Introduction
Numerous acquired molecular abnormalities and
cytogenetic abnormalities are strongly associated with
hematological malignancies (1). Core
binding factors (CBFs) are a family of heterodimeric
transcriptional regulators containing CBFβ (2). The inversion of chromosome 16, fusion of
CBFβ gene at 16q22 with the smooth muscle myosin heavy chain
(MYH11) gene at 16p13 region, forms the CBFβ-MYH11
fusion gene, inhibiting the differentiation of hematopoietic cells
by altering transcriptional regulation (3,4).
Inv(16)(p13q22) or t(16;16)(p13q22)
is a recurrent genetic abnormality, which fuses the core binding
factor-β (CBFβ) gene to the myosin heavy chain 11
(MYH11) gene. Inv(16)(p13q22)
or t(16;16)(p13q22) has been recognized as a feature of acute
myelomonocytic leukemia with abnormal eosinophils by the 2008 World
Health Organization classification and was designated as M4EO in
the French-America-British cooperative group classification
(5). Patients with inv(16)(p13q22) or t(16;16)(p13q22) mutations
have been identified to exhibit a more favorable clinical course,
an improved response to chemotherapy/stem cell transplantation, and
longer remission and survival in this AML subtype compared to those
without inv(16)(p13q22) or
t(16;16)(p13q22)(2). First described
by Nowell and Hungerford as a minute chromosome in patients with
granulocytic leukemia, the Philadelphia (Ph) chromosome is a result
of reciprocal translocation involving the long arms of chromosomes
9 and 22 (6,7). This translocation results in the
formation of a hybrid breakpoint cluster region-ABL proto-oncogene
1 (BCR-ABL) gene, which codes for a protein with enhanced
tyrosine kinase activity (8). It has
been demonstrated that the BCR-ABL fusion gene is the
hallmark of typical chronic myeloid leukemia (CML). Cases of CML
were additionally classified as chronic phase (CP), accelerated
phase (AP) or blast crisis (BC) according to criteria of WHO
classification (9). The
BCR-ABL rearrangement leads to a p210 chimeric protein in
typical CML, whereas 17–25% of patients with acute lymphocytic
leukemia and 0.9–3% patients with de novo acute myeloid
leukemia (AML) possess a p190BCR-ABL fusion protein
(1,10). The present study aimed to understand
the nature and mechanism of this particular type of leukemia
through case reports and literature review. A total of 4 patients
who were diagnosed as AML/CML with BCR-ABL and
CBFβ-MYH11 fusion genes at the First Affiliated Hospital of
Soochow University between January 2004 and December 2012 were
examined, with a detectable protein product of
p210BCR-ABL, were included in the present study. The
cases described in the present report may assist in understanding
the entity and mechanism of this particular type of leukemia.
Case report
The clinical files of the First Affiliated Hospital
of Soochow University were examined for patients who were diagnosed
with acute/chronic leukemia with BCR-ABL and/or
CBFβ-MYH11 fusion genes during January 2004 to December
2012. A total of four patients were identified with the
CBFβ-MYH11 fusion gene concomitant with BCR-ABL
expression. The patient samples were taken at different time
points: Case 1 was taken in June 2010, case 2 in August 2010, case
3 in September 2011 and case 4 in September 2010. Bone marrow (BM)
aspirates were collected into syringes containing media
supplemented with heparin. Therefore, the present report summarizes
the clinical and laboratory features of four patients with the
coexistence of BCR-ABL and CBFβ-MYH11 fusion genes,
including three males and one female with a median age of 29 years
(range, 18–40; Tables I and II). All patients exhibited constitutional
symptoms, including progressive fatigue, localized or diffuse pain
and low-grade fever. Physical examination revealed splenomegaly in
cases 1 and 4 and scattered petechiae and ecchymosis on the trunk
and lower extremities in cases 3 and 4. The peripheral blood count
revealed anemia and thrombocytopenia in three cases. Morphology and
cytochemical studies were performed on cells from the bone marrow
aspirate smears stained with Wright's stain (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 15 min and myeloperoxidase
respectively. For myeloperoxidase staining, slides had 10–15 drops
0.3% benzidine ethanol solution added to them, after 1 min, 10–15
drops of H2O2 solution were added for 5 min.
Slides were rinsed and Wright's stain was added for 30 min,
followed by another rinse (benzidine ethanol solutionand
H2O2 solutionwere purchased from Shanghai Sun
Biotech Co., Ltd., Shanghai, China). Images were captured using an
optical microscope (OLYMPUS CX-31; Olympus Corporation, Tokyo,
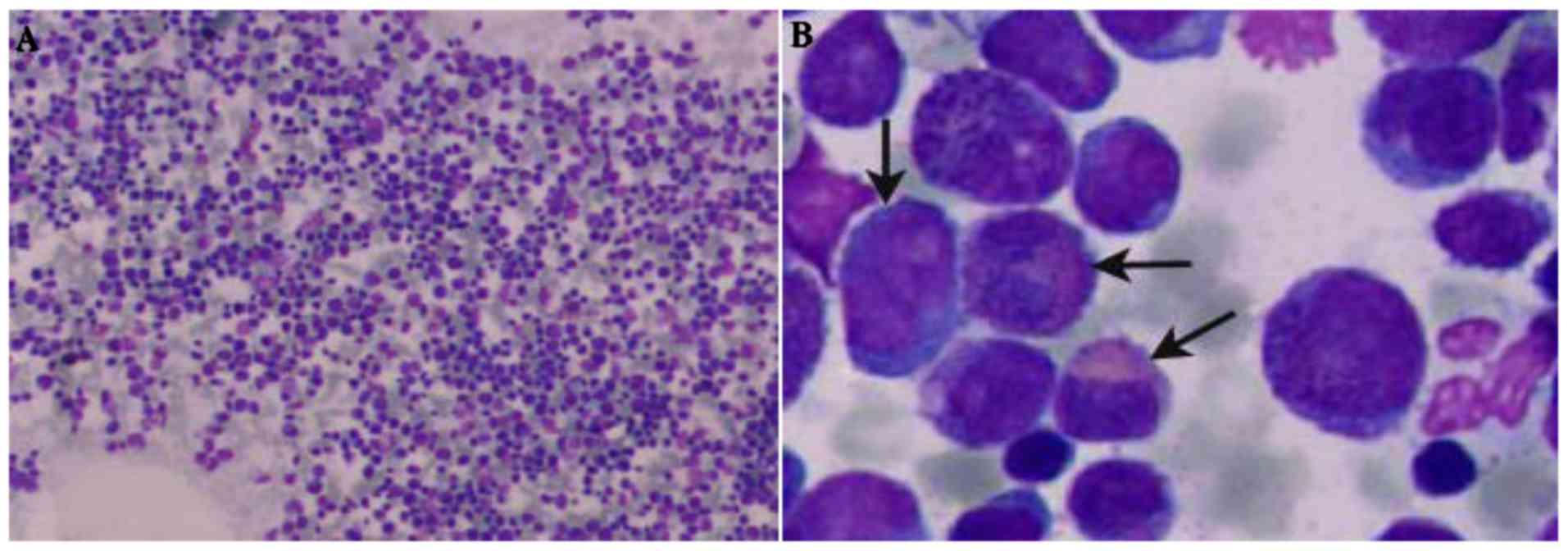
Japan) at magnifications, ×100 and ×1,000. Based on the morphology
of the BM cells (Fig. 1), three cases
were diagnosed with AML-M4 subtype with an abnormal eosinophil
component (M4EO; cases 2, 3 and 4), and case 1 was diagnosed with
CML-BC. As summarized in Table I, the
percentage of blasts in bone marrow samples was 10–49%, and the
eosinophil counts ranged from 4–14%. Immunophenotyping, the blasts
of all patients were positive for cluster of differentiation
(CD)13, CD33, CD117, CD34 and negative for B and T cell markers.
The multiplex polymerase chain reaction (PCR) detected
BCR-ABL and CBFβ-MYH11 transcripts in all 4
cases. Bone marrow mononuclear cells (BMNCs) were separated from
bone marrow of patients by Ficoll-Hypaque (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Total RNA was extracted
from BMNCs using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). The RNA was pretreated with DNase and used for
cDNA synthesis with random hexamers (Invitrogen; Thermo Fisher
Scientific, Inc.). The primers were synthesized to order by Sangon
Biotech Co., Ltd. (Shanghai, China) with the following sequences:
BCL-ABL F, 5′-AGCATTCCGCTGACCATCA-3′; BCL-ABL R,
5′-ACTCAGACCCTGAGGCTCAAAG-3′; BCL-ABL P, 5′-6-carboxyfluorescein
(6-FAM)-AAGCCCTTCAGCGGCCAGTAGCAT-carboxytetramethylrhodamine
(TAMRA)-3′. CBFβ/MYH11 F, 5′-CATTAGCACAACAGGCCTTTGA-3′; CBFβ/MYH11
R, 5′-AGGGCCCGCTTGGACTT-3; CBFβ/MYH11 P,
5′-6-FAM-TCGCGTGTCCTTCTCCGAGCCT-TAMRA-3′. The quantitative (q)PCR
conditions were as follows: 2 min at 50°C for denaturation, 10 min
at 95°C, 15 sec at 95°C and 60 sec at 60°C, followed by 40 cycles
of amplification. The relative quantification of CBFβ-MYH11
and BCR-ABL fusion genes was performed by the
2−ΔΔCq method (11). The
reverse transcription (RT)-qPCR results demonstrated that the copy
numbers of CBFβ-MYH11 and BCR-ABL fusion genes were
>5,000 copies/104 ABL copies in three patients (cases
2, 3 and 4). By contrast, in case 1 only the BCR-ABL
transcript was detected at similar levels (>5,000
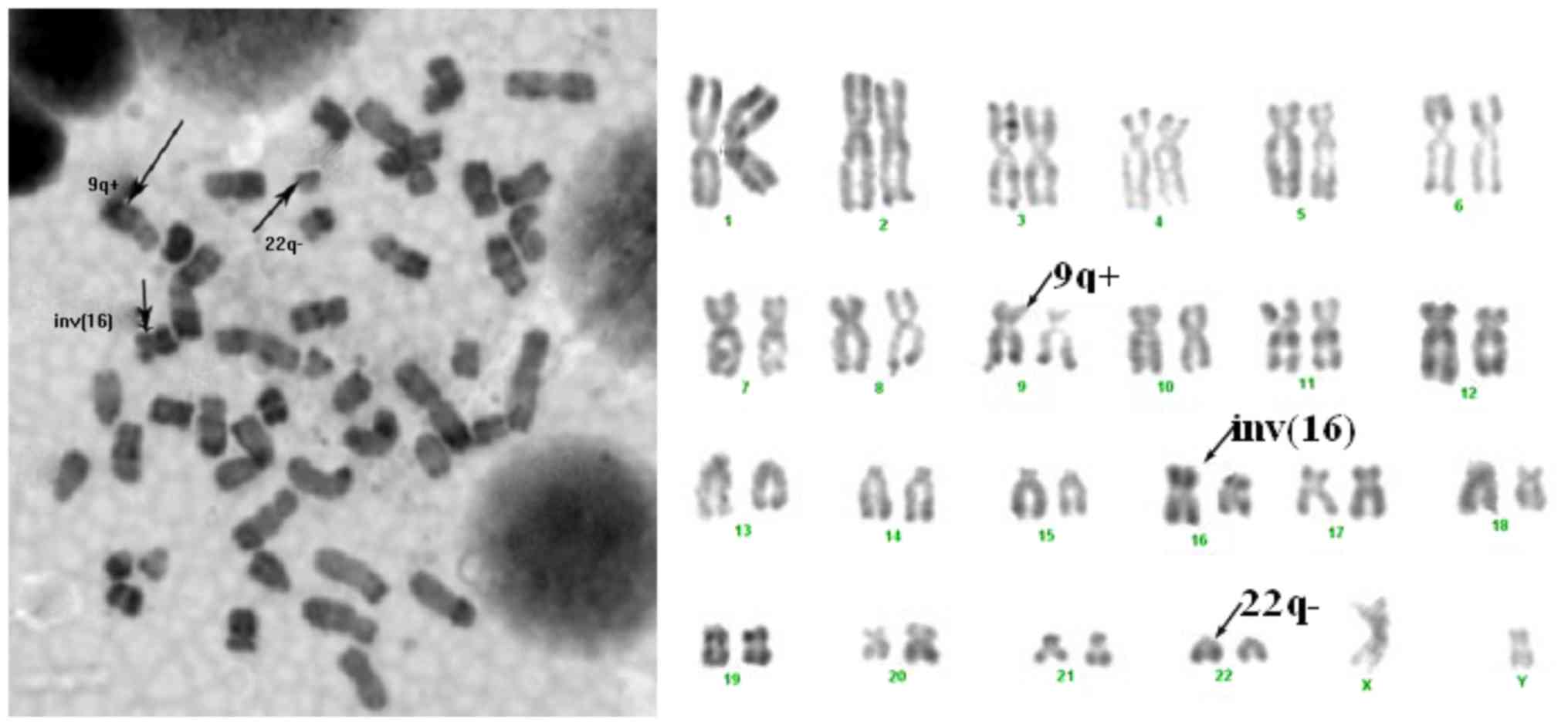
copies/104 ABL copies). Cytogenetic analysis was
performed on of the BM cells after 24 h culturing by the RBG
(R-band by Brdu using Giemsa) technique (12). At least 10 metaphases were examined
for each case. Karyotypes were described according to the standard
2009 International System for Human Cytogenetic Nomenclature
criteria (13,14).
 | Table I.Summary of clinical features and
morphology data of bone marrow samples. |
Table I.
Summary of clinical features and
morphology data of bone marrow samples.
|
|
|
|
|
| Peripheral
blood | Bone marrow, % |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Sex | Age, years | Symptoms | Signs | WBC,
×109/l | PLT,
×109/l | HB, g/l | Blast | Eosinophils | Initial
diagnosis | Outcome |
|---|
| 1 | M | 40 | Body pain | Splenomegaly | 191.8 | 389.0 | 119.0 | 10.0 | 5.5 | CML-BC | Succumbed to
disease |
| 2 | M | 36 | NA | Splenomegaly |
50.6 |
50.0 |
60.0 | 49.0 | NA | AML-M4EO | Succumbed to
disease |
| 3 | F | 18 | Fever | Petechiae,
ecchymosis |
72.2 |
22.0 |
61.0 | 46.0 | 4.0 | AML-M4EO | Succumbed to
disease |
| 4 | M | 31 | Fatigue | Splenomegaly,
petechiae, ecchymosis |
67.5 |
21.0 |
99.0 | 43.0 | 14.0 | AML-M4EO | Alive |
 | Table II.Summary of the molecular and
cytogenetic findings. |
Table II.
Summary of the molecular and
cytogenetic findings.
| Case no. | Multiplex PCR | qPCR
(copies/104 ABL copies) | FISH (%) | Cytogenetic
data |
|---|
| 1 |
p210BCR-ABL
CBFβ-MYH11 | BCR-ABL
(9958) | BCR-ABL(94)
CBFβ-MYH11 (47) | 46,X,der(Y),t(Y;1)
(q12;q23), t(9;22)(q34;q11),t(9;11)(p22,q23)[4]/47,idem,+18[3]/ 51,
idem,+4,+8,+12, +21,+mar?der(16) inv(16) (p13;q22)[4]/46,XY[2] |
| 2 |
p210BCR-ABL
CBFβ-MYH11 |
BCR-ABL(8158)
CBFβ-MYH11(5610) | BCR-ABL(92)
CBFβ-MYH11(95) |
45,X,-Y,t(9;22)t(16;16)(p13;q22)[1]/46,idem,+ph[9] |
| 3 |
p210BCR-ABL
CBFβ-MYH11 |
BCR-ABL(9496) CBFβ-MYH11
(12602) | BCR-ABL(85)
CBFβ-MYH11(91) |
46,XX,t(9;22)(q34;q11)
inv(16)(p13;q22)[9]/46,XX[2] |
| 4 |
p210BCR-ABL
CBFβ-MYH11 |
BCR-ABL(5399)
CBFβ-MYH11(10473) | BCR-ABL(46)
CBFβ-MYH11(89) |
48,XY,der(8)t(8;10)(p23;q25),
der(10),t(8;10),t(10;16)(p13;q22),
der(16),inv(16)(p13;q22)t(10;16)[4]/46,XY,idem,t(9;22)(q34;q11) |
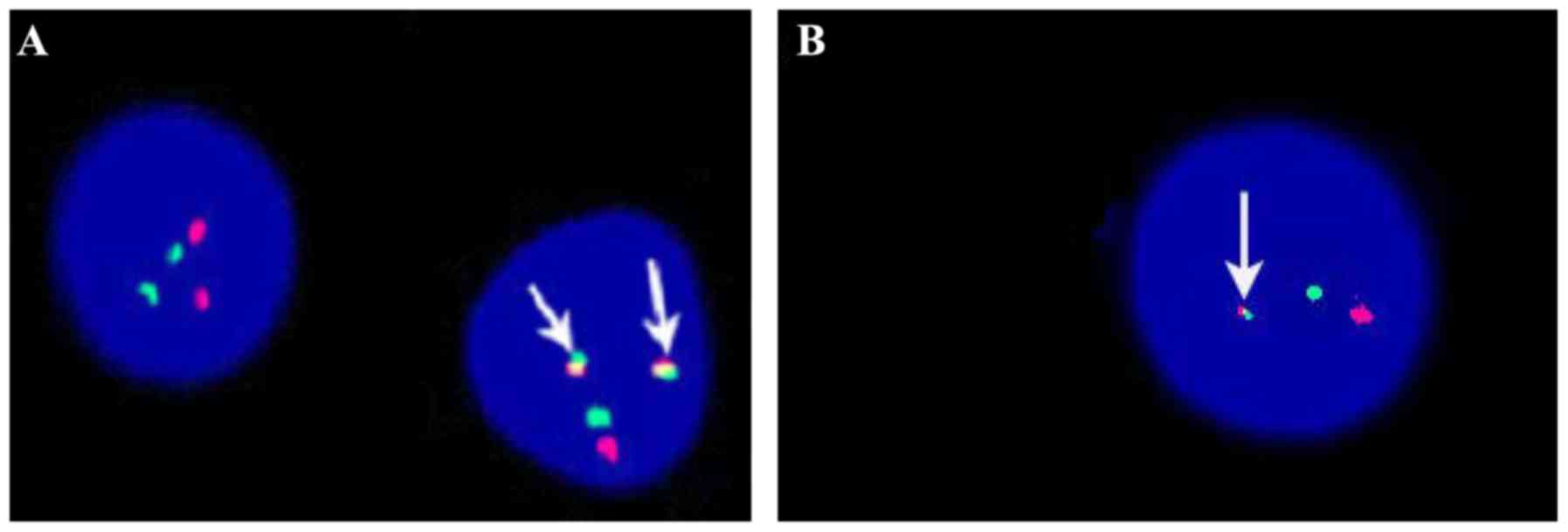
Fluorescence in situ hybridization (FISH)
analysis was performed on interphase nuclei and metaphases of the
BM cells to confirm the presence of the BCR-ABL and
CBFβ-MYH11-fused genes. The BCR-ABL dual-color,
double fusion translocation probes and the CBFβ dual color
break-apart rearrangement probes were used in the FISH analysis
according to the manufacturer's protocol (Beijing GP Medical
Technologies, Ltd., Beijing, China). Bone marrow cells were
cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% bovine fetal serum (Bovogen
Biologicals, Keilor East, VIC, Australia). The specimens were
placed on the slide, precipitated in ethanol, then ageing in 2X 0.3
M trisodium citrate, (SSC; pH 7.0) for 30 min at 37°C. Samples were
then dehydrated using increasing gradients (70, 85 and 100%) of
ethanol at room temperature for 2 min, and then dried at room
temperature. A total of 5 µg probes were mixed with the denatured
control probe, denatured for 5 min at 75°C, then hybridized
overnight at 37°C. Slides were then washed in 0.4X SSC at 72°C for
2 min and rinsed in 2X SSC at room temperature for 2 min, then in
70% ethanol for 2 min. Finally, slides were counterstained with 1
mg/ml 10 µl DAPI for 15 min. Slides were then screened using an
fluorescence microscope DMRB (OLYMPUS BX-51; Olympus Corporation,
Tokyo, Japan) at magnification, ×1,000. A minimum of 500 interphase
cells were observed. Images were captured and analyzed using the
VideoTesT (VT-FISH2.1, Nature Gene Corporation, Medford, NJ, USA).
FISH analysis also demonstrated the rearrangement of
CBFβ-MYH11 and BCR-ABL fusion signals in all cases,
and the percentage of the two fusion genes was >45% (Fig. 2). Cytogenetic analysis of all patients
revealed inv(16)(p13q22) or
t(16;16)(p13q22) and Philadelphia chromosome (Fig. 3). In addition, expression of trisomy 8
was detected in case 1, and the loss of Y chromosome was detected
in case 2. Based on physical examinations, hematological data, BM
morphology and cytogenetic and molecular analyses, a single patient
(case 1) was first diagnosed with CML-acute phase (AP), which
rapidly progressed to CML-BC, and three patients (cases 2, 3 and 4)
were diagnosed with AML-M4EO at first presentation with no evidence
of previous onset of CML. Patients were treated with various
therapeutics, including idarubicin (cases 1–4), cytarabine (cases
1–4), daunorubicin (cases 2 and 3) and tyrosine kinase inhibitors
(TKI; cases 1 and 4). A total of three patients achieved complete
remission following first round of chemotherapy (cases 1, 3 and 4).
Case 2 succumbed to respiratory failure following a pulmonary
infection. Following the fourth round of chemotherapy, case 3
abandoned treatment. A total of two patients received unrelated
donor peripheral hematopoietic stem cell and autologous peripheral
hematopoietic stem cell (case 4) transplantations. Case 1 succumbed
to Graft vs. host disease (GVHD), and case 4 was in good condition
for 24 months following transplantation. The abnormal eosinophils
observed in case 1 and case 2 presented as excessive eosinophils in
BM samples. In all cases, the blasts were of myeloid lineage and
there was evidence of monocytic differentiation, as demonstrated by
immunophenotypic analysis, in cases 3 and 4. Peripheral blood
monocytosis was exhibited in all cases. Patient data and protocol
were approved by the Ethics Committee at the First Affiliated
Hospital of Soochow University. Informed written consent was
obtained from each patient.
Discussion
The present report describes four patients with
CBFβ-MYH11 and BCR-ABL fusion genes. The inv(16) genetic abnormality was detected in case
1 at the time of blastic transformation. Although case 2 was
diagnosed with AML-M4EO based on the BM cell morphology, the
patient (case 2) should have been diagnosed CML-BP for the
following reasons: i) The patient displayed clinical characteristic
of CML, including leukocytosis and massive splenomegaly; ii) the Ph
chromosome, which typically occur in CML-BC, was detected in case
2; the rearrangement of CBFβ in addition to BCR-ABL
had been detected by FISH in the BM samples of case 3 and 4.
Additionally, karyotype analysis of the BM samples from case 4 at
diagnosis also confirmed that the chromosome translocation
inv(16) coexisted with the
Phchromosome, suggesting that the translocation of inv(16) occured following the Ph chromosome
translocation, therefore this case should have been diagnosed as
de novo Ph(+) AML-M4EO.
The morphological features of CML-BC with
inv(16) in the present study closely
resembled those of AML-M4EO, as described in the WHO classification
(15). To the best of our knowledge,
the coexistence of the BCR-ABL and CBFβ-MYH11 fusion
genes are very rare in CML and de novo AML, with only 34
cases having been identified at present (Tables III and IV) (1,16–34), including the cases described in the
present study. Extramedullary disease occurs in a wide age
spectrum, ranging from 9 to 78 years (17). CBFβ-MYH11 and BCR-ABL
affects males and females, with an increased risk in males
(17). It is suggested that males may
be more likely to exhibit the simultaneous expression of
CBFβ-MYH11 and BCR-ABL compared with females.
 | Table III.Summary of identified patients with
acute myeloid leukemia with BCR-ABL and core binding
factor-β-myosin heavy chain 11. |
Table III.
Summary of identified patients with
acute myeloid leukemia with BCR-ABL and core binding
factor-β-myosin heavy chain 11.
| Author, year | Age | Sex | Blasts, % (bone
marrow) | BCR-ABL status | Cytogenetic
findings | Clinical outcome,
time from diagnosis (months) | (Refs.) |
|---|
| Preudhomme et
al, 1992 | 64 | M | 32 | p190 |
46,XY,inv(16)(p13q22),t(9;22)(q34;q11)[30] | Alive, 12 | (18) |
| Siddiqui et
al, 2002 | 23 | M | 21 | ND |
46,XY,t(9;22)(q34;q11.2)inv(16)(p13q22) | Alive, 36 | (19) |
| Li and Hayhoe,
1988 | 39 | M | >30 | ND |
46,XY(9%)/47,XY.-18.+22,inv(16)(p13q22),del(20)(p12p13)
del(20)(q12q13),t(9;22)(q34;q11),der(16)t(16;?18)
(q24;q21),+mar(91%) | Alive, 38 | (20) |
| Wu et al,
2006 | 44 | M | 44.5 | p190 |
46,XY,t(9;22)(q34.1;q11.2)inv(16)(p13.1q22) | Succumbed to
disease | (16) |
| Miura et al,
1994 | 40 | M | 36 | M-bcr(−) |
46,XY,inv(16)(p13q22)[17]/46,XY,idem,t(9;22)(q34;q11)[3] | Alive, 27 | (21) |
| Secker-Walker et
al, 1992 | 9 | F | ND | p190 |
46,XX,inv(16)(p13q22)[21]/46,XX,t(9;22)(q34;q11)inv(16)
(p13q22)[8]/ 46,XX[10] | Molecular
remission, 1 month | (22) |
| Svaldi et
al, 2001 | 40 | F | NA | p190 |
46,XX,inv(16)[4]/46,idem(9,22)[18] | NA | (23) |
| Tirado et
al, 2010 | 13 | M | 92 | ND |
46,XY,inv(16)(p13.1q22)[2]/46,idem,del(7)
(q22q32)[16]/46,idem,t(9;22;19) | Alive, 10 | (24) |
| Cividin et
al, 2004 | 38 | F | 41 | p190 |
46,XX[22]/46,XX,inv(16)(p13q22)[1]/46,XX,idem,t(9;22)
(q34;q11)[25]/46,XX,t(2;9;22)(q32;q34;q11),inv(16)(p13q22)[23] | Alive, 12 | (1) |
| Roth et al,
2011 | 30 | F | 9 | p190 |
46,XX,t(9;22;17;19)(q34;q11.2;q25;p13.1),inv(16)(p13q22)[19] | Alive, 80 | (25) |
| Roth et al,
2011 | 35 | M | 48 | p190 |
46,XY,der(16)inv(16)(p13q22)del(16)(p11.2p13.1)[2]/46,XY,idem,t(9;22)(q34;q11.2)[18] | Alive, 17 | (25) |
| Bustamante et
al, 2012 | 49 | M | 10-15 | p190 |
46,XX,inv(16)(p13.1q22)[5]/46,idem,t(9;22)(q34;q11.2) | NA | (26) |
 | Table IV.Summary of identified patients
withchronic myeloid leukemia with BCR-ABL and core binding
factor-β-myosin heavy chain 11. |
Table IV.
Summary of identified patients
withchronic myeloid leukemia with BCR-ABL and core binding
factor-β-myosin heavy chain 11.
| Author, year | Age | Sex | Blasts, % (bone
marrow) | BCR-ABL status | Cytogenetic
findings | Clinical outcome,
time from diagnosis | (Refs.) |
|---|
| Wu et al,
2006 | 33 | M | 27 | p210 |
46,XY,t(9;22)(q34.1;q11.2)[18]/46,XY,idem,inv(16)
(p13.1q22)[2] | Succumbed following
allo-BMT | (16) |
| Wu et al,
2006 | 41 | M | 63 | p210 |
46,XY,t(9;22)(q34.1;q11.2),inv(16)(p13.1q22)[20] | Alive, 4 years | (16) |
| Wu et al,
2006 | 62 | F | 52 | p210 |
46,XX,t(9;22)(q34.1;q11.2,inv(16)(p13.1q22)[20] | Succumbed to
disease, 24 months | (16) |
| Wu et al,
2006 | 21 | M | 2 | p210 |
46,XY,t(9;22)(q34.1;q11.2)[17]/46,idem,inv(16)
(p11.2q22)[3] | Alive, 6 years | (16) |
| Wu et al,
2006 | 44 | M | 10 | p210 |
46,XY,t(9;22)(q34.1;q11.2)[90%]46,XY,inv(16)
(p13q22),t(9;22)(q34.1;q11.2)[1]
46,XY,t(6;16)(q22;q22),t(9;22)(q34.1;q11.2)[1] | Succumbed to
disease, 24 months | (16) |
| Merzianu et
al, 2005 | 43 | F | 30 | p210 |
46,XX,t(9;22)(q34;q11.2),inv(16)(p13q22)[20] | Succumbed to
disease, 3 months | (27) |
| Merzianu et
al, 2005 | 61 | F | 20 | p210 |
46,XX,t(9;22)(q34;q11.2)[3]/46,XX,t(9;22)(q34;
q11.2),inv(16)(p13q22)[7]/ 47,XX,+8,t(9;22) (q34;
q11.2)[2]/47,XX,+8,t(9;22) (q34;q11.2), inv(16) (p13q22)[4]/
46,X,add(X)(p22.3),t(9;22)
(q34;q11.2),del(12)(p11.2),inv(16)(p13q22)[1]/
47,XX,t(9;22)(q34;q11.2),inv(16)(p13q22),
+der(2)t(9;22)[1]/46,XX[2] | Succumbed to
disease, 7 months | (27) |
| Merzianu et
al, 2005 | 47 | M | 40 | p210 |
46,XY,t(9;22)(q34;q11),inv(16)(p13q22)[25] | Succumbed to
disease, 1 month | (27) |
| Merzianu et
al, 2005 | 36 | F | 70 | p2102 |
46,XX,t(9;22)(q34;q11.2),inv(16)(p13q22)[20] | Succumbed to
disease, 1 month | (27) |
| Merzianu et
al, 2005 | 48 | M | 20 | p210 |
46,XY,t(9;22)(q34;q11.2),inv(16)(p13q22)[6]/46,XY[14] | Alive, 7
months | (27) |
| Tsuboi et
al, 2002 | 44 | M | 59 | p210 |
47,XY,t(9;22)(q34;q11)inv(16)(p13q22),+der(22)
t(9;22)/48,idem,+8 | Succumbed to
disease, 3 months | (28) |
| Myint et al,
1997 | 29 | M | 90 | p210 |
46,XX,t(9;22)inv(16)(p13;q22) | Succumbed to
disease, 0 months | (29) |
| Evers et al,
1992 | 39 | M | 25 | p210 |
46,XY,-2,-9,-12,-21,+der(9)t(9;?;22),+der(12)
t(12;?2)(q24?p22),inv(16) | Succumbed to
disease, 10 months | (30) |
| Asou et al,
1992 | 51 | M | 10 | p210 |
46,XY,t(9;22)(q34;q11),inv(16)(p13q22) | Succumbed to
disease, 3 months | (31) |
| Enright et
al, 1992 | 78 | M | 19 | p210 |
46,XY,t(9;22)(q34;q11.2),inv(16)(p13q22) | Succumbed to
disease, 3 months | (32) |
| Heim et al,
1992 | 21 | M | 70 | p210 |
45,X,-Y,t(9;22)(q34.1;q11.2),inv(16)(p13.1q22) | Alive, 30
months | (33) |
| Colovic et
al, 1998 | 58 | M | 18 | p210 |
46,XY,t(9;22)(q34.1;q11.2),inv(16)(p13.1q22) | Alive, 10.5
years | (34) |
| Ninomiya et
al, 2011 | 63 | M | 31 | p190 |
46,XX,t(9;22)(q34;q11.2),inv(16)(p13.1q22) | Succumbed to
disease, 7 months following BMT | (17) |
It is well-known that the patients with CML-BC
exhibit a poor clinical outcome. By contrast, patients with Ph(+)
AML with CBFβ-MYH11(+) exhibit a relatively favorable
prognosis, similar to AML with CBFβ-MYH11(+) alone (16,17).
Notably, the outcomes of the patients of the present study were
consistent with these data. As initially diagnosed with CML-AP,
case 1 was first treated with imatinib (400 mg/day orally), but was
shortly changed to chemotherapy with cytarabine (100
mg/m2/day, days 1–7, intravenous drip) and idarubicin (8
mg/m2/day, days 1–3, intravenous drip) for 2 cycles, in
combination with imatinibas the patient rapidly progressed to
CML-BC. Although bone marrow and blood remission was achieved, the
patient quickly relapsed and the treatment course was complicated
by the lung infection. The patient underwent unrelated
donor-allogeneic hematopoietic stem cell transplantation in
November 2011. However, 15 months following transplantation, the
patient succumbed to serious GVHD. Following the standard dose of
induced chemotherapy, case 3 and 4 attained complete remission.
Subsequently, case 3 received autologous-peripheral blood stem cell
transplantation in June 2011 and remained in complete remission for
32 months following diagnosis.
Based on the data of the patient with CML in the
present study, and previous studies (35), it is suggested that
patients with CML carrying and expressing the BCR-ABL and
CBFβ-MYH11 fusion genes appear more likely to rapidly
progress to AP or BC compared with patients with CML without the
co-existence of these two genes. The product of the
CBFβ-MYH11 fusion gene may have served an important role in
the transformation of CML, and the monocytes and eosinophils may be
derived from common leukemic progenitors affected by the product of
the CBFβ-MYH11.
p210BCR-ABL is the hallmark of CML, as
identified in case 1 and case 2. However, it has also been
previously demonstrated that an additional rearrangement to the
classical p190BCR-ABL may be identified in AML (36). It appears that t(9;22)(q34;q11) with a
p210BCR-ABL rearrangement may be a molecular event
occurring not only during the development of CML, but also in AML
following the Ph chromosomal anomaly. From the results of the
previously described patients and the patients with AML with
p210BCR-ABL in the present study, the TKI imatinib used
in maintenance therapy was able to diminish the detectable levels
of BCR-ABL transcripts, suggesting that in such cases,
imatinib may be an effective treatment.
As these patients are so rare, an appropriate
diagnosis and the standard treatment for them are currently not
available. As the leukemic cell populations in the majority of
patients are composed of diverse clones with heterogeneous
phenotypes, the possibility that the p210BCR-ABL
rearrangement and CBFβ-MYH11 may reside in different
subclones in these patients cannot be excluded. These different
subclones would subsequently evolve during the clinical course,
depending on their response to treatment. Therefore, more careful
karyotyping and FISH analysis are required to dissect the
sequential molecular events occurring in a singular clone or in
various subclones in these patients, in order to more precisely
define this subtype of leukemia.
In conclusion, the present report demonstrated that
t(9;22)(q34;q11) with a p210BCR-ABL rearrangement may be
a molecular event occurring not only during the development of CML
but also in AML following a primary specific chromosomal anomaly.
Co-expression of p210BCR-ABL and CBFβ-MYH11
fusion genes may be a molecular event in various types of myeloid
leukemia. The patients with CML carrying and expressing
BCR-ABL and CBFβ-MYH11 fusion genes appear more
likely to progress rapidly to AP or BC compared with patients with
CML without the co-existence of these two genes; The product of the
CBFβ-MYH11 fusion gene may serve an important role in the
transformation of CML.
Acknowledgements
The present study was supported by grants from the
Jiangsu Provincial Special Program of Medical Science (grant no.
BL2012005), National Key Scientific Projects of China (grant no.
2011CB933501), National Scientific Foundation of China (grant no.
81070402), Jiangsu Province's Key Medical Center (grant no.
ZX201102), National Public Health Grand Research Foundation (grant
no. 201202017) and the Priority Academic Program Development of
Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Cividin M, Brizard F, Sorel N, Renaud M,
Guilhot F and Brizard A: p190(BCR-ABL) rearrangement as a secondary
change in a case of acutenmyelo-monocytic leukemia with
inv(16)(p13q22). Leuk Res. 28:97–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delaunay J, Vey N, Leblanc T, Fenaux P,
Rigal-Huguet F, Witz F, Lamy T, Auvrignon A, Blaise D, Pigneux A,
et al: Prognosis of inv(16)/t(16;16) acute myeloid leukemia (AML):
a survey of 110 cases from the French AML Intergroup. Blood.
102:462–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu P, Tarlé SA, Hajra A, Claxton DF,
Marlton P, Freedman M, Siciliano MJ and Collins FS: Fusion between
transcription factor CBF beta/PEBP2 beta and a myosin heavy chain
in acute myeloid leukemia. Science. 261:1041–1044. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundu M and Liu PP: Function of the
inv(16) fusion gene CBFB-MYH11. Current Opinion in Hematology.
8:2012001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swerdlow S, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO classification of
tumors of hematopoietic and lymphoid tissues. 4th. IARC WHO
Classification of Tumours; Lyon: 2008
|
|
6
|
Nowell PC and Hungerford DA: A minute
chromosome in human chronic granulocytic leukemia. Science.
142:14971960.
|
|
7
|
Rowley JD: Letter: a new consistent
chromosomal abnormality in chronic myelogenous leukemia identified
by quinacrine fluorescence and Giemsa staining. Nature.
243:290–293. 1973. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faderl S, Talpaz M, Kantarjian HM and
Estrov Z: Should polymerase chain reaction analysis to detect
minimal residual disease in patients with chronic myelogenous
leukemia be used in clinical decision making? Blood. 93:2755–2759.
1999.PubMed/NCBI
|
|
9
|
World Health Organization Classification
of Tumours: Pathology and genetics of tumours of haematopoietic and
lymphoid tissues. Jaffe ES, Harris NL, Stein H and Vardiman JW:
IARC WHO Classification of Tumours; Lyon: pp. 3522001
|
|
10
|
Keung YK, Beaty M, Powell BL, Molnar I,
Buss D and Pettenati M: Philadelphia chromosome positive
myelodysplastic syndrome and acute myeloid leukemia-retrospective
study and review of literature. Leuk Res. 28:579–586. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung SW, Crane JP and Burgess AC: High
resolution R banding in amniotic fluid cells using the BrdU-Hoechst
33258-Giemsa (RBG) technique. Human Genetics. 69:86–87. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaffer LG, Slovak ML and Campbell LJ:
International Standing Committee on Human Cytogenetic Nomenclature:
ISCN 2009: An international system for human cytogenetic
nomenclature. Shaffer LG, Slovak ML and Campbell LJ: Karger
Publishers; Switzerland: 2009
|
|
14
|
Li X, Li X, Xie W, Hu Y, Li J, Du W, Liu
W, Li H, Chen X, Zhang L, et al: Comprehensive profile of
cytogenetics in 2308 Chinese children and adults with de novo acute
myeloid leukemia. Blood Cells Mol Dis. 49:107–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Popescu R, Dăscălescu A, Dănăilă C,
Ghiorghiu D, Zlei M, Ivanov A, Sireteanu A, Gorduza EV and Azoicăi
D: Co-expression of the CBFβ-MYH11 and BCR-ABL fusion genes in
chronic myeloid leukaemia. Revista Romana De Medicina De Laborator.
23:221–230. 2015. View Article : Google Scholar
|
|
16
|
Wu Y, Slovak ML, Snyder DS and Arber DA:
Coexistence of inversion 16 and the philadelphia chromosome in
acute and chronic myeloid leukemias: Report of six cases and review
of literature. Am J ClinPathol. 125:260–266. 2006.
|
|
17
|
Ninomiya S, Kanemura N, Tsurumi H,
Kasahara S, Hara T, Yamada T and Moriwaki H: Coexistence of
inversion 16 and the Philadelphia chromosome comprising P190
BCR/ABL in chronic myeloid leukemia blast crisis. Int J Hematol.
93:806–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preudhomme C, Lai JL, Plantier I, Demory
JL, Zandecki M and Fenaux P: Cytogenetic and molecular remission in
a case of acute myeloid leukemia (AML) with inversion of chromosome
16 (inv(16)) and Philadelphia chromosome (Ph). Br J Haematol.
82:623–626. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiqui AD, Sheikh ZS, Liu D and Seiter
K: Coexistence of inversion 16 and the Philadelphia chromosome in
patients with acute myelogenous leukemia. Leuk Lymphoma.
43:1137–1140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YS and Hayhoe FG: Ph1 chromosome
positive acute myelomonocytic leukemia with inverted chromosome 16.
Br J Haematol. 69:5761988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miura I, Takatsu H, Yamaguchi A, Hashimoto
K, Nimura T, Nishinari T, Niitsu H and Miura AB: Standard Ph
chromosome t(9;22)(q34;q11), as an additional change in a patient
with acute myelomonocytic leukemia (M4Eo) associated with
inv(16)(p13q22). AM J Haematol. 45:94–96. 1994. View Article : Google Scholar
|
|
22
|
Secker-Walker LM, Morgan GJ, Min T,
Swansbury GJ, Craig J, Yamada T, Desalvo L, Medina JW, Chowdhury V,
Donahue RP, et al: Inversion of chromosome 16 with the Philadelphia
chromosome in acute myelomonocytic leukemia with eosinophilia:
report of two cases. Cancer Genet Cytogenet. 58:29–34. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Svaldi M, Lanthaler A, Venturi R, Coser P
and Mitterer M: Simultaneous occurrence of bcr-abl and inv16 in a
case of M1 acute myeloid leukemia. Leukemia. 15:695–699. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tirado CA, Valdez F, Klesse L, Karandikar
NJ, Uddin N, Arbini A, Fustino N, Collins R, Patel S, Smart RL, et
al: Acute myeloid leukemia with inv(16) with CBFBMYH11, 3′CBFB
deletion, variant t(9;22) with BCR-ABL1 and del(7)(q22q32) in a
pediatric patient: case report and literature review. Cancer Genet
Cytogenet. 200:54–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roth CG, Contis L, Gupta S, Agha M and
Safyan E: De novo acute myeloid leukemia with Philadelphia
chromosome (BCR-ABL)and inversion 16(CBFβ-MYH11): report of two
cases and review of the literature. Leukemia & Lymphoma.
52:531–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bustamante D, Chan KR, Czuchlewski DR and
Saadi AA: Patterns of BCR breakpoints in patients with coexisting
inv(16)(p13.1q22) and t(9;22)(q34;q11.2). Int J Hematol.
95:324–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Merzianu M, Medeiros LJ, Cortes J, Yin C,
Lin P, Jones D, Glassman A, Kantarjian H and Huh Y: inv(16)(p13q22)
in Chronic myelogenous leukemia in blast phase: A
clinicopathologic, cytogenetic and molecular study of five cases.
Am J Clin Pathol. 124:807–814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuboi K, Komatsu H, Miwa H, Iida S, Banno
S, Wakita A, Nitta M and Ueda R: Lymphoid blastic crisis of chronic
myelogenous leukemia with inv(16)(p13;q22). Leuk Res. 26:771–774.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Myint H, Ross FM, Hall JL and Hamblin TJ:
Early transformation to acute myeloblastic leukemia with the
acquisition of inv(16) in Ph positive chronic granulocytic
leukemia. Leuk Res. 21:473–474. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evers JP, Bagg A, Himoe E, Zwiebel JA and
Jacobson RJ: Temporal association of marrow eosinophilia with
inversion of chromosome 16 in recurrent blast crises of chronic
myelogenous leukemia. Cancer Genet Cytogenet. 62:134–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asou N, Sanada I, Tanaka K, Hidaka M,
Suzushima H, Matsuzaki H, Kawano F and Takatsuki K: Inversion of
chromosome 16 and bone marrow eosinophilia in a myelomonocytic
transformation of chronic myeloid leukemia. Cancer Genet Cytogenet.
61:197–200. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Enright H, Weisdorf D, Peterson L, Rydell
RE, Kaplan ME and Arthur DC: Inversion of chromosome 16 and
dysplastic eosinophils in accelerated phase of chronic myeloid
leukemia. Leukemia. 6:381–384. 1992.PubMed/NCBI
|
|
33
|
Heim S, Christensen BE, Fioretos T,
Sørensen AG and Pedersen NT: Acute myelomonocytic leukemia with
inv(16)(p13q22) complicating Philadelphia chromosome positive
chronic myeloid leukemia. Cancer Genet Cytogenet. 59:35–38. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colovic M, Jankovic G, Bila J, Djordjevic
V and Wiernik PH: Inversion of chromosome 16 in accelerated phase
of chronic myeloid leukemia: report of a case and review of the
literature. Med Oncol. 15:199–201. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin CC, Jones D, Cortes JE, Kantarjian H
and Merzianu M: Medeiros LJ and Bueso-Ramos CE: Type B core binding
factor β/smooth muscle myosin heavy chain fusion transcript in
myeloid blast phase of chronic myeloid leukemia: Correlation with
nuclear and cytoplasmic localization of the fusion protein.
Clinical Leukemia. 2:257–260. 2008. View Article : Google Scholar
|
|
36
|
Dai HP, Xue YQ, Wu LL, Pan JL, Gong YL, Wu
YF, Zhang J, Wu DP and Chen SN: p210BCR-ABLas a secondary change in
a patient with acute myelomonocytic leukemia(M4Eo) with inv(16).
Int J Hematol. 96:814–817. 2012. View Article : Google Scholar : PubMed/NCBI
|