Introduction
Taxol, also termed paclitaxel, has been widely used
in the treatment of several solid tumors, including prostate cancer
(1–3).
However, not all tumors are sensitive to paclitaxel treatment, and
the mechanisms that distinguish resistant tumors from sensitive
tumors are not well understood (4).
Therefore, identifying the molecular characteristics associated
with resistance or sensitivity to paclitaxel may help to determine
the patients that are most likely to benefit from paclitaxel
therapy.
Multiple mechanisms have been identified for
paclitaxel-mediated chemotherapy of human cancers. For example,
paclitaxel has been shown to induce apoptosis by binding to the
tubulin protein of microtubules and inhibiting the depolymerization
of microtubules (5). Several
apoptotic signaling molecules, such as phosphatidyinositol-3 kinase
(PI3K)/Akt, p53/p21 and c-Raf-1/Ras/B-cell lymphoma-2, have also
been reported to be involved in apoptosis induction by paclitaxel
(6,7).
Previous studies have shown that Akt inactivation sensitized human
ovarian cancer cells to cisplatin and paclitaxel (8–10).
Phosphatase and tensin homolog (PTEN), an endogenous tumor
suppressor, dephosphorylated the D3 position of
phosphatidylinositol-3,4,5 triphosphate to negatively control PI3K
activity, and thus inhibited a panel of cellular responses mediated
by the PI3K/Akt pathway (11).
Overexpression of PTEN in malignant cells also induced apoptosis
and suppression of survival signaling (10). The potential role of PTEN in the
clinical efficacy of prostate cancer therapy by paclitaxel and its
underlying mechanism remains to be elucidated.
The present study aimed at determining the
sensitivity of paclitaxel in PTEN-positive and PTEN-negative
prostate cancer cells. Furthermore, the present study aimed at
investigating the underlying molecular mechanism and function of
PTEN in the regulation of mammary serine protease inhibitor or
serpin (maspin) expression in paclitaxel sensitivity.
Materials and methods
Materials
Dharmacon PTEN siRNA, maspin siRNA and control siRNA
were purchased from GE Healthcare Life Sciences (Little Chalfont,
UK). Paclitaxel was purchased from Taihua Natural Plant
Pharmaceutical Co. Ltd (Xi'an, China). Gibco RPMI-1640 medium and
fetal bovine serum (FBS) were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). All other chemicals used were
of analytical grade and commercially available.
Cell culture and transfection
The prostate cancer LNCaP and 22Rv1 cell lines were
purchased from American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in RPMI-1640 medium containing 10%
FBS, 100 mg/ml streptomycin and 100 units/ml penicillin at 37°C in
a humidified 5% CO2 atmosphere. The cells were
transfected using Lipofectamine 2000 (Invitrogen, Thermo Fisher
Scientific, Inc.), according to the manufacturer's
instructions.
Cell cytotoxicity assay
The in vitro cytotoxicity of paclitaxel was
evaluated by an MTT assay (12).
Briefly, LNCaP cells and 22Rv1 cells were treated with different
concentrations (0, 5, 10, 15 and 20 nM) of paclitaxel for 48 h or
10 nm paclitaxel for different time courses (0, 24, 48 and 72 h).
The cells were washed with PBS and then 20 µl of 5 mg/ml MTT
solution was added to the cells in each well. Plates were incubated
for an additional 4 h at 37°C. The medium containing MTT was
removed and 150 µl dimethyl sulfoxide was added to dissolve the
formazan crystals. Absorbance was measured at 490 nm using a
Labsystems iEMS microplate reader (Helsinki, Finland).
Cell apoptosis analysis
The cells were treated with different concentrations
(0, 5, 10, 15 and 20 nM) of paclitaxel for 48 h or 10 nm paclitaxel
for different time courses (0, 24, 48 and 72 h). Apoptosis was
measured using an Annexin V/propidium iodide (PI) apoptosis
detection kit according to the manufacturer's instructions
(MultiSciences Biotech Co. Ltd, Zhejiang, China) and analyzed by a
FACScan cytometer equipped with Cell Quest software (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Treated cells were harvested and lysed in RIPA lysis
buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM
Na3VO4 and complete protease inhibitor
cocktail]. The insoluble materials were centrifuged at 10,000 × g
at 4°C for 10 min and the supernatants were collected. The total
proteins in supernatants were quantified by bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China), according to the manufacturer's instructions. The proteins
(10 µg) were separated on SDS-PAGE (10% gel) and transferred to
polyvinylidene fluoride membranes. The membranes were blocked,
incubated with primary antibodies (all diluted at 1:1,000 and
incubated at 4°C overnight) including anti-PTEN (cat. no. 9556;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-β-actin
(cat. no. 3700; Cell Signaling Technology) and anti-maspin (cat.
no. 271694; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
washed and incubated with the goat anti-mouse immunoglobulin G
conjugated to horseradish peroxidase (dilution, 1:5,000; cat. no.
2005; Santa Cruz Biotechnology, Inc.) at room temperature for 1 h.
The protein bands were detected using enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc.). Expressions of these
proteins were normalized to that of β-actin as a control and
analyzed using Adobe Photoshop V7.01 software (Adobe Systems Inc.,
San Jose, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from treated cells was isolated using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The mRNA was reverse transcribed with
Superscript II reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.), 1X transcription buffer containing 400 M dNTPs
and 0.5 M oligo(dT)12-18 primer (Invitrogen; Thermo
Fisher Scientific, Inc.). PCR reactions were carried out for 35
cycles (95°C, 30 sec; 58°C, 30 sec; 72°C, 30 sec) using primers
specific for maspin (forward, 5′-CCCTATGCAAAGGAATTGGA-3′ and
reverse, 5′-CAAAGTGGCCATCTGTGAGA-3′), GAPDH (forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′).
The amplified products were separated on a 2% agarose gel
containing ethidium bromide (0.5 µg/ml) and analyzed using Adobe
Photoshop V7.01 software (Adobe Systems Inc.).
Statistical analysis
Experiments were performed with three replicates.
Statistical analyses were performed using Student's t test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PTEN induces differential sensitivity
to paclitaxel treatment in prostate cancer cells
It has been shown that wild-type PTEN improves
therapeutic efficacy in the treatment of human cancer (13). To determine whether PTEN would affect
the sensitivity of prostate cancer cells to paclitaxel, the ability
of paclitaxel to inhibit cell proliferation in PTEN-negative LNCaP
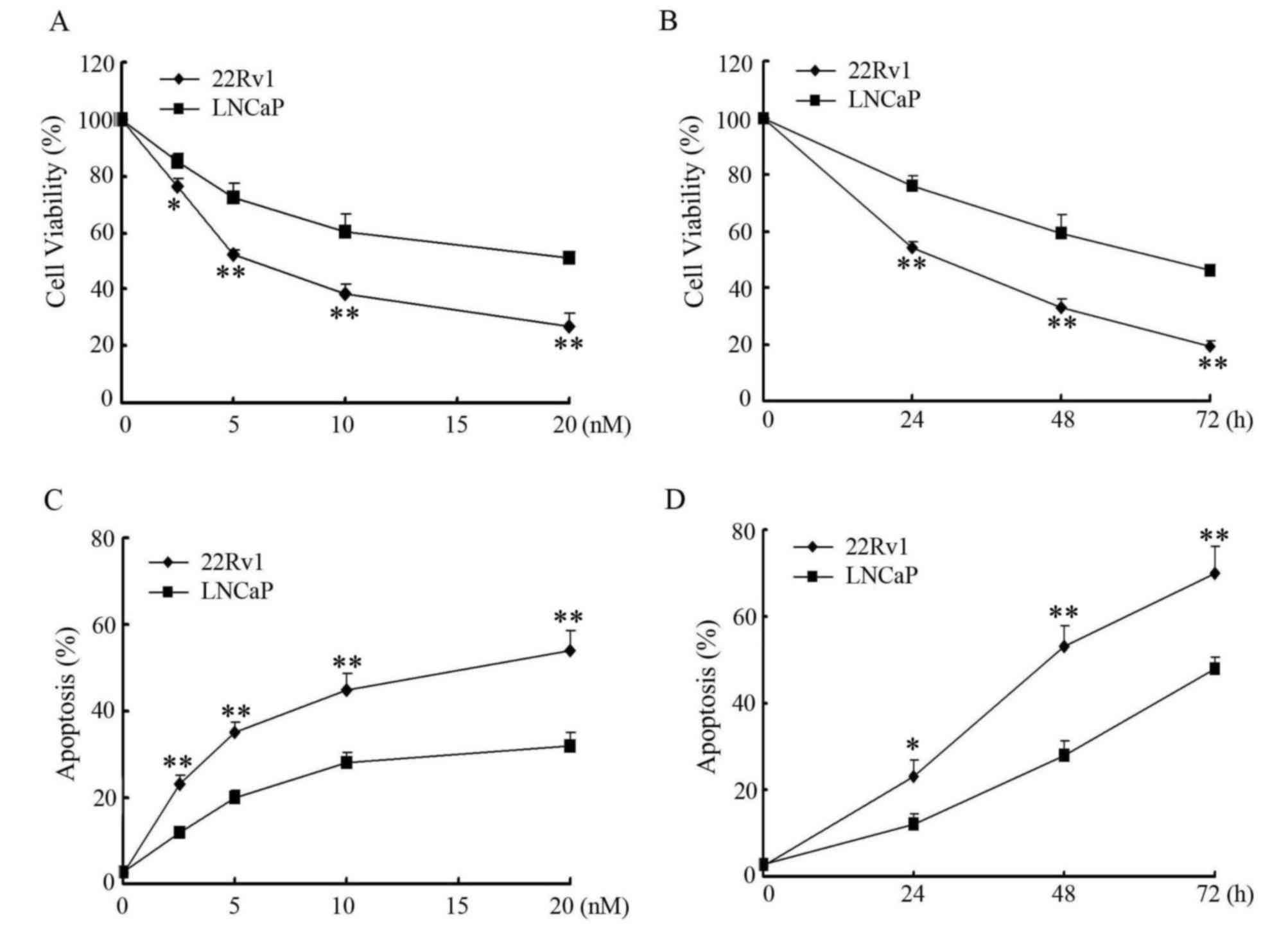
cells and PTEN-positive 22Rv1 cells was first examined. As shown in
Fig. 1A and B, paclitaxel inhibited
growth of LNCaP and 22Rv1 cells in a concentration- and
time-dependent manner. However, LNCaP cells revealed significantly
increased resistance to paclitaxel treatment compared with 22Rv1
cells (P<0.01 at 20 nM treatment for 48 h, or 10 nM treatment
for 72 h). The percentages of cell apoptosis induced by paclitaxel
in LNCaP cells and 22Rv1 cells were further determined by Annexin
V/PI staining and flow cytometry (Fig. 1C
and D). As expected, paclitaxel induced apoptosis in a
concentration- and time-dependent manner in LNCaP cells and 22Rv1
cells, and paclitaxel induced significantly increased apoptosis in
22Rv1 cells than LNCaP cells (P<0.01 at 20 nM treatment for 48
h, or 10 nM treatment for 72 h).
Paclitaxel upregulates maspin
expression in PTEN-positive prostate cancer cells
To explore the molecular mechanism involved in
paclitaxel-induced sensitivity in prostate cancer cells, PTEN
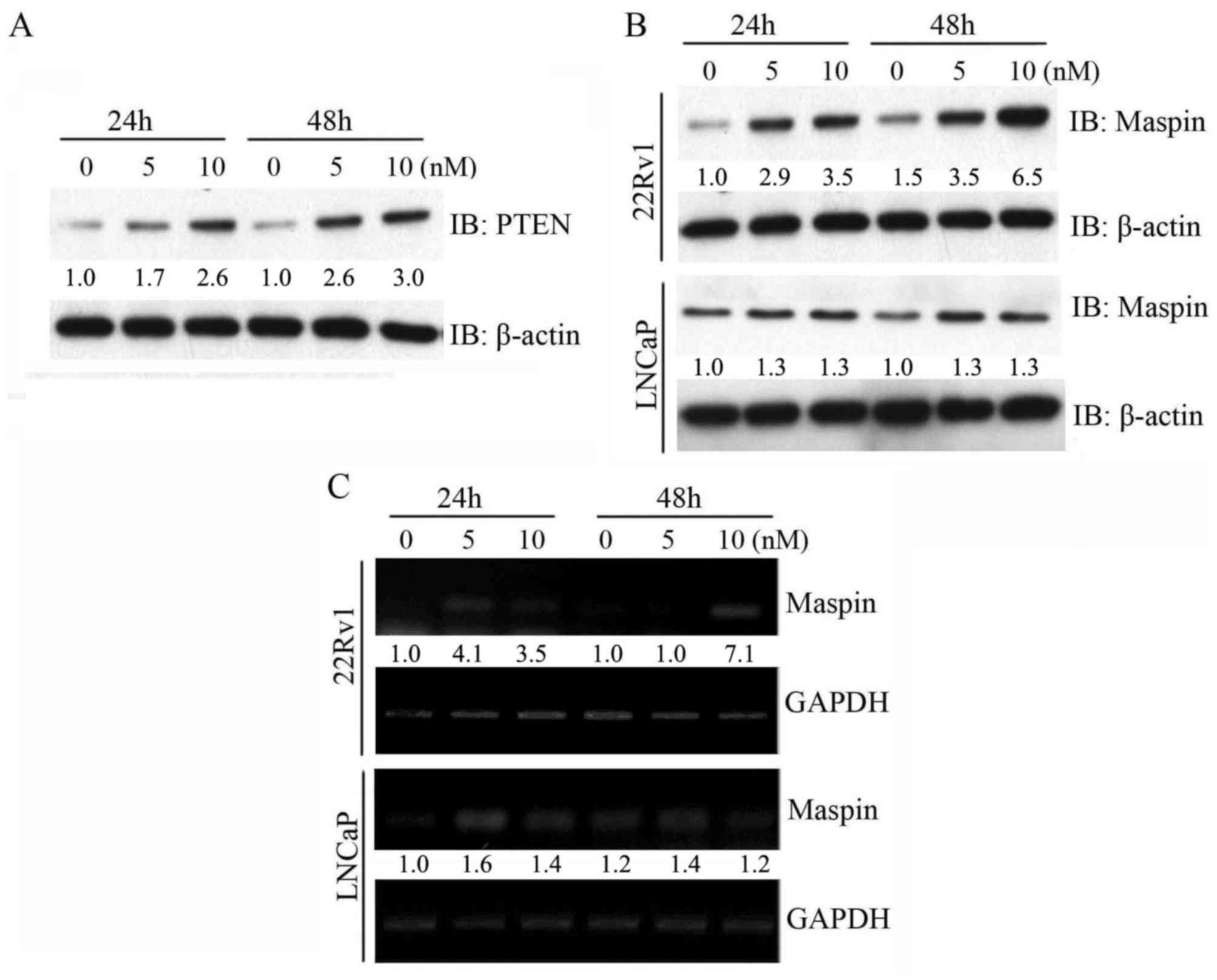
expression was determined by western blot analysis. As shown in
Fig. 2A, exposure of 22Rv1 cells to
paclitaxel resulted in a concentration- and time-dependent increase
in the PTEN protein level. Notably, the increase of maspin protein
level paralleled the elevated protein level of PTEN in 22Rv1 cells
subsequent to paclitaxel treatment. However, the maspin protein
level did not significantly change with paclitaxel treatment in
PTEN-negative LNCaP cells (Fig. 2B).
Furthermore, the induction of maspin mRNA level by paclitaxel in
22Rv1 cells and LNCaP cells was consistent with the change in
maspin protein level (Fig. 2C). These
data suggested that maspin induction by paclitaxel may occur in a
PTEN-dependent manner.
Knockdown of PTEN downregulates
paclitaxel-induced maspin expression and apoptosis in 22RV1
cells
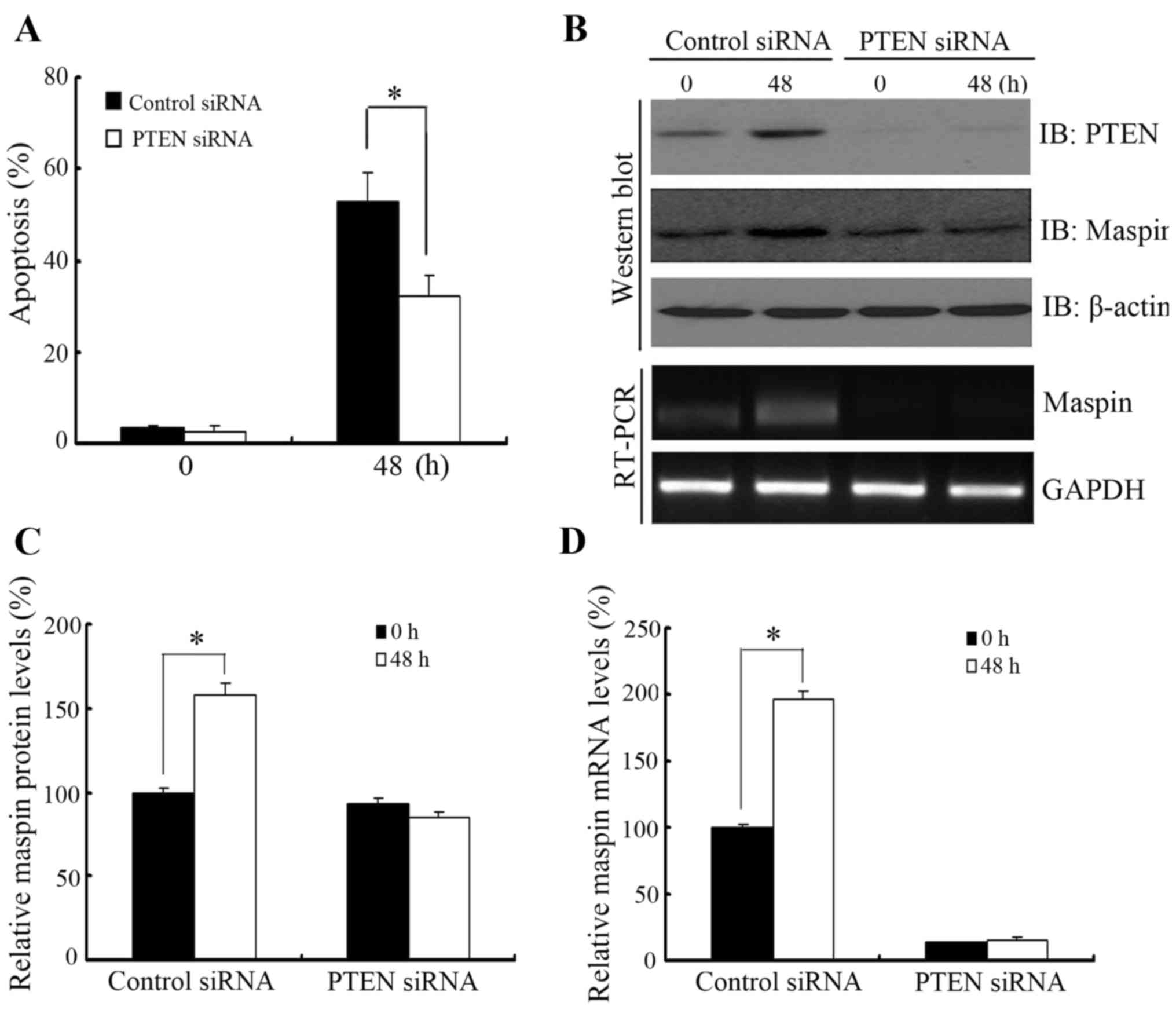
To determine whether PTEN-regulated maspin
expression was involved in paclitaxel-induced apoptosis, PTEN was
knocked down by PTEN-specific siRNA prior to paclitaxel treatment
in 22Rv1 cells. As shown in Fig. 3A,
knocking down PTEN increased resistance to paclitaxel treatment
compared with cells transfected with non-specific siRNA. In
addition, the increase in maspin protein and mRNA level subsequent
to paclitaxel treatment was impaired when 22Rv1 cells were
transfected with PTEN siRNA (Fig. 3B and
C).
PTEN overexpression upregulates
paclitaxel-induced maspin expression and apoptosis in LNCaP
cells
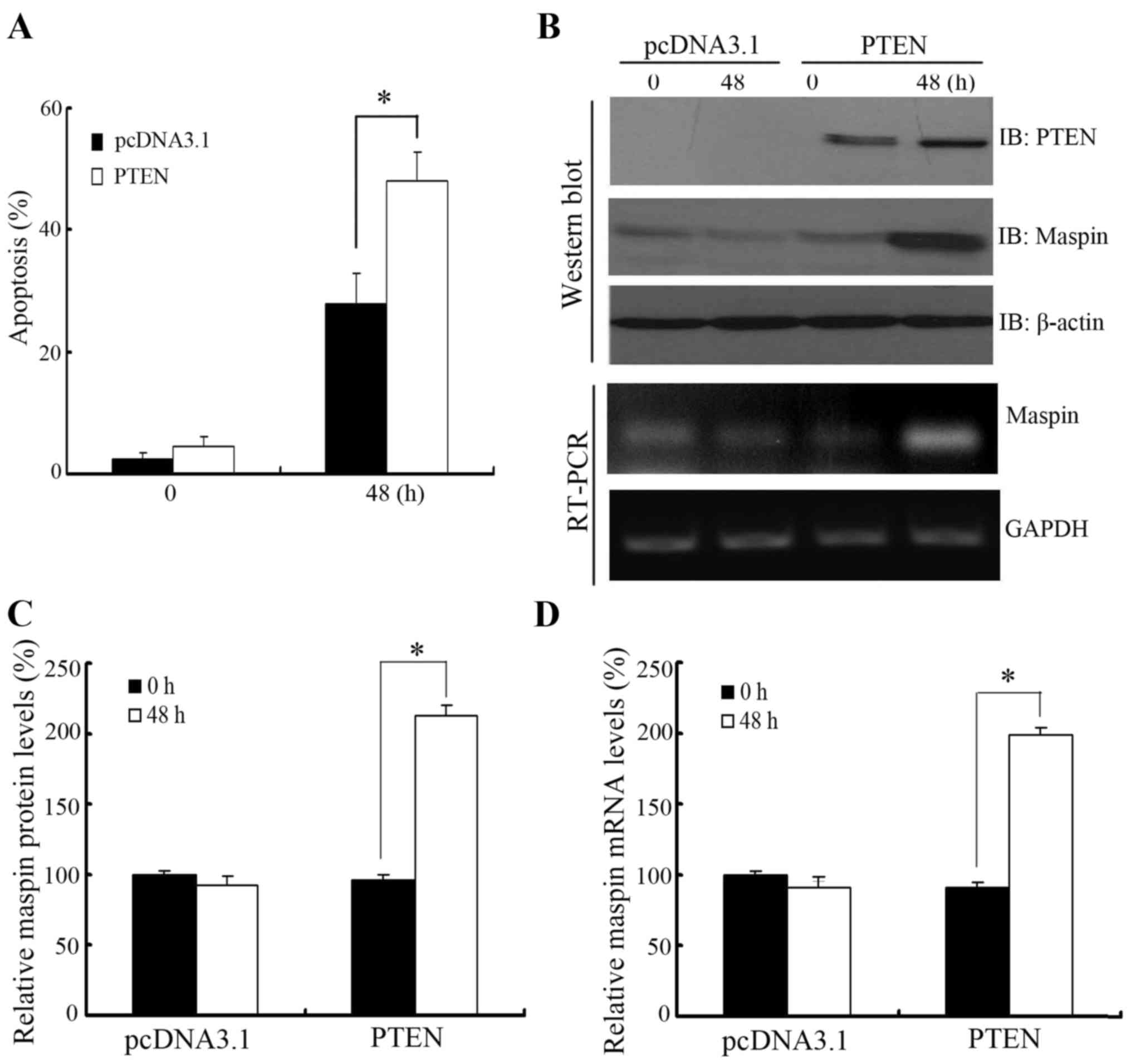
To further confirm the role of PTEN-regulated maspin
expression in paclitaxel-induced apoptosis, PTEN was overexpressed
in PTEN-negative LNCaP cells prior to paclitaxel treatment. As
shown in Fig. 4A, LNCaP cells
overexpressing PTEN showed more sensitivity to paclitaxel treatment
compared with cells transfected with the empty vector. In addition,
maspin protein and mRNA level were induced by overexpression of
PTEN in LNCaP cells when treated with paclitaxel (Fig. 4B and C).
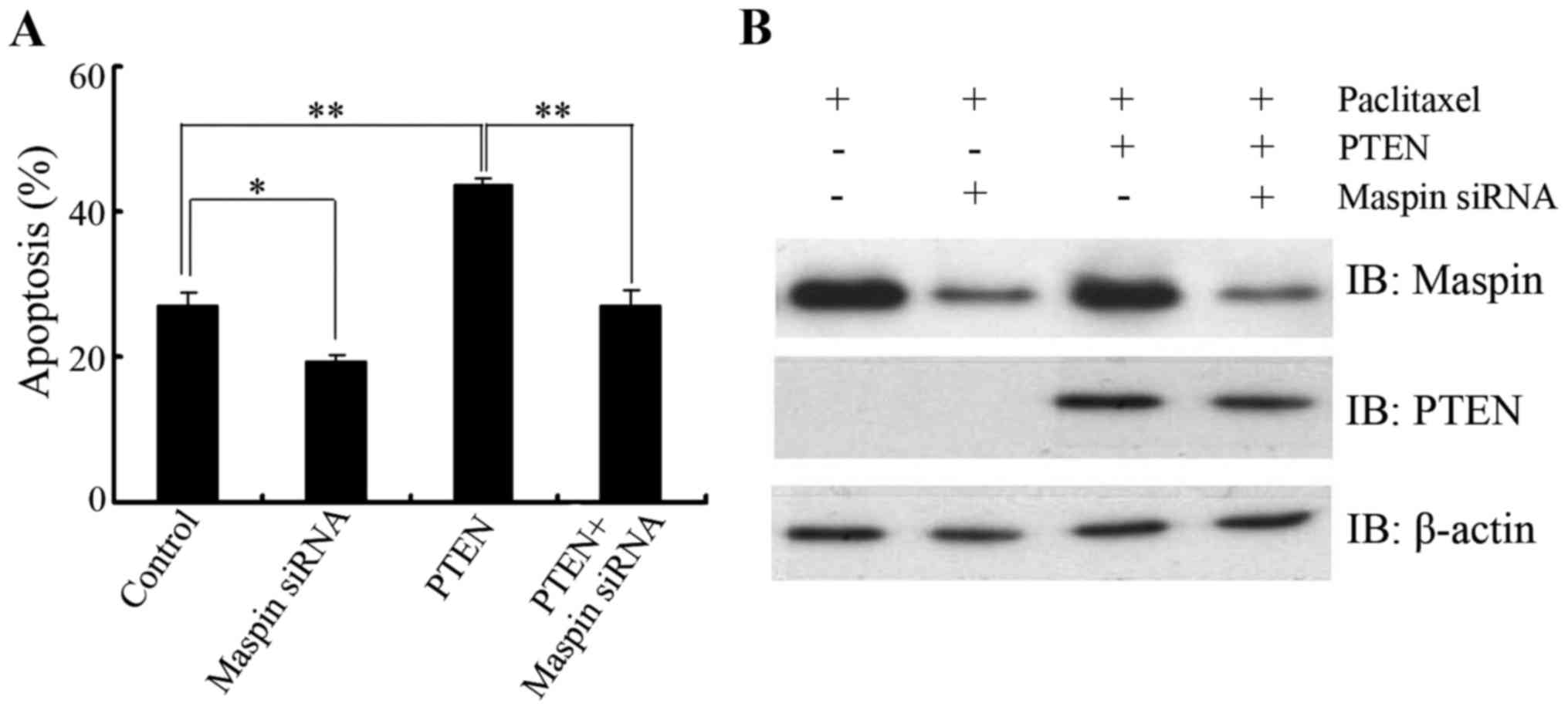
Knockdown of maspin abrogates
PTEN-induced paclitaxel sensitivity in LNCaP cells
To clarify the biological significance of PTEN in
the regulation of maspin in response to paclitaxel, LNCaP cells
were transfected with PTEN in the presence or absence of maspin
siRNA and then paclitaxel-induced apoptosis was determined. As
expected, PTEN overexpression sensitized LNCaP cells to paclitaxel
treatment. When the cells were cotransfected with maspin siRNA and
PTEN, no significant difference in apoptosis was found compared
with that of the control group (Fig. 5A
and B), which further confirmed that the PTEN/maspin pathway
may play a role in the regulation of paclitaxel sensitivity.
Discussion
Prostate cancer is the most common cancer in men and
the second leading cause of cancer-associated mortality in the
western world (14). Androgen
deprivation therapy (ADT) has become the standard treatment of
patients with metastatic prostate cancer. Although the disease
initially responds to ADT, tumors in the majority of patients
eventually relapse and evolve into castration-resistant prostate
cancer (CRPC) (15). Chemotherapy has
demonstrated a benefit in improving the survival of patients with
CRPC, and paclitaxel is a first-line chemotherapeutic agent in CRPC
(1). However, the mechanism of
apoptosis induction by paclitaxel remains to be elucidated.
The PTEN protein is a lipid phosphatase with
putative tumor-suppressing abilities. Inactivating mutations or
deletions of PTEN, which results in hyperactivation of the PI3K/Akt
signaling pathway, are frequently observed in a high proportion of
human cancers, including prostate cancer (16). PTEN deficiency is associated with a
number of aggressive tumor cell phenotypes and poor prognosis of
cancer patients (17,18). Previous studies have shown that
cellular loss of functional PTEN leads to resistance or reduced
sensitivity to chemotherapy and hormone therapy (19,20). To
explore the potential role for PTEN in paclitaxel sensitivity in
prostate cancer, the present study used two different prostate
cancer cell lines, the PTEN-positive 22Rv1 cell line and the
PTEN-negative LNCaP cell line, to assess paclitaxel sensitivity for
apoptosis induction. The present data demonstrated that 22Rv1 cells
were more sensitive to paclitaxel treatment compared with LNCaP
cells, and paclitaxel caused PTEN overexpression in 22Rv1 cells,
suggesting that paclitaxel may induce different amounts of
apoptosis in prostate cancer cells due to their different PTEN
status. To further confirm whether the presence of wild-type
functional PTEN may benefit therapeutic efficacy in the treatment
of prostate cancer, the effect of paclitaxel on the cells was
determined when PTEN was knocked down in 22Rv1 cells or PTEN was
overexpressed in LNCaP cells. As expected, knocking down PTEN in
22Rv1 cells induced resistance to paclitaxel treatment, while
overexpression of PTEN sensitized LNCaP cells to paclitaxel
treatment.
Maspin, also termed protease inhibitor 5, is
characterized as a class II tumor suppressor based on its ability
to inhibit tumor growth, metastasis and angiogenesis (21,22).
Previous studies also showed that high expression of maspin was
associated with response to chemotherapy in a number of human
primary tumors (23,24). To elucidate the tumor-suppressive
activity of maspin, certain proteins, including p53, have been
identified to regulate maspin expression. The p53 protein binds
directly to the p53-consensus-binding site present in the maspin
promoter and induces maspin expression, which elucidates the role
of p53 in cell growth, invasion and metastasis (25). In the present study, while
investigating the mechanisms underlying PTEN-induced
chemosensitivity, paclitaxel was found to upregulate maspin protein
and mRNA levels in 22Rv1 cells. In addition, ectopic expression of
PTEN was associated with elevated maspin expression in LNCaP cells
and knockdown of PTEN caused reduced maspin induction in 22Rv1
cells when treated with paclitaxel. Furthermore, the proapoptotic
effect of PTEN on paclitaxel-induced apoptosis can be abrogated by
knocking down maspin. These data suggested that maspin may be
involved in PTEN-induced chemosensitivity. Whether PTEN bound to
the maspin promoter and regulated maspin expression requires
additional investigation.
In summary, the present study showed that PTEN was
involved in paclitaxel sensitivity in prostate cancer cells.
Mechanistically, it was demonstrated that PTEN-mediated paclitaxel
sensitivity may be due to the induction of maspin expression. The
PTEN/maspin signaling pathway may have an important role in
regulating the susceptibility of prostate cancer to paclitaxel.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30801166).
References
|
1
|
Smith DC and Pienta KJ: Paclitaxel in the
treatment of hormone-refractory prostate cancer. Semin Oncol. 26 (1
Suppl 2):S109–S111. 1999.
|
|
2
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama S, Torikoshi Y, Takahashi T,
Yoshida T, Sudo T, Matsushima T, Kawasaki Y, Katayama A, Gohda K,
Hortobagyi GN, et al: Prediction of paclitaxel sensitivity by CDK1
and CDK2 activity in human breast cancer cells. Breast Cancer Res.
11:R122009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by Taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LG, Liu XM, Kreis W and Budman DR:
The effect of antimicrotubule agents on signal transduction
pathways of apoptosis: A review. Cancer Chemother Pharmacol.
44:355–361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gan L, Wang J, Xu H and Yang X: Resistance
to docetaxel-induced apoptosis in prostate cancer cells by
p38/p53/p21 signaling. Prostate. 71:1158–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann N Y Acad Sci. 1095:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng DJ, Wang J, Zhou JY and Wu GS: Role
of the Akt/mTOR survival pathway in cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 394:600–605. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu H, Cao Y, Weng D, Xing H, Song X, Zhou
J, Xu G, Lu Y, Wang S and Ma D: Effect of tumor suppressor gene
PTEN on the resistance to cisplatin in human ovarian cancer cell
lines and related mechanisms. Cancer Lett. 271:260–271. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramaswamy S, Nakamura N, Vazquez F, Batt
DB, Perera S, Roberts TM and Sellers WR: Regulation of G1
progression by the PTEN tumor suppressor protein is linked to
inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc
Natl Acad Sci USA. 96:2110–2115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang R, Banika NL and Rayb SK:
Differential sensitivity of human glioblastoma LN18 (PTEN-positive)
and A172 (PTEN-negative) cells to Taxol for apoptosis. Brain Res.
1239:216–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bilani N, Bahmad H and Abou-Kheir W:
Prostate cancer and aspirin use: Synopsis of the proposed molecular
mechanisms. Front Pharmacol. 8:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Debes JD and Tindall DJ: Mechanisms of
androgen refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cairns P, Okami K, Halachmi S, Halachmi N,
Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS and Sidransky D:
Frequent inactivation of PTEN/MMAC1 in primary prostate cancer.
Cancer Res. 57:4997–5000. 1997.PubMed/NCBI
|
|
17
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: The PI3K pathways as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leslie NR and Downes CP: PTEN function:
How normal cells control it and tumour cells lose it. Biochem J.
382:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Pore N, Cerniglia GJ, Mick R,
Georgescu MM, Bernhard EJ, Hahn SM, Gupta AK and Maity A:
Phosphatase and tensin homologue deficiency in glioblastoma confers
resistance to radiation and temozolomide that is reversed by the
protease inhibitor nelfinavir. Cancer Res. 67:4467–4473. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA
amplification contributes to cisplatin resistance in an ovarian
cancer cell line. Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Volpert O, Shi YH and Bouck N:
Maspin is an angiogenesis inhibitor. Nat Med. 6:196–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marioni G, Koussis H, Gaio E, Giacomelli
L, Bertolin A, D'Alessandro E, Scola A, Ottaviano G, de Filippis C,
Jirillo A, et al: MASPIN's prognostic role in patients with
advanced head and neck carcinoma treated with primary chemotherapy
(carboplatin plus vinorelbine) and radiotherapy: Preliminary
evidence. Acta Otolaryngol. 129:786–792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klasa-Mazurkiewicz D, Narkiewicz J,
Milczek T, Lipińska B and Emerich J: Maspin overexpression
correlates with positive response to primary chemotherapy in
ovarian cancer patients. Gynecol Oncol. 113:91–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zou Z, Gao C, Nagaich AK, Connell T, Saito
S, Moul JW, Seth P, Appella E and Srivastava S: p53 regulates the
expression of the tumor suppressor gene maspin. J Biol Chem.
275:6051–6054. 2000. View Article : Google Scholar : PubMed/NCBI
|