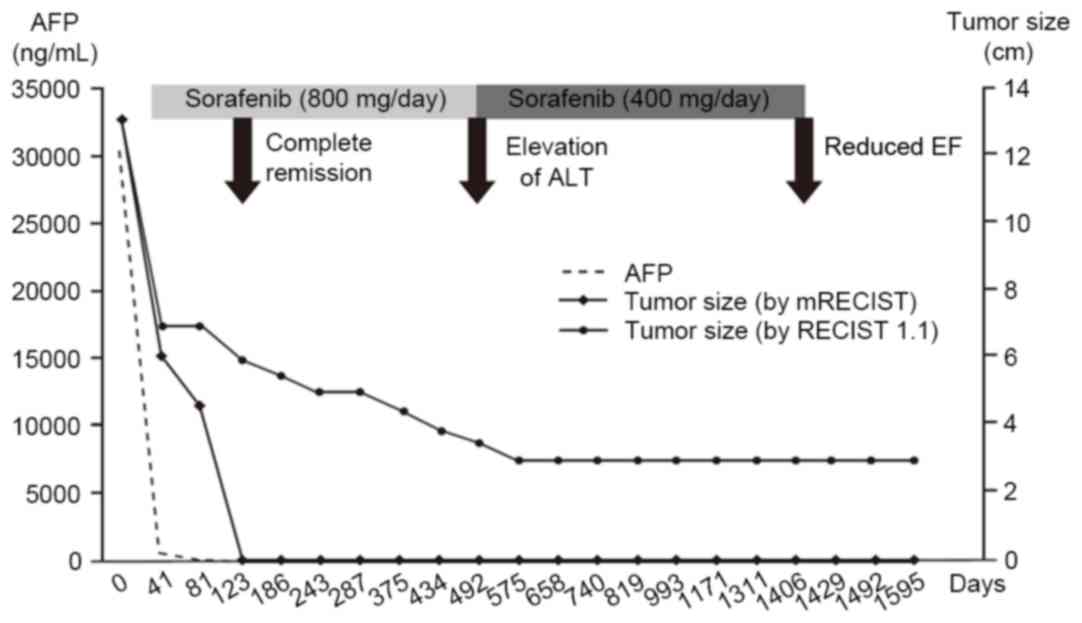
After 40 days of sorafenib therapy, the tumor size
had decreased to 6 cm and the serum AFP level had decreased to 571
ng/ml. The follow-up abdominal CT scans at 4 months demonstrated no
enhancing viable lesions in the tumor and recanalization of the
portal vein; additionally, serum AFP values normalized (8.42 ng/ml;
Fig. 1B). The sorafenib dose was
maintained at 400 mg twice daily for 16 months, after which the
sorafenib dose was reduced to 400 mg a day due to the elevation of
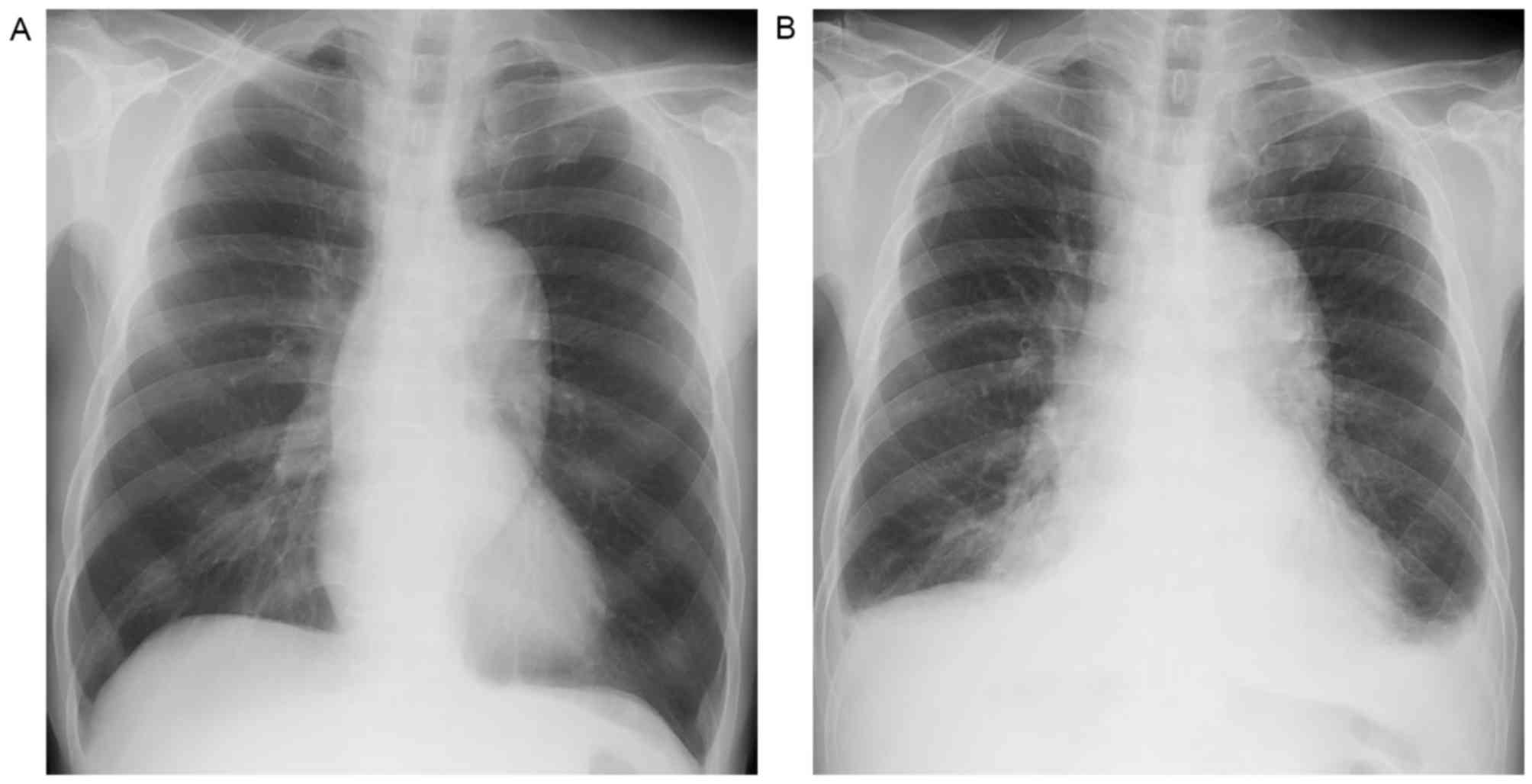
serum ALT (231 IU/ml). Sorafenib therapy was continued for 48
months until the patient experienced dyspnea due to congestive
heart failure with pleural effusion (Fig.
3). An echocardiogram revealed decreased left ventricular
systolic function (left ventricular ejection fraction, 40%),
including new-onset atrial fibrillation on the follow-up ECG.
Following the discontinuation of sorafenib and the addition of rate
control medication, the patient's dyspnea improved, with the
disappearance of the pleural effusion. Without sorafenib therapy,
the patient remained in a state of CR, followed up with serial CT
scans and tumor marker measurement, for 52 months from the time of
initiation of the sorafenib therapy (Fig.
1C). Written informed consent was obtained from the patient for
the publication of their clinical data and images.
Discussion
At present, sorafenib is the only chemotherapeutic
agent that has been proven to increase overall survival time in
patients with advanced HCC (6,7). Although
partial response was achieved in only 2 and 3.3% of patients in two
large phase III clinical trials, with no cases of CR (6,7), there
have been several reports of CR following sorafenib therapy in
patients with advanced HCC since sorafenib became available for
general clinical practice (8–11). The majority of CR cases exhibited
early tumor response following sorafenib therapy accompanied by a
rapid radiological response and the normalization of AFP within 6
months of sorafenib therapy (8–11). In
accord with previous reports, the present case exhibited a similar
clinical course, including a rapid radiologic response and the
normalization of serum AFP within 40 days of sorafenib therapy
(Fig. 4). Early AFP response
following the initiation of sorafenib therapy has previously been
identified as a positive prognostic factor for patients with
advanced HCC (15). Further
investigation regarding the contributing factors and molecular
pathophysiology of these sorafenib responders is required.
Although CR cases following sorafenib therapy have
been reported in the literature, there is limited data regarding
the long-term clinical course of these cases following the
achievement of CR (8–11). Therefore, there is no consensus on a
management strategy for CR-achieving patients. As in the present
case, if the patient can tolerate sorafenib therapy, long-term
sorafenib therapy may be an option as a maintenance treatment
following CR. However, in addition to the economic burden of
continuing sorafenib, there is limited data on safety profiles in
regard to long-term sorafenib therapy (16,17). As it
targets multiple angiogenic receptors, sorafenib causes a unique
spectrum of adverse events including skin rash, stomatitis and
cardiovascular toxicity. During sorafenib therapy, cardiovascular
adverse events, including hypertension, bleeding and cardiac or
cerebrovascular events were among the most serious observed in
clinical trials (6). Among these
cardiovascular toxicities, hypertension and bleeding are most
frequent adverse events associated with sorafenib therapy (18). Although there have been reports of
cardiotoxicity from the long-term sorafenib therapy of patients
with renal cell cancer and lung cancer, the present case is the
first report of cardiotoxicity caused by long-term sorafenib
therapy in a patient with advanced HCC; this adverse effect has
been underestimated by phase III studies due to the relatively
short-term treatment (19,20). The present case encourages the
clinician to monitor cardiac toxicity during sorafenib therapy,
particularly in elderly patients with underlying cardiovascular
diseases.
Compared with the literature to date, the present
case represents an HCC case with the longest follow-up subsequent
to CR induced by sorafenib therapy. Although the therapeutic
response was assessed by radiologic findings, rather than by
pathologic confirmation, CR was confirmed by long-term sustained
radiologic response with a normal AFP level, even subsequent to the
termination of sorafenib therapy. The present case provides the
valuable insight that long-term sorafenib therapy may be an option
subsequent to achieving CR through treatment with sorafenib, and
that efforts should be made to monitor for the appearance of less
common adverse events in patients receiving long-term sorafenib
therapy.
Acknowledgements
The present study was supported by the Kyungpook
National University Research Fund, 2010 (grant no.,
201014100000).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
VEGFR
|
vascular-endothelial growth factor
receptor
|
|
SHARP
|
sorafenib HCC assessment randomized
protocol trial
|
|
CR
|
complete remission
|
|
ALT
|
alanine aminotransferase
|
|
AFP
|
α-fetoprotein
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
European Association For The Study Of The
Liver1; European Organisation For Research And Treatment Of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn SY, Lee HS, Kweon YO, Tak WY and Park
SY: Sustained remission over 36 months of advanced hepatocellular
carcinoma after short-term sorafenib therapy. Dig Dis Sci.
58:1428–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JG: Long-term outcomes of patients
with advanced hepatocellular carcinoma who achieved complete
remission after sorafenib therapy. Clin Mol Hepatol. 21:287–294.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MS, Jin YJ, Lee JW, Lee JI, Kim YS,
Lee SY and Chae MH: Complete remission of advanced hepatocellular
carcinoma by sorafenib: A case report. World J Gastrointest Oncol.
5:38–42. 2013.PubMed/NCBI
|
|
11
|
Liu D, Liu A, Peng J, Hu Y and Feng X:
Case analysis of complete remission of advanced hepatocellular
carcinoma achieved with sorafenib. Eur J Med Res. 20:122015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J and Llovet JM: Prognostic
prediction and treatment strategy in hepatocellular carcinoma.
Hepatology. 35:519–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer Therapy Evaluation Program 3 (1998)
Common Toxicity Criteria. Version 2.0 DCTD, NCI, NIH, DHHS.
Revised: 23. 1998.
|
|
15
|
Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan
ST and Poon RT: The significance of early alpha-fetoprotein level
changes in predicting clinical and survival benefits in advanced
hepatocellular carcinoma patients receiving sorafenib. Oncologist.
16:1270–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hutson TE, Bellmunt J, Porta C, Szczylik
C, Staehler M, Nadel A, Anderson S, Bukowski R, Eisen T and
Escudier B: Sorafenib TARGET Clinical Trial Group: Long-term safety
of sorafenib in advanced renal cell carcinoma: Follow-up of
patients from phase III TARGET. Eur J Cancer. 46:2432–2440. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adjei AA, Blumenschein GR Jr, Mandrekar S,
Hillman S, Gatzemeier U and Heigener D: Long-term safety and
tolerability of sorafenib in patients with advanced non-small-cell
lung cancer: A case-based review. Clin Lung Cancer. 12:212–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdel-Rahman O and Fouad M: Risk of
cardiovascular toxicities in patients with solid tumors treated
with sorafenib: An updated systematic review and meta-analysis.
Future Oncol. 10:1981–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmidinger M, Zielinski CC, Vogl UM,
Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M and Schmidinger H:
Cardiac toxicity of sunitinib and sorafenib in patients with
metastatic renal cell carcinoma. J Clin Oncol. 26:5204–5212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duran JM, Makarewich CA, Trappanese D,
Gross P, Husain S, Dunn J, Lal H, Sharp TE, Starosta T, Vagnozzi
RJ, et al: Sorafenib cardiotoxicity increases mortality after
myocardial infarction. Circ Res. 114:1700–1712. 2014. View Article : Google Scholar : PubMed/NCBI
|