Introduction
Clinically, cervical lymph node metastases causes a
negative prognosis in patients with head and neck squamous cell
carcinoma (HNSCC), particularly those with stage T1-T2 SCC of the
tongue (1). However, cervical lymph
node metastases are difficult to detect, and the determination of
which cases should undergo neck dissection surgery remains
controversial (2,3). Various methods to predict cervical lymph
node metastasis have been proposed in an attempt to improve the
prognosis of patients with T1-T2 SCC of the tongue, including the
use of predictive clinical factors such as tumor size or invasion
depth, and pathological factors such as differentiation phenotype
and vascular invasion (4). Previous
studies have also reported correlations between the expression
levels of specific proteins and lymph node metastases (5,6).
Therefore, lymph node metastasis prediction has been examined from
a number of angles.
According to several previous studies, cluster of
differentiation (CD)147, also known as extracellular matrix
metalloproteinase (MMP) inducer, is overexpressed in cancer cells,
and induces malignant characteristics in various types of tumor,
including HNSCC (7,8). Notably, CD147 expression has been
associated with lymph node metastasis in HNSCC (9). However, the role of CD147 in the
progression of tumors, including HNSCC, is not completely
understood yet, and the detailed role of CD147 in lymph node
metastasis has not been evaluated in the context of T1-T2 SCC of
the tongue. Accordingly, detailed basic and clinical studies of
CD147 are required to improve the prognosis of patients with
HNSCC.
A large number of studies have attempted to reveal
the mechanisms underlying CD147-induced tumorigenicity (10,11). In
our previous study, it was reported that CD147 increased HNSCC cell
invasiveness, proliferation and drug resistance through
interactions with its ligand, cyclophilin A (12). Thus, further analysis of CD147 and its
ligands is important.
S100 calcium-binding protein A9 (S100A9) belongs to
the calcium-binding protein family, which comprises 21 subfamilies,
each of which has various functions that are associated with
inflammation or tumor progression (13,14).
Previous reports have indicated that S100A9 is overexpressed and
contributes to cancer progression in several types of solid tumor
(14). Notably, S100A9 was previously
reported to be a specific ligand of CD147 (11). However, the role of S100A9-CD147
interaction in HNSCC has not been elucidated to date. In the
present study, the association of CD147 expression with cervical
lymph node metastasis in T1-T2 SCC of the tongue was evaluated
retrospectively. In addition, the role of the S100A9-CD147
interaction in SCC of the tongue was assessed.
Materials and methods
Cell lines and cell culture
SAS and HSC-3 cell lines, which are derived from
human SCC of the tongue and express CD147 (15,16), were
obtained from the Japanese Collection of Research Bioresources Cell
Bank (Osaka, Japan). FaDu cells, a human hypopharyngeal SCC cell
line that expresses CD147 (17), were
a kind gift from the Department of Cell Biology and Morphology,
Akita University Graduate School of Medicine (Akita, Japan). Cells
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA) and incubated at 37°C in the presence of 5%
CO2.
Western blotting
SAS, HSC-3 and FaDu cell lines were lysed in
detergent containing 1% NP-40, 0.1 mM phenylmethylsulfonyl
fluoride, 1 mg/ml leupeptin and 1 mg/ml aprotinin, and protein
levels were determined using the Bio-Rad protein assay method
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total protein (40
µg) was separated on 8% SDS-PAGE gels and transferred to
nitrocellulose membranes using the semi-dry transfer machine
(Bio-Rad Laboratories, Inc.). Membranes were blocked with 5%
skimmed milk/TBS with Tween-20 (TBS-T) solution for 2 h at room
temperature, and incubated with primary antibodies including
against β-actin (dilution 1:1,000, cat. no. ab8227; Abcam,
Cambridge, UK) and CD147 (rabbit anti-human polyclonal, dilution
1:1,000, cat. no. sc-13976; Santa Cruz Biotechnology, Inc.), in 5%
skimmed milk/TBS-T overnight at 4°C. Subsequent to washing with
TBS-T three times, membranes were incubated for 1 h at room
temperature with a goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (dilution 1:3,000, cat.
no. 170-6515; Bio-Rad Laboratories, Inc.). The filters were rinsed
with TBS-T three times, and the blot was developed using Luminol
reagent (Santa Cruz Biotechnology Inc., Dallas, TX, USA) by
autoradiography.
Matrigel invasion assay
Cell invasiveness was evaluated in vitro
using Matrigel-coated semipermeable modified Boyden inserts with a
pore size of 8 µm (BD Biosciences, Franklin Lakes, NJ, USA). SAS
and HSC-3 cells were plated at a density of 2.5×104
cells per insert in serum-free medium with S100A9 (100 nM; ATGen
Co., Ltd., Gyeonggi, South Korea), anti-CD147 function-blocking
antibody (10 µg/ml, UM-8D6, cat. no. 10R-CD147aHU; Research
Diagnostics, Inc., Flanders, NJ, USA), for which the blocking
activity has been previously described (18,19), a
negative control mouse IgG (10 µg/ml, cat. no. X0931; Santa Clara,
CA, USA) or a combination of S100A9 with anti-CD147 or control IgG.
The lower chamber contained DMEM with 10% FBS as a chemoattractant.
To control for the effect of inhibitors on cell growth, the cells
were also plated in parallel in a 96-well plate with identical
conditions. After 48 h of treatment at 37°C in a 5% CO2
incubator, the cells on the upper side of the insert were removed
by wiping gently with a cotton swab. Cells on the reverse side of
the insert were fixed and stained using Differential Quik Stain kit
(Sysmex Corporation, Kobe, Japan) according to the manufacturer's
instructions. Invading cells in four representative fields were
manually counted using light microscopy at ×200 magnification. The
mean ± standard deviation was calculated from three independent
experiments. Cells in the 96-well plate were further assessed via
an MTT assay to identify the relative quantity of viable cells.
Produced formazan was dissolved in dimethyl sulfoxide and the
concentration was determined by optical density at 570 nm. The
numbers of invading cells were adjusted accordingly. MTT and DMSO
were purchased from Nacalai Tesque, Inc. (Kyoto, Japan).
Patients
A total of 41 patients (including 25 male and 16
female patients) with previously untreated, clinically diagnosed
stage I and II (T1 and T2 without lymph node metastasis,
respectively) SCC of the tongue and pathologically confirmed
subepithelial invasion, who underwent surgery between April 2007
and November 2012 at the Department of Otolaryngology, Akita
University Hospital (Akita, Japan), were retrospectively enrolled
in the present study. The tumors were classified according to the
2002 Union for International Cancer Control staging system
(20). All patients presented with T1
or T2 primary lesions at the clinical or radiological stage N0
(T1N0, 12 patients; T2N0, 20 patients). The patients ranged in age
from 33 to 83 years (median, 65.8 years; Table I). Patients were followed up for
>12 months after surgery.
 | Table I.Patients' characteristics and
pathological findings. |
Table I.
Patients' characteristics and
pathological findings.
| Variables | Patients, n |
|---|
| Total | 41 |
| Sex |
|
| Male | 25 |
|
Female | 16 |
| Age (years) |
|
|
Median | 65.8 |
|
Range | 33–83 |
| Tumor stage |
|
| T1 | 12 |
| T2 | 29 |
| CD147 index |
|
| 0 | 6 |
| 1 | 7 |
| 2 | 16 |
| 4 | 12 |
| Differentiation
type |
|
|
Poor | 4 |
|
Moderate | 18 |
|
Well | 19 |
| Vessel
invasion |
|
|
Positive | 32 |
|
Negative | 9 |
| Lymph vessel
invasion |
|
|
Positive | 28 |
|
Negative | 13 |
| Perineural
invasion |
|
|
Positive | 13 |
|
Negative | 28 |
| Invasion depth |
|
| ≥5
mm | 23 |
| <5
mm | 18 |
Patients with clinically diagnosed T1/T2 N0 SCC of
the tongue underwent sentinel lymph node (SLN) biopsy, and those
with positive SLNs underwent neck dissection. In the present study,
patients whose lymph node metastases were detected by SLN biopsy
and those whose lymph node metastases were detected during the
follow-up period were classified into the lymph node
metastasis-positive group.
Immunohistochemistry and
classification of pathological findings
Excised primary tumor specimens were fixed with 10%
neutral buffered formalin, and consecutive sections were cut every
5 mm and 4-µm thick tissue sections were obtained. The sections
were stained with hematoxylin and eosin, and the section containing
the invasive tumor front was selected for further analysis. The
previously described polyclonal anti-CD147 antibody was used as the
primary antibody for immunohistochemical staining. In brief,
4-µm-thick sections were deparaffinized and were initially
autoclaved for 15 min at 121°C. Sections were then were blocked
with 0.3% hydrogen peroxide in methanol for 30 min at room
temperature and with 10% normal rabbit serum/Tris (Vector
Laboratories, Inc., Burlingame, CA, USA) for 30 min at room
temperature. All sections were kept overnight at 4°C in
phosphate-buffered saline (PBS) containing the rabbit anti-human
CD147 polyclonal antibody (dilution 1:200), followed by a 1 h
incubation with biotinylated anti-rabbit IgG (ready-to-use
dilution, cat. no. ab64256; Abcam) at room temperature. The
sections were washed with PBS, and protein expression was detected
with the Vectastain avidin-biotin complex kit (Vector Laboratories,
Inc.) according to the manufacturer's instructions, and then
reacted with diaminobenzidine (Nacalai Tesque, Inc.) for 3 to 5 min
at room temperature.
A pathologist and a surgeon evaluated the CD147
immunohistochemical staining. CD147 expression in a cancer cell
nest at the invasive tumor front was identified and scored
according to the staining strength and intensity at ×200
magnification under light microscopy. Areas of CD147 staining
received a score of 0 if <10% of cells of the tumor nest were
stained, a score of 1 if ≥10% but <50% of cells of the tumor
nest were stained, and a score of 2 if ≥50% of cells of the tumor
nest were stained. The CD147 staining intensity was also scored
from 0 to 2 (negative, weak or strong staining, which we scored as
0–2, respectively), and a CD147 index (range, 0–4) was calculated,
as CD147-positive area score (0–2) × CD147 intensity score (0–2).
An index of 4 was classified as positive upon setting the cut-off
value at 3.5, which was the mean value of the CD147 index.
In addition, hematoxylin and eosin-stained samples
were examined to determine the SCC differentiation type, the
presence or absence of lymphovascular, vascular and perineural
invasion, and the depth of invasion. Samples were classified
according to the pathological findings as poorly, moderately or
well differentiated, and assessed for the presence or absence of
lymphovascular, vascular and/or perineural invasion. The invasion
depth was classified as ≥5 or <5 mm, according to a previous
study on increased cervical lymph node metastasis at invasion
depths exceeding 4–5 mm (20).
Statistical analysis
Statistical analyses were performed using JMP 11
software (SAS Institute, Inc., Cary, NC, USA). A two-tailed
Mann-Whitney U test was used to assess the statistical significance
of differences in the invasion studies. Univariate and multivariate
analyses were performed using a logistic regression model to
evaluate risk factors for metastasis. P<0.05 was considered to
indicate a statistically significant difference.
Results
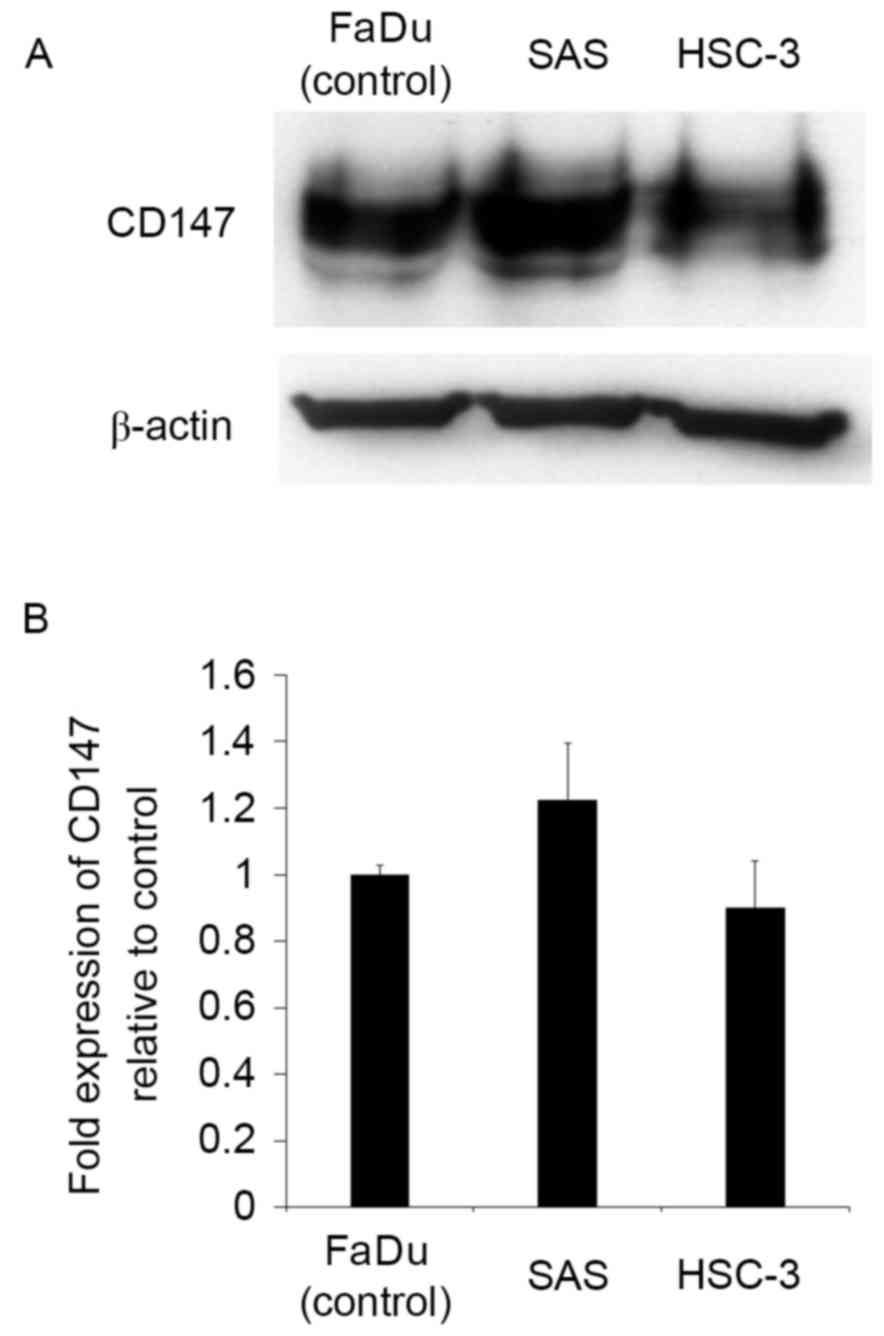
SAS and HSC-3 cells express CD147
Prior to evaluating the role of CD147 in the tongue
SCC cell lines SAS and HSC-3, its relative protein expression was
determined by western blotting. FaDu, a human hypopharyngeal SCC
cell line reported to express CD147, was used as a positive
control. SAS and HSC-3 cells were observed to express CD147 at a
high level (Fig. 1), indicating that
they were suitable for use in further studies.
S100A9 induces tongue SCC cell
invasion through its interaction with CD147
As tumor cell invasion is an important stage in the
metastasis cascade, there is a focus on invasion control as a
target for clinical tumor suppression (21,22). To
improve the understanding of the mechanisms underlying tongue SCC
cell invasion, SAS and HSC-3 cells were seeded into Matrigel
invasion chambers and stimulated with S100A9. The results are
presented in Fig. 2. Notably, the
invasiveness of SAS and HSC-3 cells increased when the cells were
co-cultured with S100A9 (P<0.05). To determine whether this
increased invasiveness was mediated by the S100A9-CD147
interaction, a CD147 function-blocking antibody was added to the
invasion assay. The antibody treatment reversed the increased
invasiveness in SAS and HSC-3 cells (P<0.05), indicating that
S100A9 induced tongue SCC cell invasion by stimulating CD147.
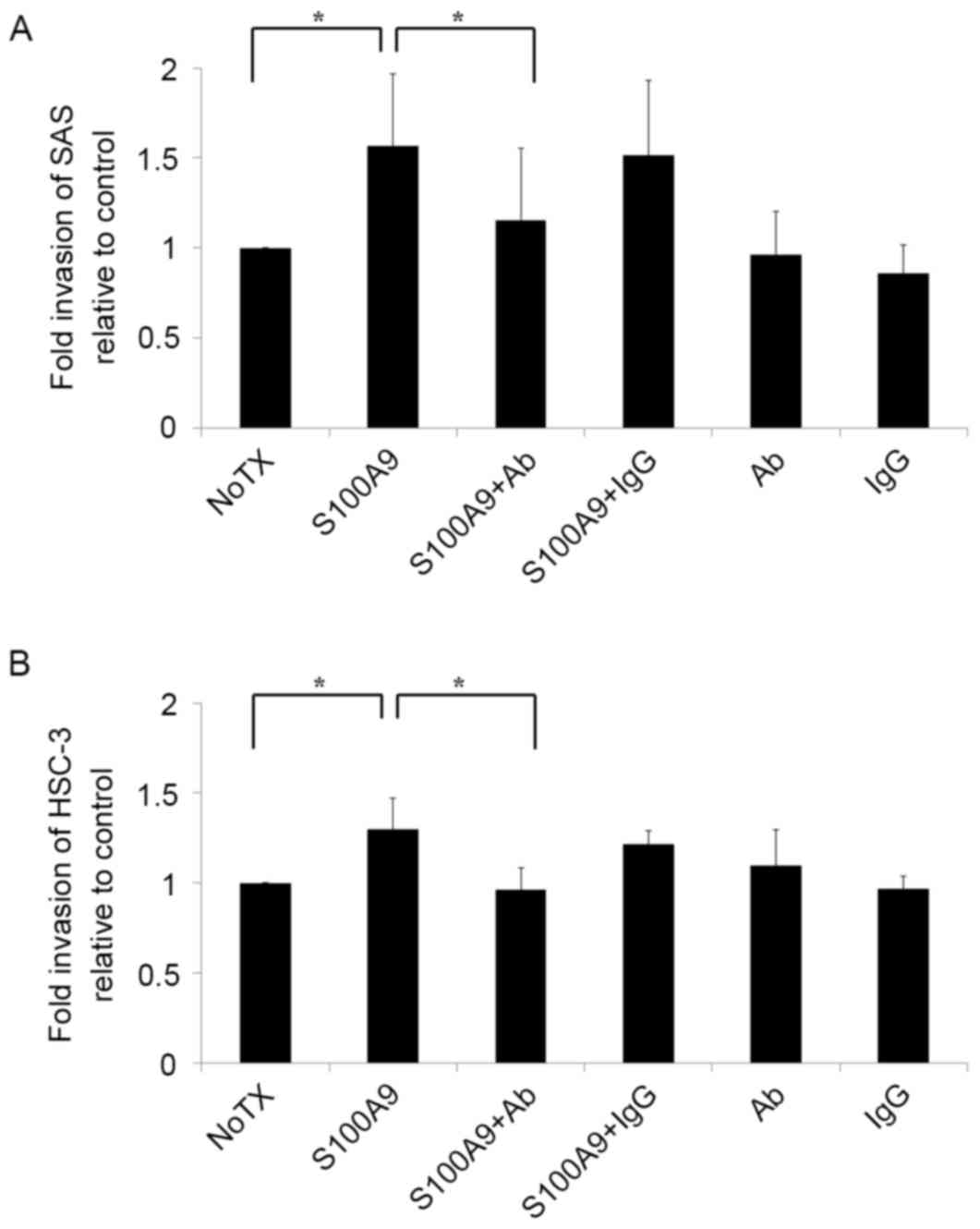 | Figure 2.S100A9 induces tongue SCC cell
invasion through its interaction with CD147. Cell invasiveness was
evaluated in vitro using a Matrigel invasion assay. (A) SAS
and (B) HSC-3 tongue SCC cells were plated in the inserts in
serum-free medium with or without S100A9 (100 nM), Ab (10 µg/ml),
IgG (10 µg/ml) or a combination of S100A9 with Ab or S100A9 with
IgG. Untreated cells were used as a control. The results are shown
as fold-changes in invasion relative to the control. Both (A) SAS
and (B) HSC-3 cell invasiveness increased in response to S100A9
stimulation, and the addition of Ab reversed this increase. The
experiment was repeated three times, and fold invasiveness relative
to control was expressed as the mean ± standard deviation.
*P<0.05 compared with the NoTX group. S100A9, S100
calcium-binding protein A9; SCC, squamous cell carcinoma; CD147,
cluster of differentiation 147; Ab, antibody; IgG, immunoglobulin
G; NoTX, no treatment. |
Analysis of pathological findings
Among the evaluated patients, the total rate of
metastasis was 29.3% (12/41), with 16.7% (2/12) and 34.5% (10/29)
of patients with T1 and T2 disease exhibiting metastasis,
respectively. In the clinical samples, the CD147 scores for cancer
nests at the invasive tumor front were as follows: 0, 6; 1, 7; 2,
16; and 4, 12 cases. Accordingly, the 12 cases scored as 4 were
defined as positive, and the remaining 27 cases as negative for
CD147 expression (Fig. 3).
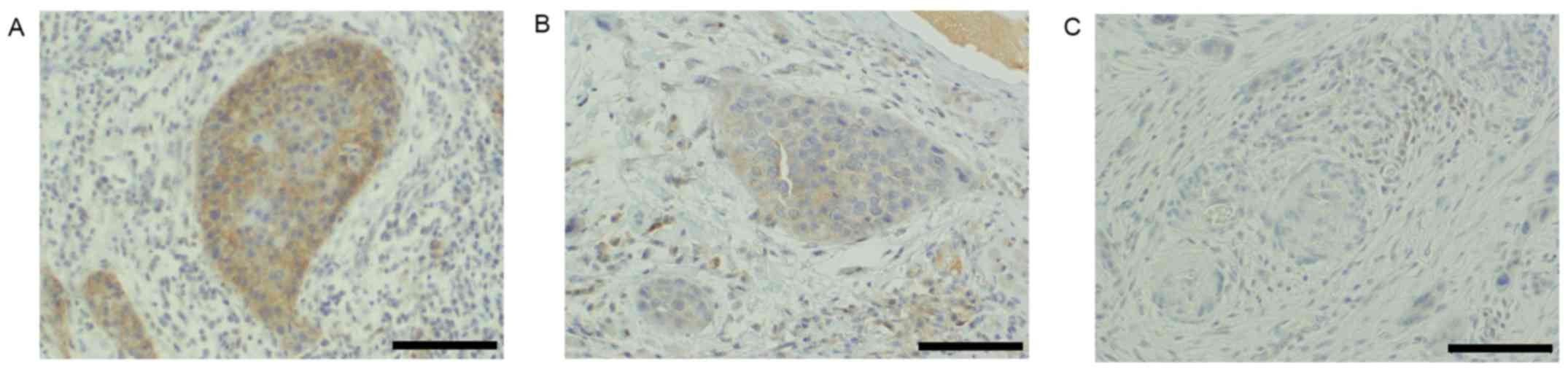 | Figure 3.CD147 expression in cancer nests at
the invasive tumor front. Immunohistochemical analysis of (A)
CD147-positive, index=4 (positive area, 2; intensity, 2), (B)
CD147-negative, index=2 (positive area, 2; intensity, 1) and (C)
CD147-negative, index=0 (positive area, 0; intensity, 0) specimens.
Magnification, ×200; scale bar, 100 µm. CD147, cluster of
differentiation 147. |
Regarding the other histopathological findings, 18
cases were poorly differentiated, 4 cases were moderately
differentiated and 19 cases were well differentiated. In addition,
32 patients exhibited vascular invasion, 28 lymphovascular invasion
and 13 perineural invasion. Regarding the depth of invasion, 23
cases had a depth of ≥5 mm and 18 had a depth <5 mm. The
patients' characteristics and pathological findings are summarized
in Table I.
The following metastasis rates were associated with
each pathological finding: CD147 positivity, 53.8%; poor or
moderate differentiation, 40.9%; vascular invasion, 34.4%;
lymphovascular invasion, 32.1%; perineural invasion, 38.5%; and
invasion depth ≥5 mm, 30.4%. The associations between
histopathological findings and metastasis are summarized in
Table II.
 | Table II.Associations between
histopathological findings and metastasis. |
Table II.
Associations between
histopathological findings and metastasis.
|
| Metastasis (n) |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive | Negative | Metastasis rate
(%) |
|---|
| CD147 |
|
|
|
|
Positive | 7 | 6 | 53.8 |
|
Negative | 4 | 24 | 14.3 |
| Differentiation
type |
|
|
|
|
Moderate/poor | 9 | 13 | 40.9 |
|
Well | 3 | 16 | 15.8 |
| Vascular
invasion |
|
|
|
|
Positive | 11 | 21 | 34.4 |
|
Negative | 1 | 8 | 11.1 |
| Lymphovascular
invasion |
|
|
|
|
Positive | 9 | 19 | 32.1 |
|
Negative | 3 | 10 | 23.1 |
| Perineural
invasion |
|
|
|
|
Positive | 5 | 8 | 38.5 |
|
Negative | 7 | 21 | 25.0 |
| Invasive depth
(mm) |
|
|
|
| ≥5 | 7 | 16 | 30.4 |
|
<5 | 5 | 13 | 27.8 |
CD147 serves as a predictor of lymph
node metastasis in patients with clinical stage N0, T1-T2 SCC of
the tongue
Table III shows the
results of univariate and multivariate analyses of the risk factors
for lymph node metastasis. The univariate analysis identified a
significantly higher incidence of lymph node metastasis among
CD147-positive cases (P=0.013). However, the differentiation type,
vascular invasion, lymphovascular invasion, perineural invasion and
invasion depth did not significantly impact the lymph node
metastasis risk. A multivariate analysis confirmed that CD147
expression was an independent risk factor for lymph node metastasis
in patients with clinical stage N0, T1-T2 SCC of the tongue
(P=0.028).
 | Table III.Univariate and multivariate analyses
for risk factors related to metastasis. |
Table III.
Univariate and multivariate analyses
for risk factors related to metastasis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| CD147 (positive vs.
negative) | 0.013a | 6.720 | 1.50–30.07 | 0.028a | 7.842 | 1.24–49.41 |
| Differentiation
type (poor or moderate vs. well) | 0.087 | 3.690 | 0.83–16.51 | 0.079 | 5.974 | 0.81–43.98 |
| Vessel invasion
(positive vs. negative) | 0.202 | 4.190 | 0.46–37.94 | 0.197 | 5.343 | 0.42–68.13 |
| Lymphatic vessel
invasion (positive vs. negative) | 0.554 | 1.580 | 0.35–7.18 | 0.632 | 0.598 | 0.07–4.89 |
| Perineural invasion
(positive vs. negative) | 0.381 | 1.880 | 0.46–7.66 | 0.442 | 0.470 | 0.07–3.23 |
| Invasive depth (≥5
vs. <5 mm) | 0.853 | 1.140 | 0.29–4.44 | 0.705 | 0.705 | 0.11–4.32 |
Discussion
A number of rich lymphatic intercommunications
associated with other regions of the mouth predispose tongue
cancers to metastasize to the cervical lymph nodes (regional lymph
nodes) at a relatively early stage (2); therefore, control of these lymph node
metastases is an extremely important prognostic factor. Similarly,
for SCC of the tongue, the presence or absence of metastasis to the
cervical lymph nodes is among the most critical factors in early
SCC of the tongue, particularly at stages T1 and T2 (1). Although neck dissection surgery is the
most effective means of controlling cervical lymph node metastasis,
it is difficult to preoperatively predict such metastases (23). This has led to controversy, as certain
clinicians recommend prophylactic neck dissection, whereas others
suggest avoiding unnecessary neck dissection to prevent
complications (2,3). Accordingly, an evaluation method that
can accurately detect the presence of potential lymph node
metastases is required.
Various attempts have been made to predict potential
cervical lymph node metastasis, including classifications based on
clinical findings such as the tumor diameter, invasiveness and
thickness (20), as well as recent
trials investigating biological characteristics such as specific
protein expression in cancer cells (6,24).
Regarding the latter, associations have been reported between
podoplanin, Smad interacting protein 1 and E-cadherin expression,
and cervical neck lymph node metastasis in patients with
early-stage SCC of the tongue (6,24). Based
on these previous reports, the present study investigated the
association of CD147 expression in cancer nests at the deepest
invasion site (a component of the invasive tumor front) with
cervical lymph node metastasis in patients with early-stage SCC of
the tongue. The results indicated that high CD147 expression in
cancer nests at the invasive tumor front was an independent risk
factor for cervical lymph node metastasis.
CD147 was originally investigated as inducer of MMPs
(25). MMPs mediate the dissolution
of the basement membrane and extracellular matrix, and are thus
indispensable for cancer cell invasion into the vascular system and
metastasis (26). The roles of MMPs
were previously examined in various solid carcinomas, including
head and neck cancers (27–29). Additionally, our group previously
described the role of CD147 in HNSCC cell invasiveness and
migration (12,30). In addition, associations of CD147
expression with cervical lymph node metastasis and poor prognosis
have been reported in hypopharyngeal (31) and laryngeal (7) cancers. Therefore, the potential
correlation between CD147 and tumor progression, including
metastasis within the head and neck region, has garnered
attention.
Although specific protein expression patterns in
tumors can predict lymph node metastasis and, thus, a poor
prognosis, the evaluation methods remain controversial. Various
methods for evaluating specific protein expression in tumor tissues
have been reported (6,24). However, previous studies on metastasis
have reported changes and reductions in the functions of cell
adhesion molecules, as well as changes in cellular properties
advantageous to metastasis, along the invasive tumor front
(32–35). These studies, therefore, have
highlighted the usefulness of evaluating the invasive tumor front
of malignant tumors. The importance of evaluating the expression of
specific proteins at the invasive tumor front of early-stage SCC of
the tongue was also previously reported (32).
According to previous studies, CD147 is expressed in
invadopodia, which occur on cells at the frontline of tumor
invasion, and controls the function of invadopodia by inducing MMPs
(33). These findings suggest the
induction of large quantities of MMP at the invasive tumor front in
response to CD147 overexpression. Therefore, patients with high
levels of CD147 expression in cancer nests at the invasive tumor
front may be at a higher risk of metastasis due to increased
invasion following the increased degradation of the basement
membrane and extracellular matrix.
Notably, in the present study, the results of a cell
invasion assay demonstrated that CD147 increased the invasive
ability of tongue SCC cells through its interaction with S100A9.
Upregulated S100A9 expression has been reported in various types of
malignant tumors, including SCC of the tongue (13,36).
Accordingly, it is possible that, in tongue SCC cells, a high level
of CD147 expression induces metastasis through increased
interactions with S100A9. However, elucidation of the detailed
mechanism by which CD147 promotes tumor progression is required to
determine the clinical role of this protein.
When evaluating a cancer nest at the invasive tumor
front, it may be necessary to identify the invasive tumor front
from an excised whole-tumor specimen. Therefore, this method may
not not suitable for preoperative predictions of metastatic
potential in clinical N0 cases, in which biopsy specimens comprise
only a part of the tumor. However, this method could be used as a
predictor of late metastasis, as stage T1-T2 SCC of the tongue is
often followed up subsequent to primary tumor resection without
neck dissection. In addition, this method typically requires time
for immunostaining prior to diagnosis; however, a technique that
enables immunostaining during surgery has been reported (37). If this technique is incorporated, the
evaluation of CD147 expression at the invasive tumor front may be a
criterion for deciding whether neck dissection should be performed
during a primary lesion resection in cases of T1-T2 SCC of the
tongue. Therefore, it is necessary to carefully examine the
clinical usefulness of CD147.
In conclusion, it has been determined that CD147
exerts its effects on the invasiveness and metastasis of SCC of the
tongue through its interactions with S100A9, and that its
overexpression correlates with an increased risk of cervical lymph
node metastasis. These findings support the possible use of CD147
overexpression as a marker of metastatic potential in cases of SCC
of the tongue.
Acknowledgements
The authors thank Mr. Manabu Kawamura for his help
in immunostaining. The present study was supported by the Japanese
Society for Promotion of Science JSPS KAKENHI (grant no.
15K19706).
References
|
1
|
Haddadin KJ, Soutar DS, Oliver RJ, Webster
MH, Robertson AG and MacDonald DG: Improved survival for patients
with clinically T1/T2, N0 tongue tumors undergoing a prophylactic
neck dissection. Head Neck. 21:517–525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang SF, Kang CJ, Lin CY, Fan KH, Yen TC,
Wang HM, Chen IH, Liao CT, Cheng AJ and Chang JT: Neck treatment of
patients with early stage oral tongue cancer: Comparison between
observation, supraomohyoid dissection, and extended dissection.
Cancer. 112:1066–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim YC, Lee JS, Koo BS, Kim SH, Kim YH and
Choi EC: Treatment of contralateral N0 neck in early squamous cell
carcinoma of the oral tongue: Elective neck dissection versus
observation. Laryngoscope. 116:461–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SC, Zhang S, Ishii G, Endoh Y, Kodama
K, Miyamoto S, Hayashi R, Ebihara S, Cho JS and Ochiai A:
Predictive markers for late cervical metastasis in stage I and II
invasive squamous cell carcinoma of the oral tongue. Clin Cancer
Res. 10:166–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueda G, Sunakawa H, Nakamori K, Shinya T,
Tsuhako W, Tamura Y, Kosugi T, Sato N, Ogi K and Hiratsuka H:
Aberrant expression of beta- and gamma-catenin is an independent
prognostic marker in oral squamous cell carcinoma. Int J Oral
Maxillofac Surg. 35:356–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanata K, Yamaguchi N, Yoshikawa K, Mezaki
Y, Miura M, Suzuki S, Senoo H and Ishikawa K: Soluble EMMPRIN
(extra-cellular matrix metalloproteinase inducer) stimulates the
migration of HEp-2 human laryngeal carcinoma cells, accompanied by
increased MMP-2 production in fibroblasts. Arch Histol Cytol.
70:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou
T and Rosenthal EL: CD147 and AGR2 expression promote cellular
proliferation and metastasis of head and neck squamous cell
carcinoma. Exp Cell Res. 318:1788–1798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu T, Zhou M, Peng L, Kong S, Miao R, Shi
Y, Sheng H and Li L: Upregulation of CD147 promotes cell invasion,
epithelial-to-mesenchymal transition and activates MAPK/ERK
signaling pathway in colorectal cancer. Int J Clin Exp Pathol.
7:7432–7441. 2014.PubMed/NCBI
|
|
11
|
Hibino T, Sakaguchi M, Miyamoto S,
Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R and
Huh NH: S100A9 is a novel ligand of EMMPRIN that promotes melanoma
metastasis. Cancer Res. 73:172–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi M, Suzuki S and Ishikawa K:
Cyclophilin A-EMMPRIN interaction induces invasion of head and neck
squamous cell carcinoma. Oncol Rep. 27:198–203. 2012.PubMed/NCBI
|
|
13
|
Fang WY, Chen YW, Hsiao JR, Liu CS, Kuo
YZ, Wang YC, Chang KC, Tsai ST, Chang MZ, Lin SH and Wu LW:
Elevated S100A9 expression in tumor stroma functions as an early
recurrence marker for early-stage oral cancer patients through
increased tumor cell invasion, angiogenesis, macrophage recruitment
and interleukin-6 production. Oncotarget. 6:28401–28424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markowitz J and Carson WE III: Review of
S100A9 biology and its role in cancer. Biochim Biophys Acta.
1835:100–109. 2013.PubMed/NCBI
|
|
15
|
Suzuki S and Ishikawa K: Combined
inhibition of EMMPRIN and epidermal growth factor receptor prevents
the growth and migration of head and neck squamous cell carcinoma
cells. Int J Oncol. 44:912–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erdem NF, Carlson ER, Gerard DA and Ichiki
AT: Characterization of 3 oral squamous cell carcinoma cell lines
with different invasion and/or metastatic potentials. J Oral
Maxillofac Surg. 65:1725–1733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
Involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bordador LC, Li X, Toole B, Chen B, Regezi
J, Zardi L, Hu Y and Ramos DM: Expression of emmprin by oral
squamous cell carcinoma. Int J Cancer. 85:347–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koga K, Nabeshima K, Aoki M, Kawakami T,
Hamasaki M, Toole BP, Nakayama J and Iwasaki H: Emmprin in
epithelioid sarcoma: Expression in tumor cell membrane and
stimulation of MMP-2 production in tumor-associated fibroblasts.
Int J Cancer. 120:761–768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumours. 6th. John Wiley & Sons;
Hoboken, NJ: 2002
|
|
21
|
Asakage T, Yokose T, Mukai K, Tsugane S,
Tsubono Y, Asai M and Ebihara S: Tumor thickness predicts cervical
metastasis in patients with stage I/II carcinoma of the tongue.
Cancer. 82:1443–1448. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koppikar P, Choi SH, Egloff AM, Cai Q,
Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM
and Grandis JR: Combined inhibition of c-Src and epidermal growth
factor receptor abrogates growth and invasion of head and neck
squamous cell carcinoma. Clin Cancer Res. 14:4284–4291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neri S, Hashimoto H, Kii H, Watanabe H,
Masutomi K, Kuwata T, Date H, Tsuboi M, Goto K, Ochiai A and Ishii
G: Cancer cell invasion driven by extracellular matrix remodeling
is dependent on the properties of cancer-associated fibroblasts. J
Cancer Res Clin Oncol. 142:437–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honda K, Ishiyama K, Suzuki S, Oumi E,
Sato T, Kawasaki Y, Saito H and Ishikawa K: Sentinel lymph node
biopsy using computed tomographic lymphography in patients with
early tongue cancer. Acta Otolaryngol. 135:507–512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakamoto K, Imanishi Y, Tomita T, Shimoda
M, Kameyama K, Shibata K, Sakai N, Ozawa H, Shigetomi S, Fujii R,
et al: Overexpression of SIP1 and downregulation of E-cadherin
predict delayed neck metastasis in stage I/II oral tongue squamous
cell carcinoma after partial glossectomy. Ann Surg Oncol.
19:612–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo H, Li R, Zucker S and Toole BP:
EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis,
also binds interstitial collagenase to the tumor cell surface.
Cancer Res. 60:888–91891. 2000.PubMed/NCBI
|
|
27
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brown PD, Bloxidge RE, Stuart NS, Gatter
KC and Carmichael J: Association between expression of activated
72-kilodalton gelatinase and tumor spread in non-small-cell lung
carcinoma. J Natl Cancer Inst. 85:574–578. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davies B, Waxman J, Wasan H, Abel P,
Williams G, Krausz T, Neal D, Thomas D, Hanby A and Balkwill F:
Levels of Matrix Metalloproteases in bladder cancer correlate with
tumor grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
30
|
Wu J, Hao ZW, Zhao YX, Yang XM, Tang H,
Zhang X, Song F, Sun XX, Wang B, Nan G, et al: Full-length soluble
CD147 promotes MMP-2 expression and is a potential serological
marker in detection of hepatocellular carcinoma. J Transl Med.
12:1902014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
Involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Q, Liu Y, Huang Y, Huang D, Li Y, Wu
J and Duan M: Expression of COX-2, CD44v6 and CD147 and
relationship with invasion and lymph node metastasis in
hypopharyngeal squamous cell carcinoma. PLoS One. 8:e710482013.doi:
10.1371/journal.pone.0071048. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tumuluri V, Thomas GA and Fraser IS: The
relationship of proliferating cell density at the invasive tumour
front with prognostic and risk factors in human oral squamous cell
carcinoma. J Oral Pathol Med. 33:204–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grass GD, Bratoeva M and Toole BP:
Regulation of invadopodia formation and activity by CD147. J Cell
Sci. 125:777–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bryne M, Boysen M, Alfsen CG, Abeler VM,
Sudbø J, Nesland JM, Kristensen GB, Piffko J and Bankfalvi A: The
invasive front of carcinomas. The most important area for tumour
prognosis? Anticancer Res. 18:4757–4764. 1998.PubMed/NCBI
|
|
36
|
Sewell DA, Yuan CX and Robertson E:
Proteomic signatures in laryngeal squamous cell carcinoma. ORL J
Otorhinolaryngol Relat Spec. 69:77–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Toda H, Minamiya Y, Kagaya M, Nanjo H,
Akagami Y, Saito H, Ito M, Konno H, Motoyama S and Ogawa J: A novel
immunohistochemical staining method allows ultrarapid detection of
lymph node micrometastases while conserving antibody. Acta
Histochem Cytochem. 44:133–139. 2011. View Article : Google Scholar : PubMed/NCBI
|