Introduction
Deregulated expression of cytokines by tumor cells
and their surrounding stromal cells including fibroblasts and
immune cells have been found to be essential for cancer cells to
acquire aggressive phenotype (1).
These resulting highly tumorigenic cells are now referred to as
cancer stem cells (CSCs) or tumor initiating cells, often
associated with stem cell (SC) properties including resistance to
chemotherapy, increased capacity of anchorage independent growth,
expression of SC antigens (2). CSCs
have been found and characterized in various cancers on the basis
of their SC markers and functional properties such as sphere
forming ability or in vivo tumorigenicity. There were
several SC markers have been identified as universal markers for
most cancer types. CD44, CD133 and ATP-binding cassette transporter
G2 (ABCG2), among many SC markers, have been used individually or
in combination with other markers to identify and isolate CSC from
cancers of breast (3), colon
(4), skin (5), ovary (6)
and pancreas (7). Although initially
CD44 was broadly considered as a CSC marker in various cancers
(8), more detailed recent reports
revealed that the variant 6 isoform (CD44v6) is found to
specifically expresses in CSCs of brain (9) and colon cancers (10), and in an earlier clinical study
(11) CD44v6 was found in metastatic
lesions of PC suggesting this isoform may be associated with
metastasis.
Another prospective cell surface antigen is CD133,
which is now established as a putative CSC marker for most
prevalent solid human cancers including brain (12), colon (4), head and neck cancers (13). In the case of PC, CD133 has been
defined not only as a CSC marker, in vitro and in
vivo functional studies also established the CD133 positive
cancer cells (sometimes in combination with other markers) as a
core population responsible for drug resistance, invasion,
tumorigenicity and metastasis (14).
In their cohort study Maeda et al examined clinical
relevance of CD133 in PC via immunohistochemistry, in which CD133
expression in PC tumor samples correlated with lymph node
metastasis and poor prognosis (15).
Overexpression of ABCG2 in various cancer cells has been associated
with multi-drug resistance due to its ability to efflux the drugs
outside the cell, and reports also demonstrated that ABCG2 can be
used as a CSC marker independently (16). Although essential roles of CSC in PC
progression have been proved beyond doubt, however little is known
about the cytokines that increase CSC properties in this
cancer.
TNFα and TGFβ-1, among others, have been found to be
most abundant cytokines that play crucial roles not only in
augmenting cancer cells invasion and migration capacities, but also
promote their ‘stemness’ as demonstrated by mechanistically
overexpression or suppression and exogenously stimulating
approaches (17,18). For example, targeting TNFα by
monoclonal antibody (mAB) attenuated tumor growth and made the
tumor cells sensible to drug treatment in a mouse model of PC
(19). Clinical observation also
support those cellular and animal studies, since overexpression of
these cytokines have been found in many different human tumor
samples and patient blood and correlated with poor prognosis
(20). For example Lin et al
reported that high level of TGFβ-1 in serum of PC patients was
associated with increased risk of death (21). Elevated serum concentrations of TNFα
and TGFβ-1 have been observed in blood from PC patients (22). Moreover, recent reports further
expanded our understanding of these cytokines in the CSC biology
(17). For example treatment with
TGFβ for 7 days resulted in increased self-renewal capacity of
patient-derived glioma-initiating cells (GICs) via inducing
leukemia inhibitory factor, and prevented GICs differentiation and
promoted in vivo oncogenesis (23). In their blood cancer study, Kagoya
et al revealed a potential role of TNFα in leukemia
initiating cells' (LICs) maintenance, in which constitutive NF-κB
activity is maintained through autocrine TNFα secretion by LICs
(24). However, the possible effects
of TNFα and TGFβ-1 on CSC populations of PC have not yet been
studied.
In this report, we examined the effects of TNFα and
TGFβ-1 on PC cell line MiaPaCa-2 cells, and our phenotypic and
functional data showed that these cytokines substantially increase
CSC populations in this cell line when worked together and
significantly increase self-renewal and proliferation and probably
ABCG2 dependent drug resistance.
Materials and methods
Reagents
Culture media and reagents for maintaining parental
MiaPaCa-2 cells and tumor spheres are as following: DMEM, DMEM-F12,
recombinant epidermal growth factor (rEGF), recombinant basic
fibroblast growth factor (rbFGF), B27 and N2 supplements (Life
Technologies, Grand Island, NY, USA), insulin, fetal bovine serum
(FBS), L-glutamine, TrypLEX, Poly-HEMA (all from Sigma, St. Louis,
MO, USA, unless otherwise specified). Antibodies for flow
cytometry: CD133/1 (AC133)-APC (Miltenyi Biotech, Cambridge, MA,
USA), EGF-R-PE, purified anti-CD32 anti-CD16 (BD Biosciences, San
Diego, CA,), CD44v6-AlexaFluor 488, VEGF-R1/Flt1-PE, ABCG2-PE
(R&D Systems, Minneapolis, MN, USA) (BD Biosciences). For MTT
assay: 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium
bromide (MTT), daunorubicin and gemcitabine (Eli Lilly).
Cell culture
MiaPaCa-2 cells were purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA), and maintained in
DMEM supplemented with 10% FBS, glutamine, and antibiotics
(penicillin and streptomycin). Cells were incubated in a humidified
incubator containing 5% CO2 at 37°C. To obtain tumor
spheres from MiaPaCa-2 cells and their cytokine-treated
subcultures, the cells growing in adherent culture condition were
detached using TrypLEX when cell confluence reached to 80–90%, and
were washed with ice-cold PBS. Single cells were seeded at a
density of 2,000 cells per well in Poly-HEMA-coated or ultralow
attachment 24-well plates. DMEM/F12 was used as the basic
sphere-culture medium, which supplemented with 50 ng/ml rEGF, 20
ng/ml rbFGF, 5 µg/ml insulin, 1X B27 supplement without vitamin A,
1X N2 supplement and 1% FBS. Sphere cells were incubated in an
incubator containing no CO2 at 37°C. After 10–12 days,
the sphere cells were harvested by gentle centrifugation and
dissociated with TrypLEX.
Sphere formation assay
The culture condition was similar to the above
description, except seeding density. To evaluate sphere forming
ability, cells were seeded at a density of 200 cells per well in
Poly-HEMA-coated 96-well plates. 5 days later, fresh sphere-culture
medium was added to each well. On day 11–12, the numbers of spheres
were counted under an inverted microscope (DMIL; Leica, Mannheim,
Germany), and sphere forming efficiency was calculated by dividing
number of spheres to initially seeded cells, and by multiplying
with 100%.
Flow cytometry
Prior to the labeling, cells were incubated with
anti-CD32/anti-CD16 cocktail to block the Fc receptors. Then, for
surface and intracellular staining, cells were subsequently
incubated with mAbs specific for surface markers according to the
manufacturer's protocols, then fixed and permeabilized with
Fixation/Permeabilization solution (BD Pharmingen; BD Biosciences)
and incubated for 20 min in the dark at room temperature. Cells
were then washed with Perm/Wash Buffer (BD Biosciences) and stained
with mAbs specific for intracellular cytokines. Afterwards, cells
were washed with PBS, re-suspended in flow solution, and
immediately analyzed by flow cytometry on FACSCalibur (BD
Biosciences) using CellQuest Pro software (BD Biosciences). All
cells were gated based on FSC and SSC properties, from this gate,
cells were analyzed for expression of CSC markers. Anti-Mouse Ig,
κ/Negative Control Compensation Particles Set (BD Biosciences),
unstained cells, single fluorochrome stained cells, and cells
stained as fluorescence-minus-one (FMO) controls were used to set
up the machine.
MTT cell proliferation and
cytotoxicity assay
The sensitivities of the four subcultures to
daunorubicin and gemcitabine were assessed by MTT assay. Each
subculture divided in two groups, one is control group where no
drug will be added, and second group is experimental group where a
drug will be added. Cells were seeded in 96-well plates at a
density of 1,000 cells/100 µl in culture media and plus their own
corresponding cytokines. After 24 h incubation, 100 µl culture
media were added to control groups and equal volume of culture
media containing daunorubicin or gemcitabine, with a final
concentration of 200 ng/ml or 6.8 µg/ml respectively, were added to
experimental groups. After another 24 h incubation, 20 µl MTT
solution (5 mg/ml) was added to each well, and incubated for 4 h at
37°C, then supernatant was carefully removed and let the plates
dried out at room temperature. Then 100 µl of DMSO was added to
each well. The absorbance of each well was measured at 492/630 nm
using a microplate reader.
Wound healing assay
Appropriate number of cells from four subcultures
were seeded in adherent culture dishes and allowed them to grow
until reached to confluency. A straight stretch was made using a
pipette tip in the central area of the confluent culture. The cells
were rinsed with fresh culture media to remove detached cells. New
media with low serum concentration were added to each subculture.
Phase contrast images were recorded at indicated time points.
Statistics
The Wilcoxon signed-rank test was used to obtain
P-values when compared the proportions of CSC populations between
untreated and cytokine treated cells. For the rest of the data
Student's t-test was used to determine statistically significant
difference and the results were represented as the mean ± SD.
P<0.05 was considered as significant.
Results
Treatment of MiaPaCa-2 with TNFα and
TGFβ-1 increase CSC populations in adherent culture condition
TGFβ-1 has long been used as a master stimulator of
epithelial-mesenchymal transition (EMT), in which epithelial cells
lose their coble-stone like morphology and convert to
spindle-shaped, fibroblastoid cells (25). Although less frequently used than
TGFβ-1, TNFα has also been accepted as an EMT stimulator (26). In some reports combination of these
two cytokines were used and exhibited more profound effects on
cells than used alone (27), and the
cells undergone EMT often share CSC characteristics (25). Therefore, we cultured MiaPaCa-2 cells
with low concentrations of TNFα (2 ng/ml) and TGFβ-1 (2 ng/ml) and
combination of both in adherent culture dishes. So we had four
subcultures including untreated parental cells designated as
follows: pMia (untreated parental MiaPaCa-2), pMiaTN
(TNFα-treated), pMiaTB (TGFβ-1-treated), and pMiaTT (TNFα +
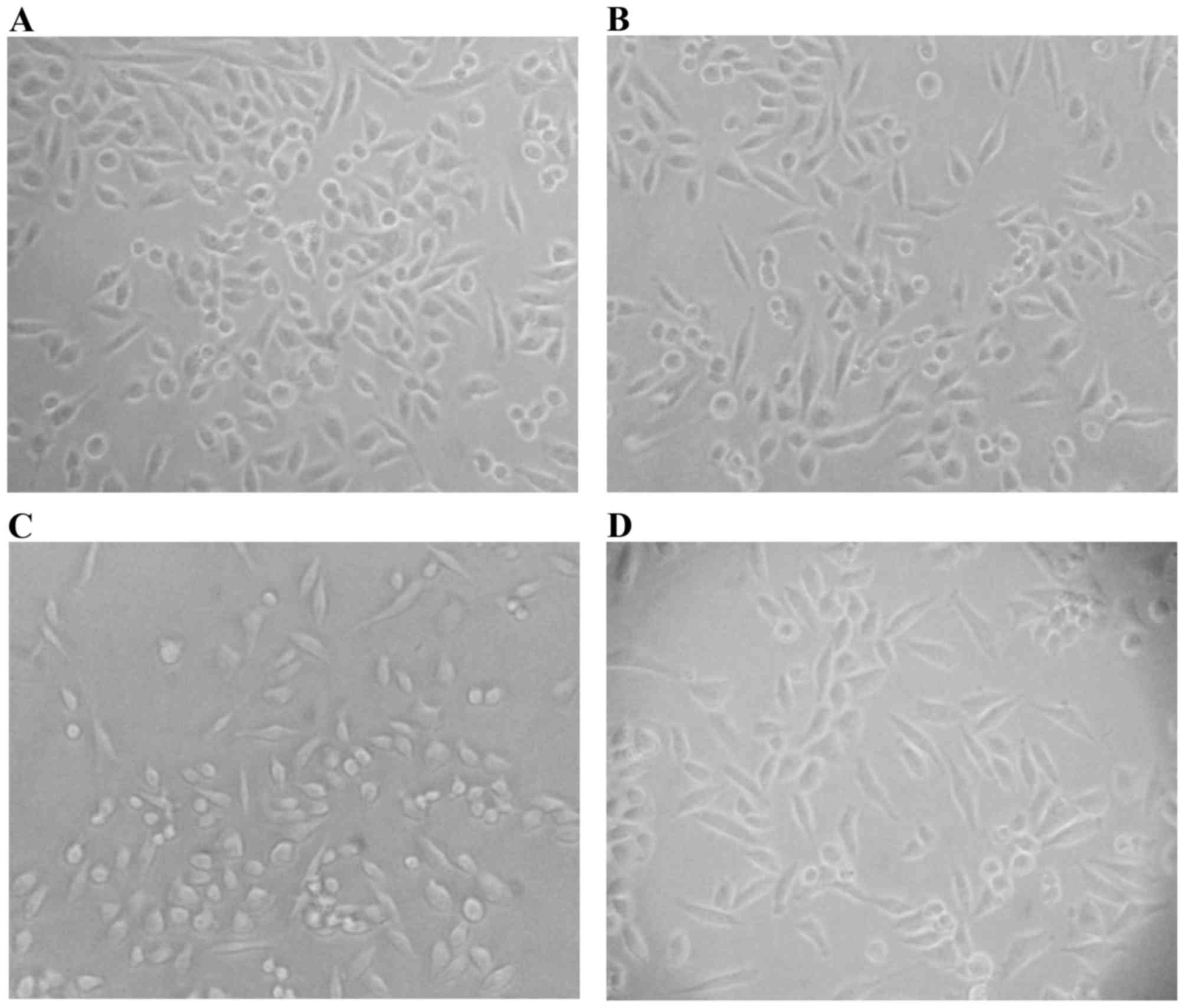
TGFβ-1-treated). Two days later, the proportion of spindle-shaped
cells moderately increased among TNFα alone (Fig. 1B) and in combination with TGFβ-1 (TNFα
+ TGFβ-1) treated cells (Fig. 1D).
Interestingly, the proportion of small and round-shaped cells was
increased after treatment with TGFβ-1 alone (Fig. 1C). After in total three days of
incubation, the cells were subjected to flow cytometry analyses to
assess a series of markers and a single case of flow cytometry data
obtained at the same day and comparison of the proportions of the
phenotypic markers between untreated (pMia or Mia) and double
treated (pMiaTT or MiaTT) cells were shown by flow dot plots and
graphs respectively. As shown in Fig.
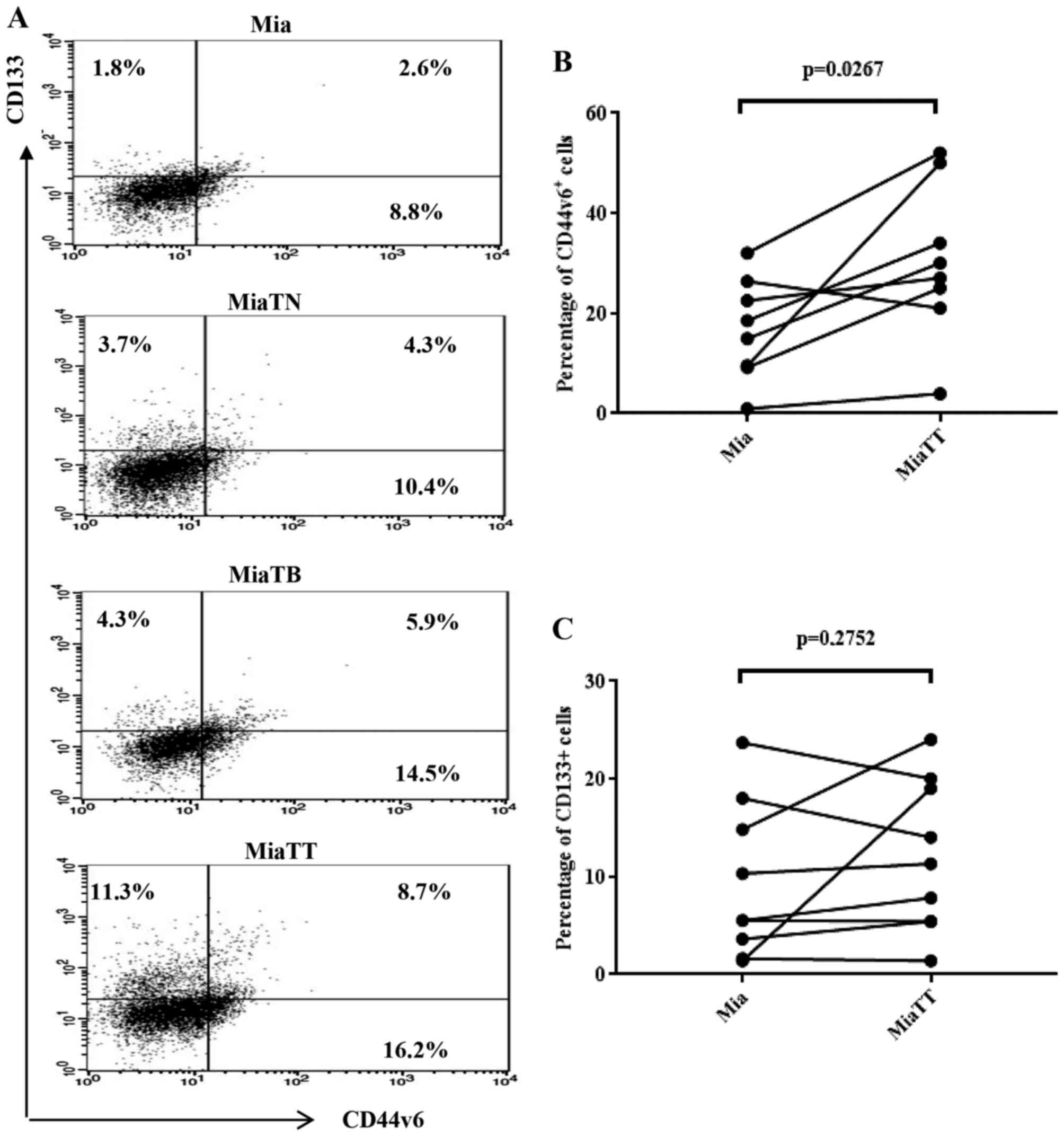
2A combinatorial treatment resulted in the most substantial
increase of CD44v6 positive cells (20.7%), CD133 positive cells
(3.5%) and double positive (CD44v6/CD133) cells (2.4%) among four
subcultures, while individual treatment also considerably increased
when compared to untreated parental pMia cells. Specifically,
pMiaTN cells included 8.8% of CD44v6 positive cells, 2.0% of CD133
positive cells, and 0.8% of CD44v6/CD133 positive cells, while
pMiaTB cells included 13.9% of CD44v6 positive cells, 1.3% of CD133
positive cells, and 1.2% of CD44v6/CD133 positive cells. Parental
pMia cells included 3.7% of CD44v6 positive cells, 0.5% of CD133
positive cells, and 0.2% of CD44v6/CD133 positive cells. The
significant changes in the proportions of CD44v6 and CD133
expressing cells between pMia and pMiaTT cells were shown in
Fig. 2B and C. In the case of ABCG2
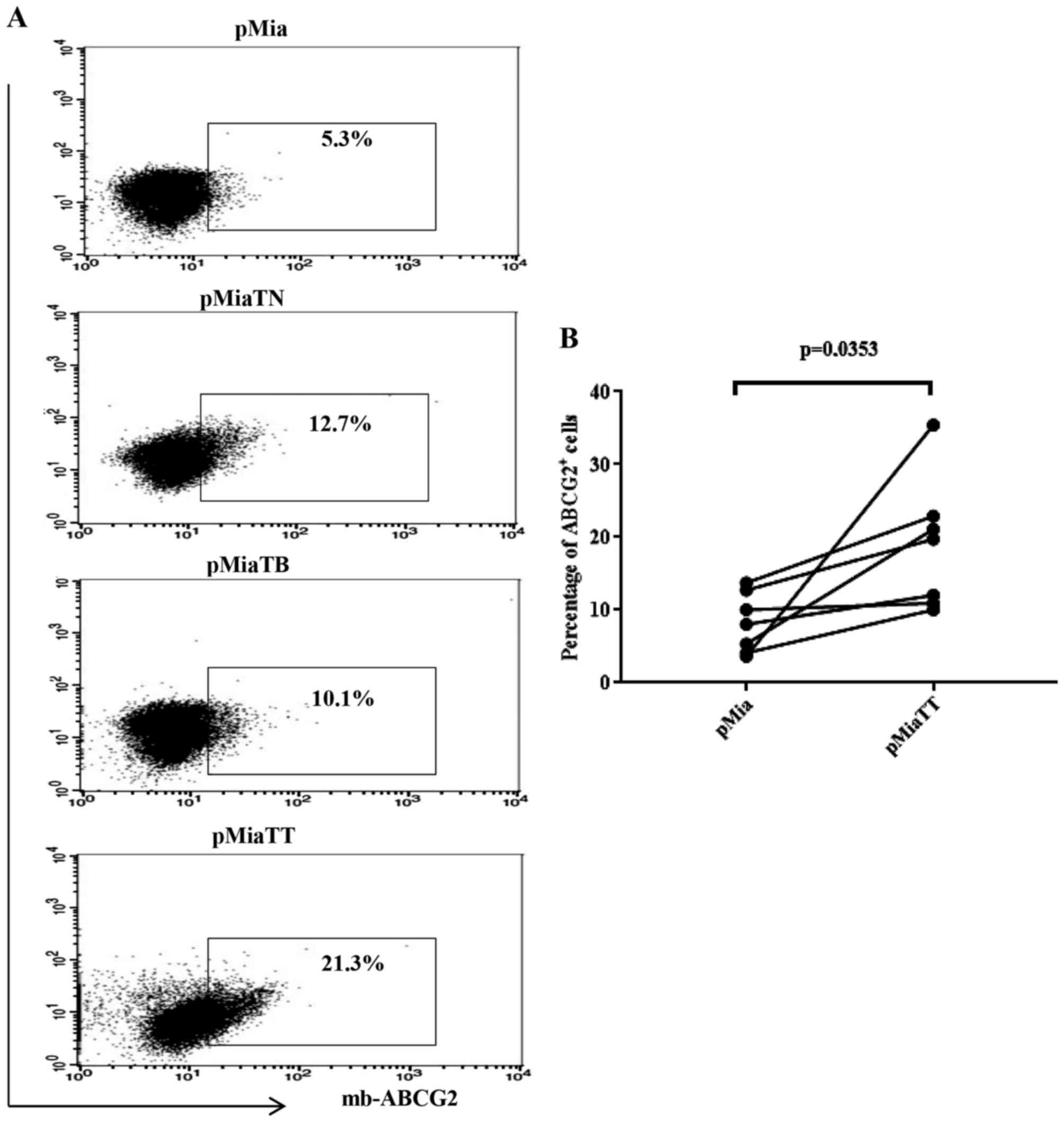
(Fig. 3), pMiaTT cells still showed
the greatest proportion of ABCG2 positive cells (21.3%) when
compared to control pMia cells (5.3%) and individually treated
pMiaTN cells (12.7%) and pMiaTB cells (10.1%). Fig. 3B shows a significant change in the
proportion of ABCG2 expressing cells between pMia and pMiaTT cells.
Taken together, these data suggest that TNFα and TGFβ-1 could
synergistically increase CSC populations among MiaPaCa-2 cells.
Pretreatment of MiaPaCa-2 cells with
TNFα and TGFβ-1 increase CSC populations in sphere forming culture
condition
Meanwhile, we succeeded culturing of tumorspheres
from MiaPaCa-2 cells, and furthered the assessment of CSC marker
expression on sphere cells derived from the four individual
subcultures designated as follows: Mia (derived from untreated
parental MiaPaCa-2), MiaTN (TNFα-pretreated), MiaTB
(TGFβ-1-pretreated), and MiaTT (TNFα + TGFβ-1-pretreated).
Procedures of obtaining of tumor spheres described in the materials
and methods. After 12–14 days of culture, the tumor spheres were
harvested and dissociated with TrypLEX, and flow cytometry analyses
were performed. In consistent with the data obtained from adherent
culture, pretreatment with TNFα + TGFβ-1 gave rise to the highest
number of CSCs (Fig. 4A).
Specifically, 24.9% of CD446v positive cells and 20% of CD133
positive cells and 8.7% of double positive cells, while MiaTN cells
possessed 14.7% of CD44v6 positive cells, 8% of CD133 positive
cells, and 4.3% of double positive cells. MiaTB cells possessed
20.4, 10.2 and 5.9% of respective CSC populations. Control Mia
cells included 11.4, 4.4 and 2.6% of respective CSC populations.
The significant change (Fig. 4B) in
the proportion of CD44v6 expressing cells between Mia and MiaTT
cells was obtained, however, there was no significant change in
terms of CD133 expressing cells between Mia and MiaTT cells as
shown in Fig. 4C. In general, cells
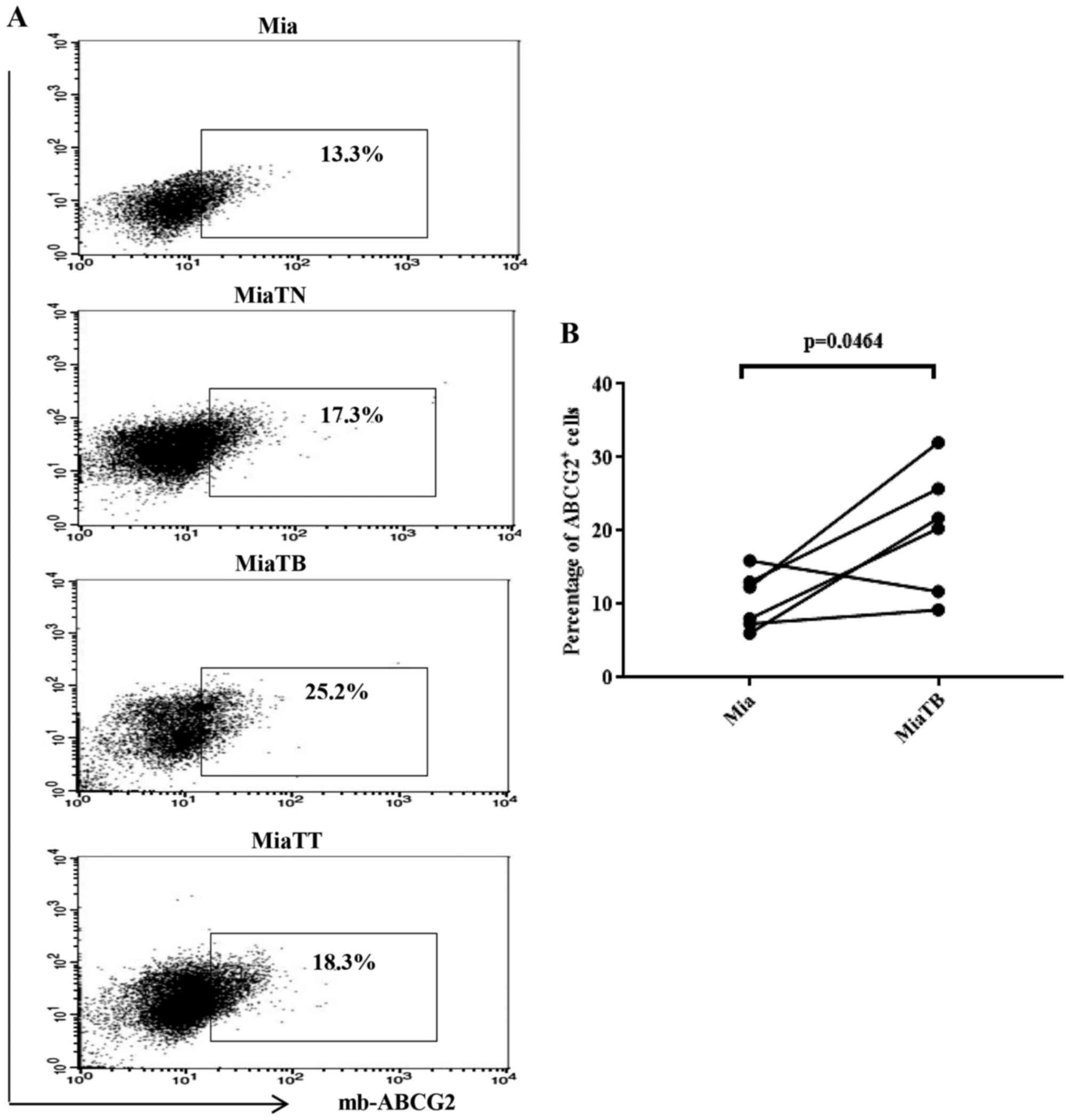
pretreated with cytokines showed higher proportion of ABCG2
positive cells compared to the control (Fig. 5A), however, in this case MiaTB cells
possessed the highest proportion of ABCG2 positive cells which
reached 25.2%, while MiaTT and MiaTN cells had similar percentages
of ABCG2 positive cells 18.3% and 17.3% respectively. Control Mia
cells possessed 13.3% of ABCG2 positive cells. Accordingly,
comparison of the proportion of ABCG2 positive cells between Mia
and MiaTB cells was reached to statistical significance (Fig. 5B). Of note, the proportions of CSCs in
sphere-forming cultures were substantially increased compared to
adherent cultures as sphere-forming culture media have the ability
to enrich SC populations (Figs. 2,
3, 4,
and 5).
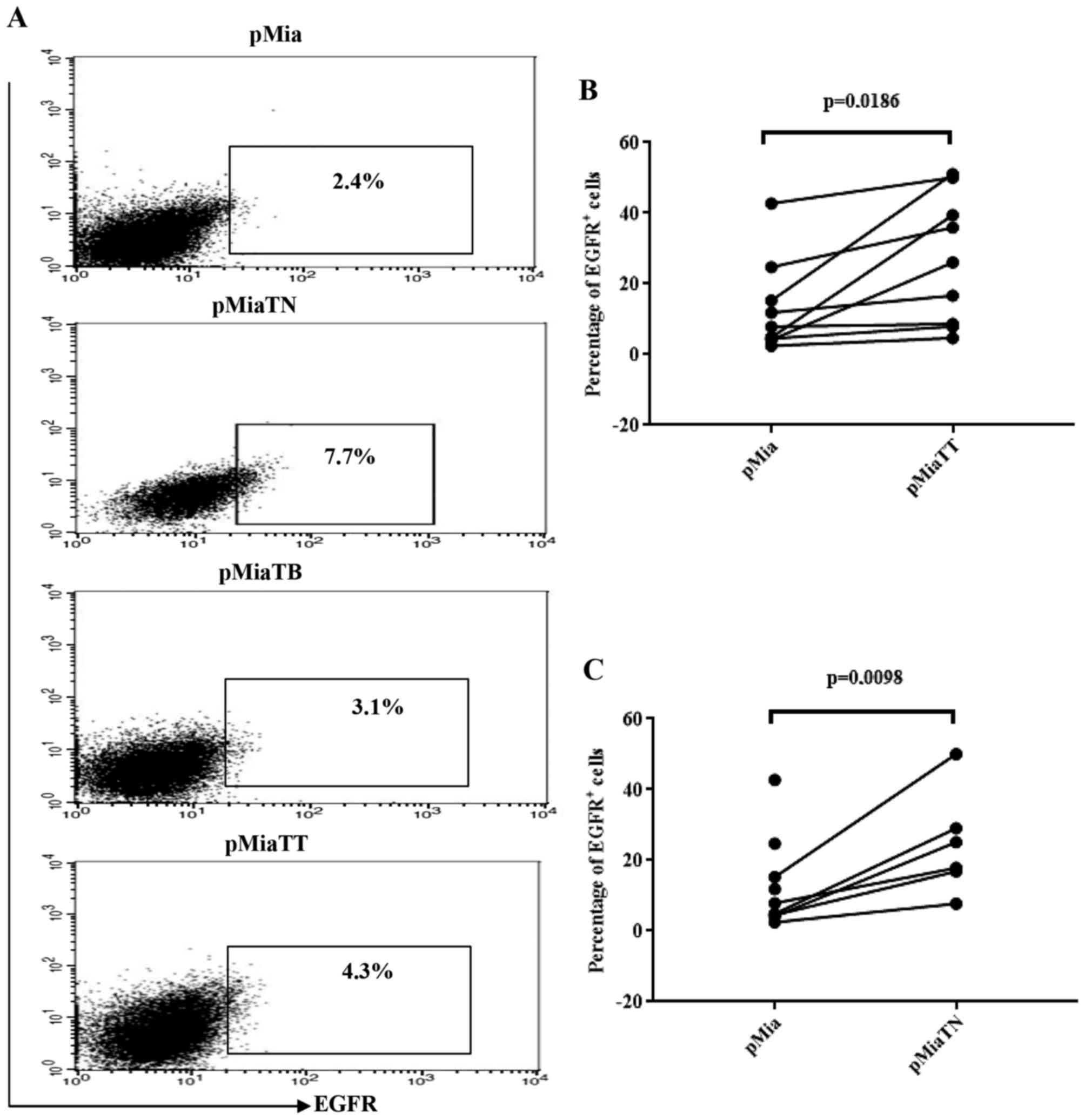
Treatment with TNFα increases the
proportion of EGFR positive cells among adherent MiaPaCa-2
cells
Previously, induction of EGFR expression by TNFα at
mRNA and protein level in six PC cell lines has been reported
(28). However, in that study
MiaPaCa-2 was not included. So we tested using flow cytometry
whether TNFα and TGFβ-1 have any effect on EGFR expressing
population in MiaPaCa-2 cells. As indicated in Fig. 6A, TNF-α treatment, in concert with
previous works, increased EGFR positive cells (7.7%) compared to
untreated (2.4%) and TGFβ-1 treated cells (3.1%), while treatment
with combination of cytokines increased EGFR expressing cells
nearly by two-fold (4.3%) when compared to untreated cells.
Fig. 6B and C show that the
comparisons of the proportion of EGFR positive cells between pMia
and pMiaTT cells and between pMia and pMiaTN cells were reached to
statistical significance.
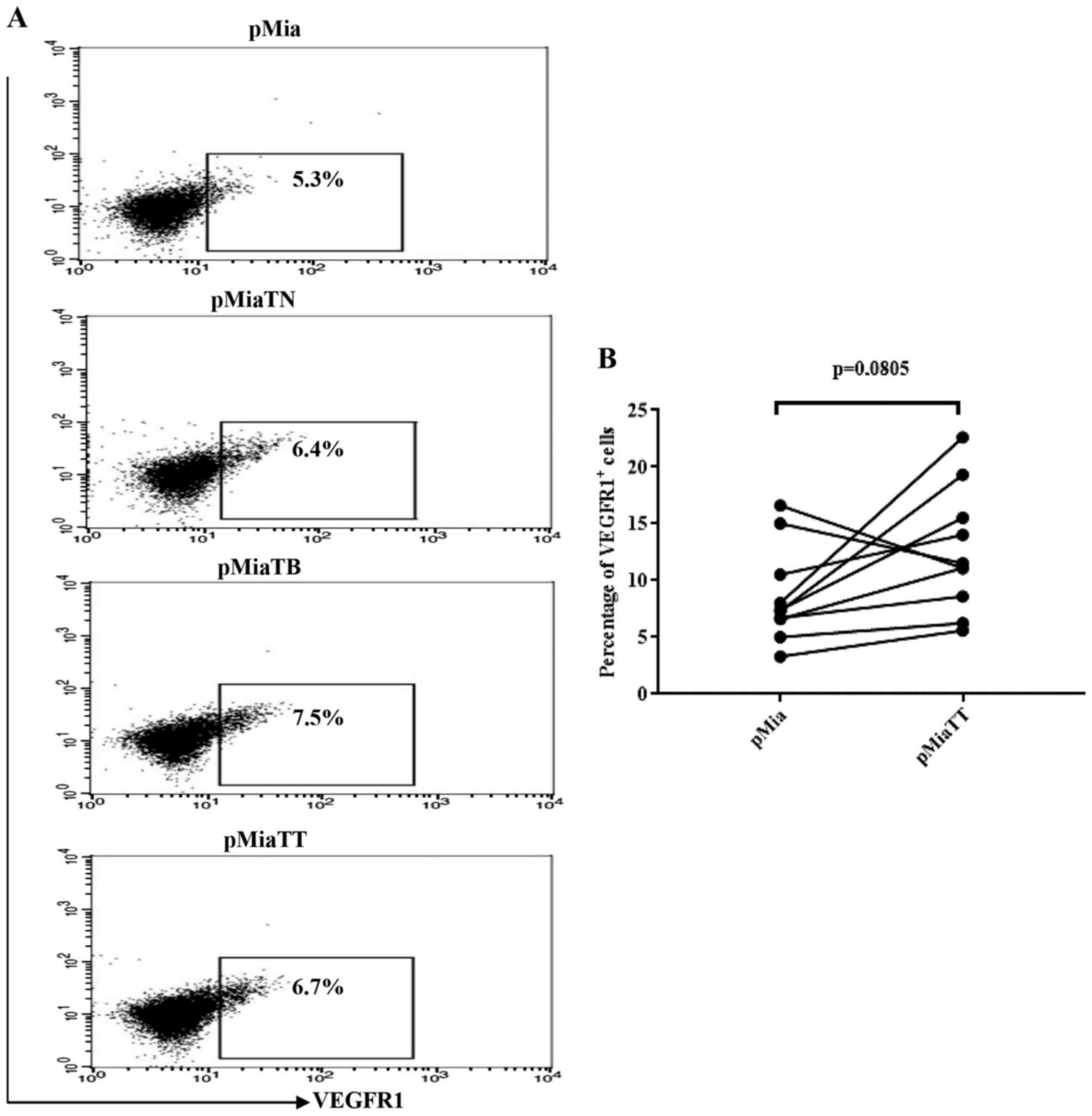
TNFα and TGFβ-1 treatment do not
increase the proportion of vascular endothelial growth factor
receptor 1 (VEGFR1) expressing cells among MiaPaCa-2 cells in
adherent culture
Because presence of VEGFR1 on PC cell lines
including MiaPaCa-2 have been demonstrated by RT-PCR and western
blot analysis, and upon stimulation with VEGF-A or VEGF-B Panc-1
cells exhibited increased migration and invasion abilities
(29), therefore we tested whether
TNFα and TGFβ-1 could increase the proportion of VEGFR1 expressing
cells among MiaPaCa-2. As indicated in Fig. 7A, the flow cytometry data revealed
that neither individual nor combinatorial treatment led to
substantial increase of the proportion of VEGFR1 expressing cells.
Likewise, there was no significant change in the proportion of
VEGFR1 expressing cells between pMia and pMiaTT cells (Fig. 7B).
TNFα and TGFβ-1 treatment do not
promote migration of MiaPaCa-2 cells
In order to confirm our phenotypic data on VEGFR1 at
functional level, we performed wound healing assay. Indeed, the
migration assay revealed that treatment of MiaPaCa-2 cells with
TNF-α and TGFβ-1 neither individually nor combinatorially promoted
migration capacity of the cells (data not shown) suggesting TNFα
and TGFβ-1 may not bestow a migratory capacity on MiaPaCa-2 cells
as shown in other types of cancer cells.
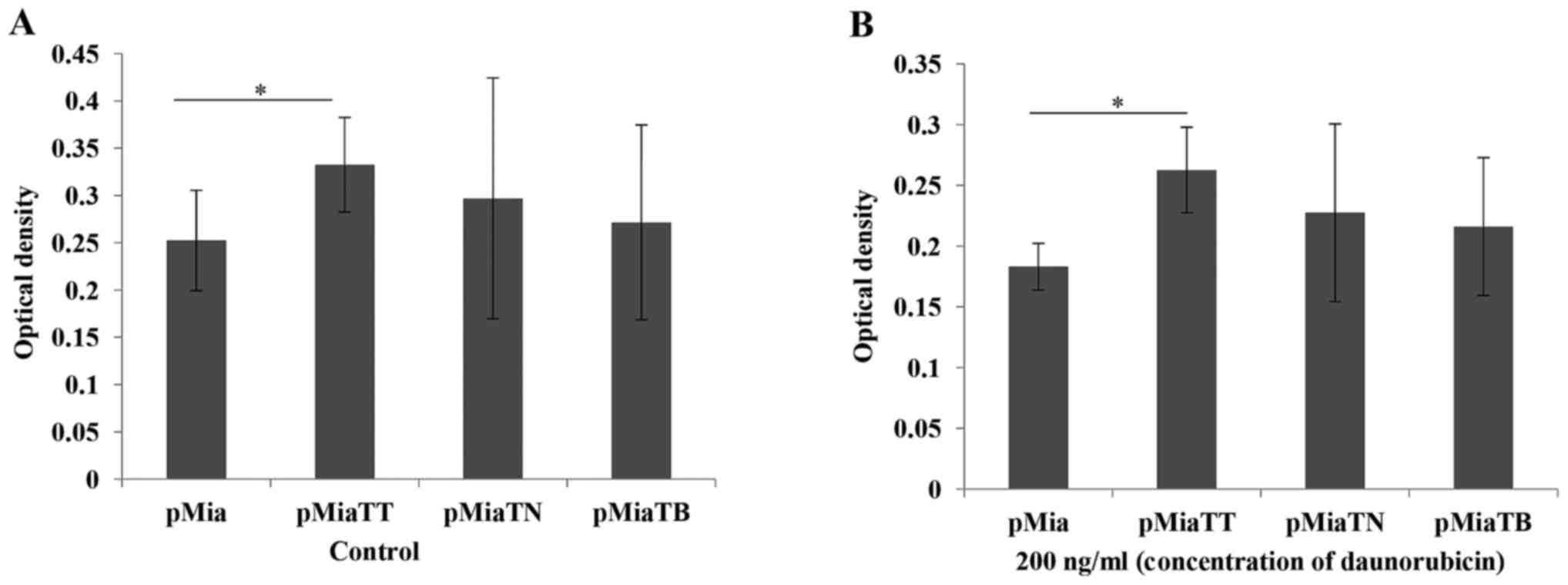
TNFα + TGFβ treatment lead to
increased proliferation and daunorubicine resistance, but not
gemcitabine
In our study ABCG2 expressing cells were increased
upon exposure to TNFα + TGFβ, therefore we carried out cytotoxicity
assay in order to examine the phenotypic data. We first chose
daunorubicin, because it is one of the substrates of ABCG2
(16). As indicated in Fig. 8B combinatorial treatment significantly
(P=0.00407) increased daunorubicin resistance compared to untreated
cells. At the same time we also tested the proliferation ability of
the cells upon treatment with these cytokines. As we expected
combinatorial treatment significantly (P=0.039416) increased the
proliferation ability of the cells (Fig.
8A). Then we further tested if this cytokine treatment will
also give the cells more resistance to gemcitabine which is a one
of the most frequently used chemodrugs in basic research and
clinical setting in particular for PC. Surprisingly, the cytokine
treatments with both individually and combinatorially did not
increase drug resistance to gemcitabine (data not shown).
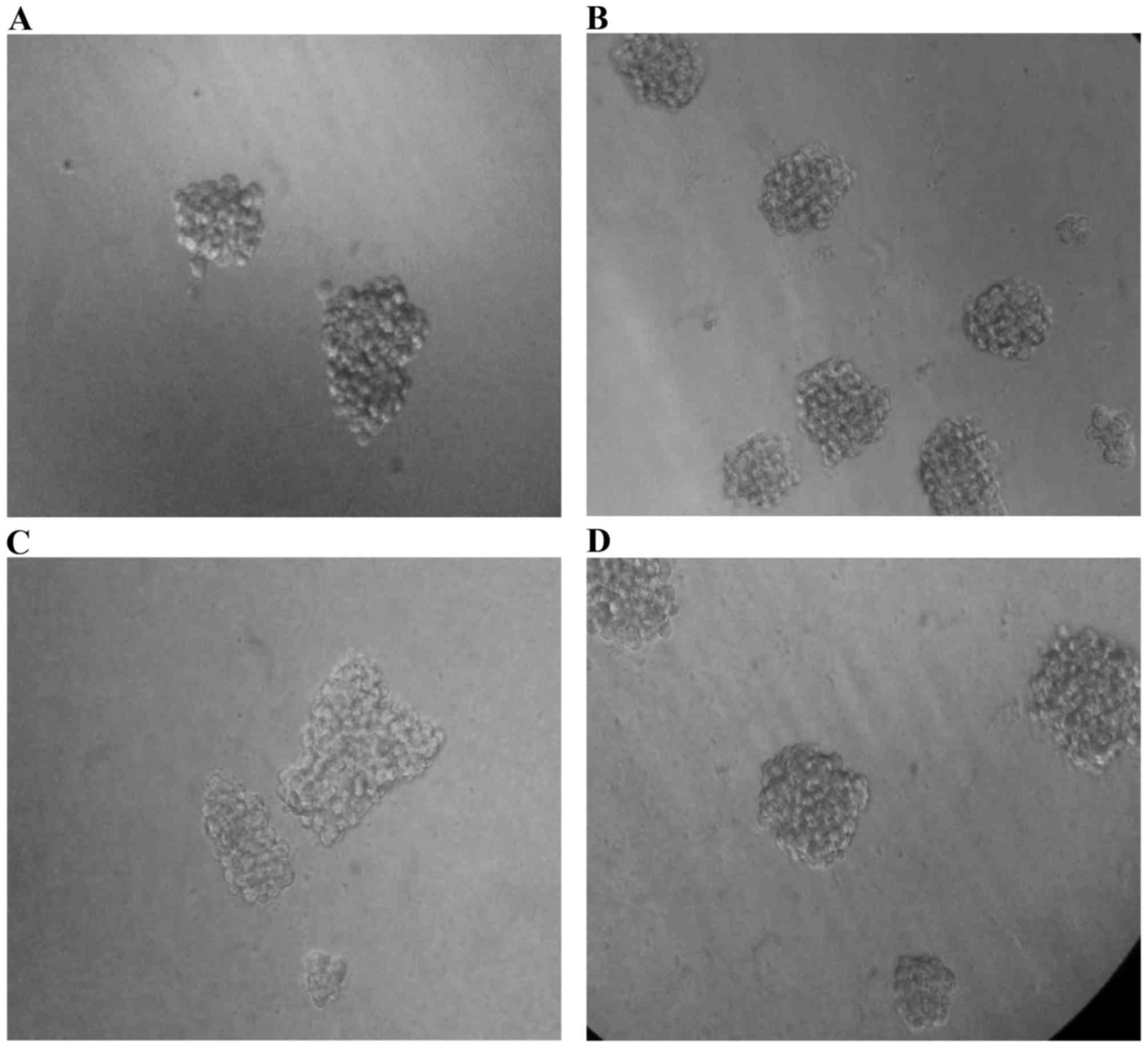
Pretreatment of MiaPaCa-2 cells with
TNFα alone and TNFα + TGFβ promote sphere forming ability
To assess self-renewal capacity of these four
subcultures, we performed sphere formation assay. The four
subcultures were plated in sphere forming condition, and after 11
to 12 days of incubation the numbers of spheres were counted under
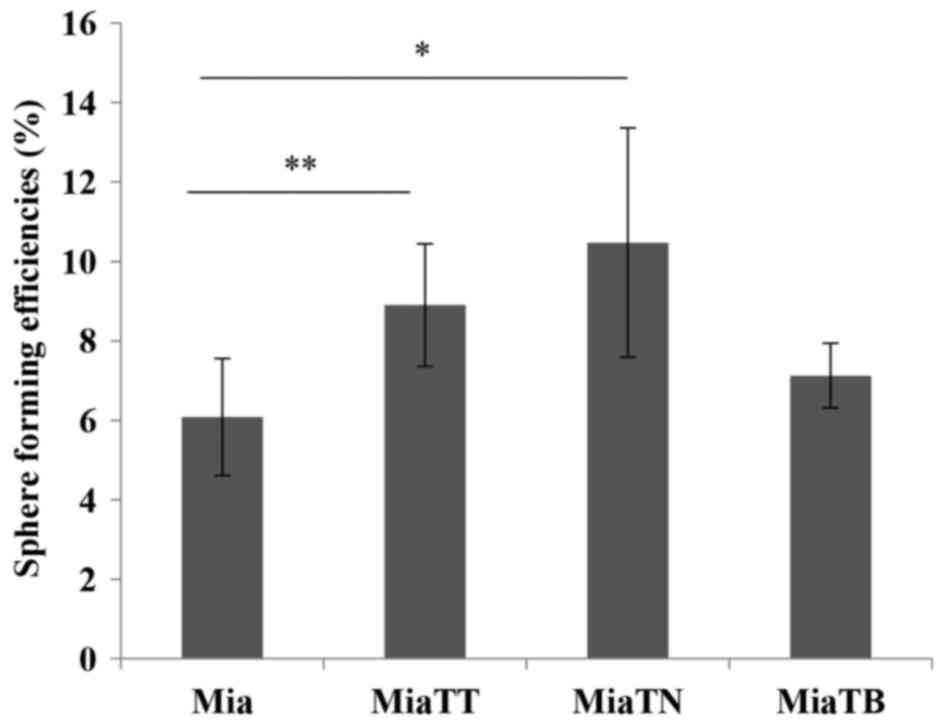
an inverted microscope (Fig. 9).
Interestingly, the cells pre-treated with TNFα generated spheres
with the highest efficiency (P=0.0475578), while the cells
pretreated with combination of cytokines (TNFα + TGFβ-1) still had
a significant (P=0.0385558) increase in sphere forming ability when
compared to untreated cells. TGFβ-1 pretreated cells generated
slightly higher number of spheres than untreated parental cells
(Fig. 10).
Discussion
Pancreatic CSCs have been defined based on the
expression of putative CSCs markers including CD133, CD44, CD24,
CXCR4, EpCAM, and ABCG2, among others (7,14,30–32). These
distinct cell populations often associated with increased
resistance to chemodrugs, anchorage independent growth potential,
in vivo tumorigenesity and metastatic activity. Although
there are still lacks of evidence about the stromal factors that
increase the CSC populations in PC, however, the preclinical and
clinical studies can provide important clues about the potential
candidate factors responsible for augmenting CSC populations in
PC.
Elevated levels of TNFα and TGFβ-1 in PC patients'
blood and tumor tissues have been found in a number of clinical
studies (20). Preclinical studies
also constantly demonstrate the involvement of these cytokines in
various aspects of cancer cell progression including invasion,
migration, and metastasis (17,18). Thus,
we aimed to examine whether TNFα and TGFβ-1 have effects on CSC
populations expressing above mentioned CSC markers in PC.
Here, we found that TNFα and TGFβ-1 can increase the
proportion of CSC populations, which defined by expression of
putative CSC markers: CD44v6, CD133, and ABCG2. These increases in
CSC populations were seen both in adherent culture and sphere
forming culture conditions, in the latter case the cells pretreated
with these cytokines.
Tumor-promoting roles of TNFα and TGFβ-1have been
shown in numerous works, and recent reports even linked these two
cytokines with regulation of CSC properties of breast cancer
(33), gilaoblastoma (23), leukemia (24) and PC (34). For example, Wang et al revealed
that TGFβ-1-induced EMT could increase CD44/CD24 expressing CSC
population in PANC-1 cells, another well known PC cell line
(34). A quite recent report revealed
that TNFα can maintain leukemia initiating cells via autocrine
fashion by forming a positive feedback loop with NF-κB (24). The importance of TNFα in PC
progression has been well exemplified in a study of Egberts et
al, in which several PC cell lines (MiaPaCa-2 was not included)
acquired invasive properties in vitro upon treatment with
TNFα, and they further injected these cells into mice and
subsequently treated with TNFα, and observed strong enhancement of
tumor growth and metastasis. They also showed a reduction of tumor
growth and metastasis after inhibition of TNFα with its inhibitors
(19). There were also few reports
showed synergistic effects of TNFα and TGFβ-1 on cancer cell
progression (27,35).
To our knowledge this is the first study to show
increased properties of CSCs in MiaPaCa-2 cells upon exposure to
TNFα and TGFβ-1. These data suggest that cytokines secreted by
tumor associated stromal and immune cells have an important role in
PC progression through propagating CSC populations within
pancreatic tumor mass, it can also explain, at least in part, why
there were elevated concentrations of these cytokines in advanced
PC patients. However, it remains unknown, in our study, as to why
the proportion of smaller and round-shaped cells was increased upon
TGFβ-1 treatment. Because it is contrary to most studies in which
TGFβ-1 treated cells often exhibit mesenchymal like morphology. For
example, in their study Wang et al showed that PANC-1 cells
acquired mesenchymal morphology upon exposure to TGFβ-1 (34). This may due to the different PC cell
lines respond distinctly to TGFβ-1 signaling.
A recent report highlighted the importance of EGFR
signaling, in corporation with HH/GLI signaling, for the oncogenic
phenotype of basal cell carcinoma and tumor initiating PC cells
(36), and in an earlier study
overexpression of EGFR at both mRNA and protein level in response
to TNFα stimulation has been found (28) suggesting there should be a close
relationship between EGFR and CSC properties of PC. Thus, we
examined EGFR expressing population using flow cytometry. In
consistent with the previous reports, treatment with TNFα
substantially increased the proportion of EGFR expressing
population among MiaPaCa-2 cells compared to untreated and TGFβ-1
alone and combinatorial treatment.
Because activation of VEGFR1 upon stimulation with
VEGF-A and VEGF-B was associated with increased migration and
invasiveness of PC cells (29), we
examined the effect of TNFα and TGFβ-1 on VEGFR1 expressing
MiaPaCa-2 cells. Surprisingly, exposure to TNFα and TGFβ-1
individually and combination of both cytokines only marginally
increased the number of VEGFR1 expressing cells, suggesting TNFα
and TGFβ-1 are may not implicate in migration and invasion of
MiaPaCa-2 cells. To test this assumption we further performed wound
healing assay. Indeed, the wound healing assay supported our
phenotypic data, as neither cytokine individually or
combinatorially promoted migration of MiaPaCa-2 cells. Overall,
these findings suggest that TNFα and TGFβ-1 may not contribute
directly to invasion and metastasis of MiaPaCa-2 cells. At this
point, we should admit that there was a limitation in our
experiments regarding migration of cytokine-treated cells. That is
we used very low concentration of TNFα. In many previous cancer
works the concentration of TNFα were ranged from 10 to 50 ng/ml.
The reason we chose this particular concentration (2 ng/ml) for
TNFα was the morphological changes have already occurred in
MiaPaCa-2 cells at this concentration in the beginning of the
experiments.
The substantially increased proportion of ABCG2
expressing cells among MiaPaCa-2 upon treatment with combination of
both cytokines gave us an implication that TNFα and TGFβ-1may play
a role in drug resistance of the cells. Therefore, we treated the
four subcultures with daunorubicin, as it is one of the main
substrates of ABCG2 (16). As
expected treatment with combination of the cytokines significantly
increased daunorubicin resistance. We subsequently tested if the
cytokine treated cells also acquired a resistance to gemcitabine, a
widely used chemodrug to treat PC and other solid cancers. However,
quite unexpectedly, TNFα and TGFβ-1 treatment did not improve
gemcitabine resistance of the cells. There are two possible
explanations may account for this failure. Firstly, the proportion
of the CSC populations in MiaPaCa-2 cells after treatment with
these cytokines is still not adequate to show a dramatic change in
drug (against gemcitabine) resistance, and MTT assay may not
sensible enough to detect the small changes that occurred in the
cells. Another explanation is MiaPaCa-2 itself is intrinsically
resistant to gemcitabine (37) and
there is little room for further increasing drug resistance
(against gemcitabine). Our microscopic observations agree with the
second explanation, while not denying the first one, because unlike
treatment with daunorubicin, which dramatically inhibited
proliferation of MiaPaCa-2 cells and led to apoptotic death,
gemcitabine treatment exhibited little effect on cell proliferation
and led to unremarkable apoptotic death (unpublished observation).
At the same time we also showed that the proliferation rate of the
cells was significantly increased upon treatment with TNFα +
TGFβ-1.
In order to assess our phenotypic data about CSC
markers at the functional level, we performed sphere forming assay,
and interestingly, the highest sphere forming ability was seen in
TNFα pretreated cells, while pretreated with both cytokines still
yielded significant number of spheres. The increased number of EGFR
expressing cells may explain the reason as to why pretreatment with
TNFα increased self-renewal of the cells, despite lower proportion
of CD44v6 and CD133 expressing cells compared to TGFβ-1 and TNFα +
TGFβ-1treated cells. It is noteworthy that the self-renewal assay
was conducted in vitro condition, in which included
recombinant EGF, a direct ligand for EGFR, therefore it is not
surprising that the cells pretreated with TNFα exhibited the
highest sphere forming capacity. Therefore, this finding should be
validated through in vivo tumorigenesis study in the
future.
Since these two cytokines, TNFα and TGFβ-1, have
been considered as pleiotropic molecules which are enable to
initiate multiple signaling cascades in a cell, so we can assume
multiple signaling pathways as candidate mechanisms for these
increases of CSC populations in MiaPaCa-2 cells. Among them
aberrant NF-κB and STAT3 signalings can be considered as most
promising candidate mechanisms, since constitutive activations of
NF-κB and STAT3 and their positive feedback relationships with CSC
markers such as CD44 and CD133 have been shown in some cancer types
including PC (38–40). Furthermore, TNFα is a well known
activator of both NF-kB and STAT3, and a recent study showed that
TGFβ-1is also enable to activate NF-kB in PC cells (41) suggesting simultaneous presence of TNFα
and TGFβ-1 in the tumor microenvironment may further augment the
CSC populations of PC through activating those master transcription
factors and our data support this hypothesis to some degree as
combination of TNFα and TGFβ-1 has increased CSC populations of
MiaPaCa-2 cells greater than when used them individually.
In summary, our data provide a mechanistic link
between TNFα, TGFβ-1 and CSC properties of PC. This link was
demonstrated by synergistically increased CSC populations among
MiaPaCa-2 cells upon treatment with TNFα and TGFβ-1 and increased
sphere forming ability, and probably ABCG2 dependent drug
resistance. These findings highlight the importance of stromal
factors during tumor progression and imply a better understanding
of the more functional roles and downstream signaling pathways of
TNFα and TGFβ-1 in PC will give further insight into underlying
mechanisms of PC progression.
Acknowledgements
The present study was supported by the Ministry of
Education and Science of Republic of Kazakhstan (grant no.
612.017.1:616-006).
Glossary
Abbreviations
Abbreviations:
|
PC
|
pancreatic cancer
|
|
TNFα
|
tumor necrosis factor α
|
|
TGFβ-1
|
transforming growth factor β-1
|
|
CSC
|
cancer stem cell
|
|
ABCG2
|
ATP-binding cassette transporter
G2
|
References
|
1
|
Lin W and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell
characteristics. Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AKT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monzani E, Facchetti F, Galmozzi E,
Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D,
Santinami M, et al: Melanoma contains CD133 and ABCG2 positive
cells with enhanced tumourigenic potential. Eur J Cancer.
43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kryczek I, Liu S, Roh M, Vatan L, Szeliga
W, Wei S, Banerjee M, Mao Y, Kotarski J, Wicha MS, et al:
Expression of aldehyde dehydrogenase and CD133 defines ovarian
cancer stem cells. Int J Cancer. 130:29–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molejon MI, Tellechea JI, Loncle C, Gayet
O, Duconseil P, Lopez-millan MB, Moutardier V, Gasmi M, Garcia S,
Turrini O, et al: Deciphering the cellular source of tumor relapse
identifies CD44 as a major therapeutic target in pancreatic
adenocarcinoma. Oncotarget. 6:7408–7423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jijiwa M, Demir H, Gupta S, Leung C, Joshi
K, Orozco N, Huang T, Yildiz VO, Shibahara I, de Jesus JA, et al:
CD44v6 regulates growth of brain tumor stem cells partially through
the AKT-mediated pathway. PLoS One. 6:e242172011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rall CJ and Rustgi AK: CD44 isoform
expression in primary and metastatic pancreatic adenocarcinoma.
Cancer Res. 55:1831–1835. 1995.PubMed/NCBI
|
|
12
|
Liu G, Yuan X, Zeng Z, Benfante A, Iovino
F, Biffoni M, Apuzzo T, Sperduti I, Volpe S and Cocorullo G:
Analysis of gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133+ cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maeda S, Shinchi H, Kurahara H, Mataki Y,
Maemura K, Sato M, Natsugoe S, Aikou T and Takao S: CD133
expression is correlated with lymph node metastasis and vascular
endothelial growth factor-C expression in pancreatic cancer. Br J
Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikushima H and Miyazono K: TGFbeta
signalling: A complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balkwill F: TNF-α in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egberts JH, Cloosters V, Noack A,
Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C,
Tepel J, et al: Anti-tumor necrosis factor therapy inhibits
pancreatic tumor growth and metastasis. Cancer Res. 68:1443–1450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roshani R, McCarthy F and Hagemann T:
Inflammatory cytokines in human pancreatic cancer. Cancer Lett.
345:157–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Kikuchi S, Tamakoshi A, Obata Y,
Yagyu K, Inaba Y, Kurosawa M, Kawamura T, Motohashi Y and Ishibashi
T: JACC Study Group: Serum transforming growth factor-beta1 levels
and pancreatic cancer risk: A nested case-control study (Japan).
Cancer Causes Control. 17:1077–1082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poch B, Lotspeich E, Ramadani M, Gansauge
S, Beger HG and Gansauge F: Systemic immune dysfunction in
pancreatic cancer patients. Langenbecks Arch Surg. 392:353–358.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Miyazawa K and Miyazono K: Autocrine TGF-beta signaling maintains
tumorigenicity of glioma-initiating cells through Sry-related
HMG-box factors. Cell Stem Cell. 5:504–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa
M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y and Kurokawa
M: Positive feedback between NF-κB and TNF-α promotes
leukemia-initiating cell capacity. J Clin Invest. 124:528–542.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY,
Sun GH and Sun KH: TNF-α induces epithelial-mesenchymal transition
of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol
Cancer Res. 10:1109–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borthwick LA, Gardner A, De Soyza A, Mann
DA and Fisher AJ: Transforming growth factor-β1 (TGF-β1) driven
epithelial to mesenchymal transition (EMT) is accentuated by tumour
necrosis factor α (TNFα) via crosstalk between the SMAD and NF-κB
pathways. Cancer Microenviron. 5:45–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmiegel W, Roeder C, Schmielau J, Rodeck
U and Kalthoff H: Tumor necrosis factor alpha induces the
expression of transforming growth factor alpha and the epidermal
growth factor receptor in human pancreatic cancer cells. Proc Natl
Acad Sci USA. 90:863–867. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wey JS, Fan F, Gray MJ, Bauer TW, McCarty
MF, Somcio R, Liu W, Evans DB, Wu Y, Hicklin DJ and Ellis LM:
Vascular endothelial growth factor receptor-1 promotes migration
and invasion in pancreatic carcinoma cell lines. Cancer.
104:427–438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao B, Azmi AS, Aboukameel A, Ahmad A,
Bolling-Fischer A, Sethi S, Ali S, Li Y, Kong D, Banerjee S, et al:
Pancreatic cancer stem-like cells display aggressive behavior
mediated via activation of FoxQ1. J Biol Chem. 289:14520–14533.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
32
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Yu Y, Tsuyada A, Ren X, Wu X,
Stubblefield K, Rankin-Gee EK and Wang SE: Transforming growth
factor-β regulates the sphere-initiating stem cell-like feature in
breast cancer through miRNA-181 and ATM. Oncogene. 30:1470–1480.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Wu J, Zhang Y, Xue X, Tang D, Yuan
Z, Chen M, Wei J, Zhang J and Miao Y: Transforming growth factor
beta-induced epithelial-mesenchymal transition increases cancer
stem-like cells in the PANC-1 cell line. Oncol Lett. 3:229–233.
2012.PubMed/NCBI
|
|
35
|
Asiedu MK, Ingle JN, Behrens MD, Radisky
DC and Knutson KL: TGFβ/TNF(alpha)-mediated epithelial-mesenchymal
transition generates breast cancer stem cells with a claudin-low
phenotype. Cancer Res. 71:4707–4719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eberl M, Klingler S, Mangelberger D,
Loipetzberger A, Damhofer H, Zoidl K, Schnidar H, Hache H, Bauer
HC, Solca F, et al: Hedgehog-EGFR cooperation response genes
determine the oncogenic phenotype of basal cell carcinoma and
tumour-initiating pancreatic cancer cells. EMBO Mol Med. 4:218–233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer.
Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun L, Mathews LA, Cabarcas SM, Zhang X,
Yang A, Zhang Y, Young MR, Klarmann KD, Keller JR and Farrar WL:
Epigenetic regulation of SOX9 by the NF-κB signaling pathway in
pancreatic cancer stem cells. Stem Cells. 31:1454–1466. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin L, Jou D, Wang Y, Ma H, Liu T, Fuchs
J, Li PK, Lü J, Li C and Lin J: STAT3 as a potential therapeutic
target in ALDH+ and CD44+/CD24+ stem cell-like pancreatic cancer
cells. Int J Oncol. 49:2265–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chow JY, Ban M, Wu HL, Nguyen F, Huang M,
Chung H, Dong H and Carethers JM: TGF-beta downregulates PTEN via
activation of NF-kappaB in pancreatic cancer cells. Am J Physiol
Gastrointest Liver Physiol. 298:G275–G282. 2010. View Article : Google Scholar : PubMed/NCBI
|