Introduction
Nasopharyngeal carcinoma (NPC) may be distinguished
from other types of head and neck cancer based on its unbalanced
endemic distribution, pathology and clinical attributes (1). NPC is an endemic neoplasm in southern
China, with incidence rates of 20–30 per 100,000 being reported in
certain areas of Guangdong province (2,3).
Currently, the prognosis of patients with NPC is evaluated based
primarily on the Union for International Cancer Control/American
Joint Cancer Committee (UICC/AJCC) tumor node metastasis (TNM)
staging system (4). However, there is
a discrepancy between actual clinical outcome and anatomically
based TNM stage, indicating that clinical staging is insufficient
for the precise prediction of prognosis (5). It is therefore critical to investigate
alternative factors in order to accurately predict the outcome for
patients with NPC.
18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography
(PET)/computerized tomography (CT) is a synergistic combination of
functional and anatomical imaging, and serves a growing role in the
diagnosis, staging and prognosis of patients with NPC (6,7).
18F-FDG uptake using maximum standardized uptake values
(SUVmax) has been reported to be correlated with tumor
proliferation rates, metastatic potential, sensitivity to
radiotherapy/chemotherapy and clinical outcomes (8,9).
Furthermore, previous studies have revealed that patients with NPC
who exhibit high SUVmax generally exhibit less favorable outcomes
(10,11). However, due to the heterogeneity of
these patients, the use of SUVmax alone to complement the TNM
classification and refine risk stratification remains inadequate
(12).
It has been suggested that cancer-associated
inflammation represents a hallmark of malignant tumors (13,14).
Infiltrating leukocytes in the tumor microenvironment promote tumor
development, invasion and metastasis (15,16).
Previous studies have identified that complete blood count (CBC)
parameters associated with systemic inflammation, including
leukocytes and their differential counts, are clearly correlated
with prognosis in patients with a variety of neoplasms (17–20),
including NPC (21,22). However, previous studies on NPC
usually utilize limited endpoints, including overall survival (OS)
and progression-free survival (PFS) (21,22). The
prognostic value of peripheral leukocytes and differential counts
of neutrophils and monocytes in patients with NPC has not been
sufficiently evaluated.
Previous studies have reported that a substantial
component of 18F-FDG uptake in tumor tissues is a result
of activity localized to peri-tumoral inflammatory cells (23–25).
However, studies on the association between PET parameters and
cancer-associated inflammation are lacking. The present study
investigated the association between PET SUVmax, peripheral
inflammatory markers and TNM stage. The prognostic power of SUVmax
and inflammation for predicting various survival endpoints in
patients with NPC was also investigated. The combined use of PET
parameters and blood inflammatory markers may improve prognostic
stratification and individually tailored treatment in patients with
NPC.
Materials and methods
Patient selection
The present study included 121 patients who had been
newly diagnosed with NPC between February 2009 and December 2013 at
the Nanfang Hospital of Southern Medical University (Guangzhou,
China). The inclusion criteria were: Biopsy-proven primary NPC; a
pretreatment whole-body 18F-FDG PET/CT scan and CBC
assessment; non-disseminated NPC; and receipt of definitive
radiotherapy at the South Hospital of Southern Medical University.
The exclusion criteria were: Simultaneous second primary tumors;
clinical evidence of infection or other systemic inflammatory
conditions; and incomplete treatment. Approval was granted from the
Southern Medical University Institutional Review Board to proceed
with this retrospective study. Written informed consent was
obtained from all patients prior to enrollment in the present
study.
All patients underwent PET/CT and CBC within 2 weeks
prior to therapy. Other evaluations included a complete patient
history, physical examination, biochemistry profiles and a magnetic
resonance imaging (MRI) scan of the nasopharynx and neck. The
results of the 18F-FDG PET and MRI scans were analyzed
in the present study. Staging was performed based on the 7th
version of the UICC/AJCC TNM staging system (4).
Treatment
All patients were treated with definitive
radiotherapy. The radiation dose ranges to the nasopharynx, lymph
node-positive area and lymph node-negative area were 66–76, 60–70
and 50–60 Gy, respectively. The majority of the patients (79/121)
were treated with intensity modulated radiation therapy, and the
remaining patients were treated with 3-dimensional conformal
radiation therapy. Overall, 16/121 patients (13.2%) were treated
with radiotherapy alone, whilst 105/121 (86.8%) received
platinum-based chemotherapy. Concurrent chemotherapy consisted of
cisplatin (75 mg/m2), cisplatin (75 mg/m2)
with 5-fluorouracil (4.0 g/m2) or paclitaxel liposome
(135 mg/m2) on weeks 1, 4 and 7 of radiotherapy.
Neoadjuvant or adjuvant chemotherapy consisted of cisplatin (75
mg/m2) with 5-fluorouracil (4.0 g/m2) or
paclitaxel liposome (135 mg/m2) every 3 weeks for 2 or 3
cycles.
PET/CT
All examinations were performed using a Discovery LS
PET/CT scanner (GE Healthcare Bio-Sciences, Waukesha, WI, USA).
Patients fasted for at least 6 h prior to the scan, and blood
glucose was monitored immediately prior to the study to ensure
patients had a normal blood glucose level (<7 mmol/l). An
intravenous injection of 232–524 MBq (6.27–14.16 mCi) of 18F-FDG
was administered. Patients then waited for ~60 min prior to the
whole-body PET/CT being performed, in accordance with published
guidelines for tumor imaging with 18F-FDG PET/CT (26).
Image acquisition using whole-body
18F-FDG PET/CT included 6–8 bed positions for each
unenhanced CT and PET scan, covering the entire range from the
vertex of the skull to the mid thigh using head fixation. The PET
images were reconstructed using a standard iterative algorithm
(27) (ordered-subset expectation
maximization), with the CT data used for attenuation correction
(28). The captured PET and CT images
were sent to the Xeleris workstation (GE Healthcare) for
registration and fusion.
All images of fused PET/CT were analyzed
comparatively by 2 experienced nuclear medicine physicians. The
region of interest was drawn along the margin of the lesion for the
measurement of SUVmax normalized to body weight. SUVmax at the
primary tumor (SUVmax-P) and regional lymph nodes (SUVmax-N) was
automatically calculated by the Xeleris workstation software (GE
Healthcare; ADW4.1).
CBC
The CBC test was performed within a 2-week period
prior to therapy, and determined by a fully automated hematology
analyzer Sysmex XE-5000 (Sysmex Corporation, Kobe, Japan).
Leukocyte, neutrophil and monocyte counts were recorded.
Follow-up
Patients were regularly followed up until mortality
or the patients last visit. The patients were scheduled to visit
the clinics every 3 months in the first 3 years, and every 6 months
thereafter. The date of last follow-up was June 2015, and the
median follow-up duration was 37 months (range, 4–74 months).
Physical examination and nasopharyngoscopy were performed on each
visit. Nasopharyngeal and neck MRIs, chest X-rays, and abdominal
sonograms were performed when clinical indications suggested it was
necessary. Locoregional recurrence was established by biopsy or
PET/CT scans. Distant metastases were diagnosed based on clinical
symptoms, physical examination and imaging methods, including chest
plain film or CT scan, bone scan, and abdominal sonography or
PET/CT scan.
Statistical analysis
The primary endpoint was PFS, and the secondary
endpoints were OS, distant metastasis-free survival (DMFS) and
locoregional recurrence-free survival (LRFS). PFS was calculated
from the first day of treatment to the date of relapse at any site,
mortality or the last follow-up appointment. For the remaining
endpoints, the duration was measured from the first day of
treatment to the date of the target event or censored at the last
follow-up date. The Spearman's rank correlation test was applied to
assess the association of PET SUVmax, circulating inflammation
makers and TNM stage. The receiver operating characteristic (ROC)
curve analysis was subjected to the selection of cut-off points to
stratify patients at a high risk of progression. Kaplan-Meier
analysis and log-rank test were used to compare the difference
between survival rates. Multivariate Cox proportional hazards
models were used to identify independent prognostic factors.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Patients' clinical characteristics are shown in
Table I. Results of the follow-up
revealed that out of the 121 patients, 19 had developed distant
metastasis, 7 exhibited locoregional recurrence, 3 showed distant
metastasis and locoregional recurrence and mortality had occurred
in 12 patients. The 3-year PFS, 3-year OS and 3-year DMFS for all
121 patients were 77.9, 88.9 and 82.6%, respectively. A total of 9
patients were unavailable for follow-up.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | N (%) |
|---|
| Age
(years)a | 44 (17–76) |
| Gender |
|
|
Male | 98 (81.0) |
|
Female | 23 (19.0) |
| Histology |
|
| WHO
I | 9 (7.4) |
| WHO
IIA-B | 112 (92.6) |
| Tumor stage |
|
| I | 27 (22.3) |
| II | 18 (14.9) |
|
III | 55 (45.5) |
| IV | 21 (17.4) |
| Node stage |
|
| 0 | 27 (22.3) |
| 1 | 33 (27.3) |
| 2 | 49 (40.5) |
| 3a | 3 (2.5) |
| 3b | 9 (7.4) |
| Clinical
classification |
|
| I | 4 (3.3) |
| II | 18 (14.9) |
|
III | 67 (55.4) |
|
IVa | 20 (16.5) |
|
IVb | 12 (9.9) |
| Treatment
outcome |
|
| Distant
metastasis | 19 (15.7) |
|
Locoregional recurrence | 7 (5.8) |
|
Mortality | 12 (9.9) |
Association among PET parameters,
peripheral inflammatory markers and TNM stage
The association between SUVmax and CBC inflammatory
makers is shown in Table II.
Increased SUVmax-P was associated with increased leukocytes
(r=0.203, P=0.025), neutrophils (r=0.238, P=0.009) and monocytes
(r=0.185, P=0.043). Patients with increased SUVmax-N had
significantly increased monocytes (r=0.206, P=0.024). However, no
significant association of SUVmax-N with leukocytes and neutrophils
was observed.
 | Table II.Association between positron emission
tomography parameters and peripheral inflammatory markers. |
Table II.
Association between positron emission
tomography parameters and peripheral inflammatory markers.
|
| Maximal
standardized uptake values at the primary tumor | Maximal
standardized uptake values at regional lymph nodes |
|---|
|
|
|
|
|---|
| Cell type | r-value | P-value | r-value | P-value |
|---|
| Leukocytes | 0.203 | 0.025 | 0.068 | 0.46 |
| Neutrophils | 0.238 | 0.009 | 0.023 | 0.802 |
| Monocytes | 0.185 | 0.043 | 0.206 | 0.024 |
In addition, the association of TNM stage with PET
parameters and CBC variables is shown in Table III. Higher T stage was significantly
associated with increased SUVmax-P (r=0.526, P<0.001) and
neutrophils (r=0.207, P=0.023). Similarly, N stage was positively
correlated with SUVmax-N (r=0.622, P<0.001) and monocytes
(r=0.222, P=0.014). Compared with patients with early disease,
those with advanced NPC had increased SUVmax-P (r=0.264, P=0.003),
SUVmax-N (r=0.280, P=0.002) and monocytes (r=0.245, P=0.007).
 | Table III.Association between tumor node
metastasis stage, positron emission tomography parameters and
peripheral inflammatory markers. |
Table III.
Association between tumor node
metastasis stage, positron emission tomography parameters and
peripheral inflammatory markers.
|
| Tumor stage | Node stage | Clinical stage |
|---|
|
|
|
|
|
|---|
| Factor | r-value | P-value | r-value | P-value | r-value | P-value |
|---|
| Maximal
standardized uptake values at the primary tumor | 0.526 | <0.001 | 0.070 | 0.447 | 0.264 | 0.003 |
| Maximal
standardized uptake values at regional lymph nodes | 0.061 | 0.503 | 0.622 | <0.001 | 0.280 | 0.002 |
| Leukocytes | 0.146 | 0.110 | 0.045 | 0.621 | 0.128 | 0.160 |
| Neutrophils | 0.207 | 0.023 | 0.012 | 0.894 | 0.143 | 0.118 |
| Monocytes | 0.167 | 0.067 | 0.222 | 0.014 | 0.245 | 0.007 |
Univariate analysis of PET parameters
and peripheral inflammatory markers as prognostic factors for PFS,
OS, DMFS and LRFS
As PFS was the primary endpoint, the present study
took the values of PET parameters and CBC variables showing the
best trade-off between sensitivity and specificity for PFS as the
cut-off values, which were determined by ROC analysis. The cut-off
values of SUVmax-P, SUVmax-N, leukocytes, neutrophils and monocytes
were 12.35, 10.15, 8.19, 5.18 and 0.59, respectively.
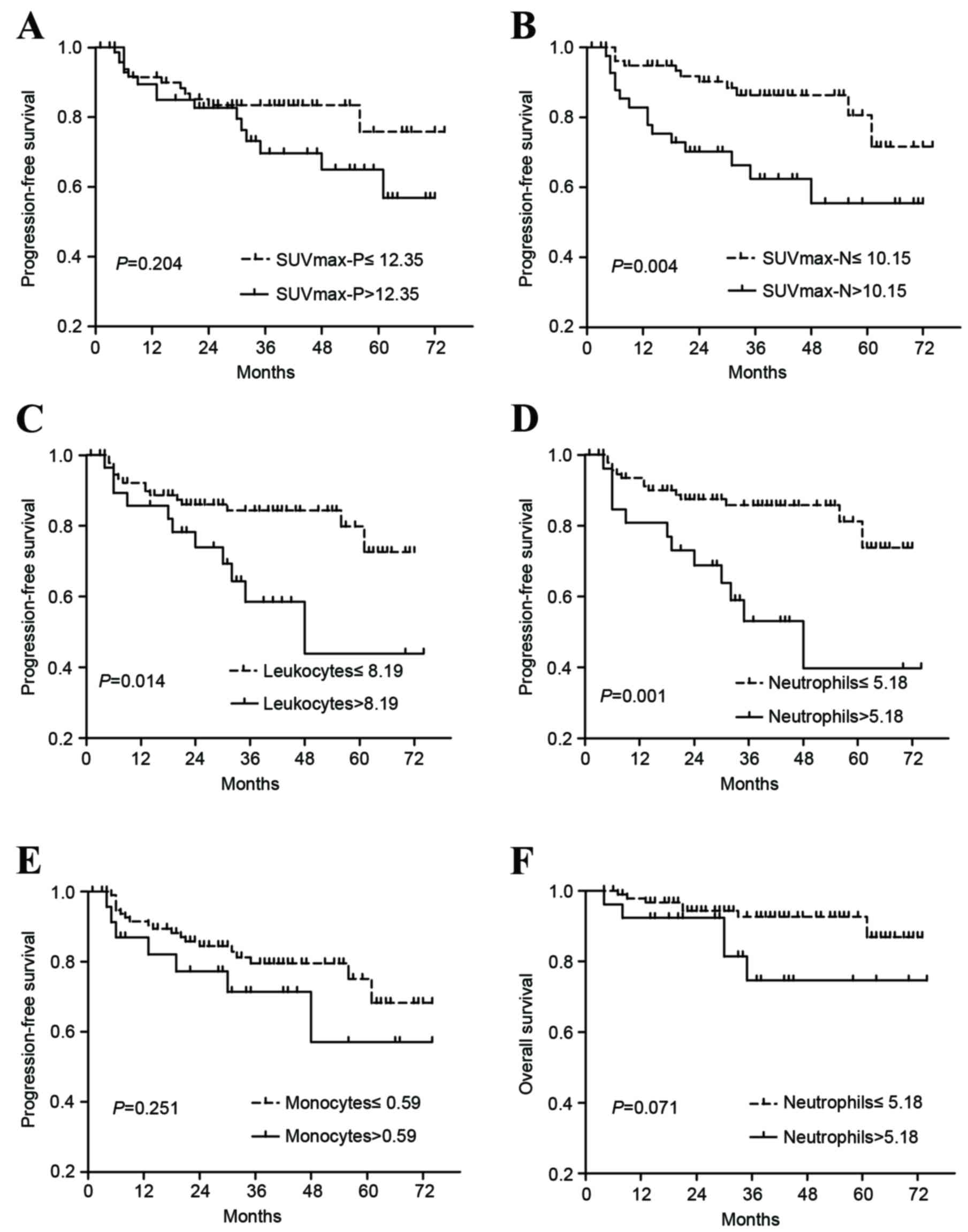
As revealed in Fig. 1,
Kaplan-Meier survival curves for PFS differed significantly when
patients were stratified according to the cut-off points of
SUVmax-N (3-year PFS 86.4 vs. 62.4%, P=0.004), leukocytes (84.3 vs.
58.5%, P=0.014) and neutrophils (85.7 vs. 53.1%, P=0.001).
Significance was not identified between PFS and SUVmax-P or between
PFS and monocytes (P>0.05). In addition, patients with increased
values of neutrophils demonstrated a tendency towards poorer OS
(P=0.071). No significant association was identified between the
remaining parameters and OS (data not shown).
As shown in Fig. 2,
SUVmax-N (P=0.003) and neutrophils (P=0.013) were significantly
associated with DMFS. Compared with patients who had SUVmax-N
≤10.15 (3-year DMFS, 89.2%), those with SUVmax-N >10.15 had a
3-year DMFS of 70.1%. Furthermore, patients with neutrophils ≤5.18
(3-year DMFS, 86.8%) had an improved DMFS compared with those with
neutrophils >5.18 (3-year DMFS, 67.0%). The 3-year LRFS rates
differed significantly when patients were stratified according to
the cut-off points of leukocytes (98.8 vs. 83.8%, P<0.001),
neutrophils (98.8 vs. 82.4%, P<0.001) and monocytes (97.8 vs.
84.7%, P=0.007). Patients with increased SUVmax-P (>12.35)
experienced poorer LRFS compared with patients with SUVmax-P ≤12.35
(3-year LRFS, 98.3 vs. 90.9%), but this difference was not
statistically significant (P=0.096). SUVmax-P did not exhibit a
significant difference for DMFS and SUVmax-N demonstrated no
significant difference in LRFS (data not shown).
Multivariate Cox regression
analysis
To discriminate the independent prognostic
indicators of various outcomes, significant factors identified in
univarite analysis were subsequenly put into a Cox proportional
hazards model. Adjustments were also made for age, gender,
histological type, T stage and N stage. The results of multivariate
survival analysis are shown in Table
IV. Increased values of SUVmax-N (hazard ratio, 2.572, P=0.026)
and neutrophils (hazard ratio, 3.684, P=0.033) retained their
independent prognostic significance for poorer PFS. In addition,
SUVmax-N (hazard ratio, 3.065, P=0.026) and neutrophils (hazard
ratio, 2.888, P=0.032) were independent predictive factors for
DMFS. By contrast, leukocytes, neutrophils and monocytes lost their
prognostic significance for LRFS (data not shown).
 | Table IV.Multivariate Cox regression
analyses. |
Table IV.
Multivariate Cox regression
analyses.
|
| Progression-free
survival | Distant
metastasis-free survival |
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age: >45
years | 2.052
(0.869–4.846) | 0.101 | 1.694
(0.630–4.553) | 0.296 |
| Gender: Female | 0.711
(0.210–2.415) | 0.585 | 0.909
(0.259–3.189) | 0.881 |
| Histology:
WHOIIa-b | 1.442
(0.186–11.193) | 0.727 | 1.170
(0.148–9.243) | 0.882 |
| T stage: T3-4 | 1.159
(0.468–2.872) | 0.749 | 1.055
(0.348–3.199) | 0.925 |
| N stage: N2-3b | 2.661
(1.065–6.649) | 0.036 | 2.478
(0.880–6.983) | 0.086 |
| Maximal
standardized uptake values at regional lymph nodes: >10.15 | 2.572
(1.121–5.898) | 0.026 | 3.065
(1.145–8.201) | 0.026 |
| Leukocytes:
>8.19 | 0.846
(0.261–2.745) | 0.781 | – | – |
| Neutrophils:
>5.18 | 3.684
(1.114–12.181) | 0.033 | 2.888
(1.093–7.634) | 0.032 |
Furthermore, when patients were stratified by
SUVmax-N and neutrophils, it was revealed that patients with lower
levels of SUVmax-N and neutrophils (SUVmax-N ≤10.15 and neutrophils
≤5.18) had significantly improved prognosis in PFS (96.4 vs. 58.5%,
P<0.001; Fig. 3A), OS (95.7 vs.
81.1%, P=0.044; Fig. 3B), DMFS (96.4
vs. 67.0%, P<0.001; Fig. 3C) and
LRFS (100 vs. 90.2%, P=0.036; Fig.
3D) compared with those with SUVmax-N >10.15 or neutrophils
>5.18.
Discussion
NPC treatment remains challenging due to a high
tendency to relapse, particularly in the form of distant
metastasis. Identification of prognostic factors is important for
risk stratification and the potential improvement of treatment
outcomes (29). It is particularly
beneficial if identification prognostic factors are achieved
noninvasively. To the best of our knowledge, this is the first
study to evaluate PET SUVmax and inflammation simultaneously as
prognostic markers in patients with NPC.
Inflammation is closely associated with malignant
tumors. Multiple mechanistic similarities are now recognized
between inflammatory and malignant cells in terms of the underlying
metabolic pathways (30,31). High glucose metabolism and consequent
high 18F-FDG accumulation are not unique phenomena for
malignant cells. Inflammation also demonstrates increased
18F-FDG uptake, which is mainly caused by inflammatory
cells (32,33). Previous studies (23–25) have
reported that the infiltrating inflammatory cells, particularly
macrophages, serve an important role in 18F-FDG uptake
in tumor tissue. However, to the best of our knowledge there have
been no studies addressing the association between PET SUVmax and
circulating inflammatory cells in cancer. The present study
initially identified that SUVmax-P had a weak association with
peripheral leukocytes, neutrophils and monocytes. SUVmax-N was
significantly associated with monocytes. Patients with active
infections were excluded from the present study. Blood leukocytes,
neutrophils and monocytes may partially reflect the infiltration of
inflammatory cells around tumor tissue. This may partially explain
the present observation that SUVmax may be associated with blood
inflammatory cells.
In agreement with previous studies (10,34), the
present study identified that increased SUVmax-N was significantly
associated with a higher N stage. SUVmax-P was positively
correlated with T stage. Similar to the T and N classification in
the TNM stage system, metabolic parameters of the primary tumor and
regional lymph nodes appear to represent different prognostic
properties. In the present study, SUVmax-N was an independent
prognostic marker for PFS and DMFS, but demonstrated no significant
difference in locoregional control. Patients with increased
SUVmax-P had reduced LRFS, but this difference was not
statistically significant, possibly due to the small sample size
for locoregional recurrence. Compared with SUVmax-P, SUVmax-N had
reduced predictive value for LRFS, but increased value for DMFS. In
line with the present results, Chan et al (12) reported that SUVmax-N appeared to be
more powerful in predicting distant failure than SUVmax-P. This
phenomenon has also been noted in previous studies of non-NPC head
and neck cancer (35–37).
It is generally accepted that inflammation may
contribute to the initiation and progression of cancer. Systemic
inflammation may also protect circulating metastatic cancer cells
from natural killer cell-mediated killing, thereby overcoming
immunosurveillance (38). Neutrophils
in the peripheral or tumor microenvironment have been demonstrated
to produce proangiogenic factors, including vascular endothelial
growth factor, to stimulate tumor development (39,40). A
number of studies have shown associations between differential
counts of leukocytes and the prognosis in various types of cancer,
including melanoma (17), advanced
non-small cell lung (18) and gastric
cancer (19). A limited number of
studies exist on the prognostic role of peripheral leukocytes and
differential counts of neutrophils and monocytes in NPC. He et
al (21) and Sun et al
(22) investigated the association of
neutrophils and lymphocytes with PFS and OS in NPC; however, the
authors did not evaluate the endpoints for distant metastasis and
locoregional recurrence. The prognostic roles of leukocyte and
monocyte count were not addressed in above two studies. The present
study assessed the prognostic power of leukocyte, neutrophil and
monocyte counts for predicting PFS, OS, DMFS and LRFS in patients
with NPC. It was revealed that neutrophil count (≤5.18) was a
negative prognostic marker for PFS, DMFS and LRFS, and demonstrated
a trend of poorer OS. Following multivariate adjustment, neutrophil
count maintained independent prognostic significance for PFS and
DMFS. Although leukocyte count was a significant prognostic factor
for PFS and LRFS and monocyte count was significantly associated
with LRFS, they both lost independent prognostic significance in
subsequent multivariate analysis. Therefore, compared with
leukocytes and monocytes, neutrophils may have an increased
predictive value for NPC.
Although the majority of previous studies have
focused on the prediction of poor prognosis, with the goal of
identifying patients who may benefit from adjuvant chemotherapy, it
is equally important that prognostic classifiers can identify
patients with good prognosis who may not require further radical
treatment, as the benefit of adjuvant chemotherapy in patients with
NPC remains unclear (41,42). The present data demonstrated that
prognostic stratification was greatly improved following the
combination of SUVmax-N and neutrophils in NPC. The combined
assessment provides a novel tool for reaching optimal clinical
decisions, enabling clinicians to identify low-risk patients
(SUVmax-N ≤10.15 and neutrophils ≤5.18) for mild treatment without
unnecessary radical therapy. By contrast, patients with an
increased level of SUVmax-N or neutrophils, may benefit from
higher-dose radiation, adjuvant therapy or molecular-target
therapy. However, the mechanism by which the combined assessment
improves the prognostic stratification of patients with NPC is
unclear. As is commonly reported, reprogramming energy metabolism
and inflammation are the emerging hallmarks of cancer (14). Circulating inflammatory cells may
represent a high degree of inflammatory cell infiltration in the
tumor microenvironment, which may enhance tumor progression and
increase glucose uptake. Markedly increased uptake of glucose can
be documented readily by noninvasively visualizing glucose uptake
using 18F-FDG PET. The inflammation-metabolism-cancer
connection may account for the present results, which observed that
PET SUVmax and inflammation had a coordinated value for NPC
prognosis.
The principal limitations of the present study are
its retrospective nature, small sample-size, insufficient follow-up
for certain patients and the inclusion of the patients from a
single institution. A larger, multicentre prospective design is
required for further validation.
In summary, PET SUVmax may be significantly
associated with TNM stage and cancer-associated inflammation. The
present study has screened out SUVmax-N and neutrophils as
independent prognostic indicators for PFS and DMFS. These findings
provide additional evidence supporting the use of
18F-FDG PET and CBC in the clinical management of
patients with NPC. Further studies are required to clarify whether
the combined assessment of 18F-FDG PET functional
parameters and peripheral inflammatory markers can improve patient
outcomes through an optimized biomarker-guided and imaging-guided
treatment strategy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172586).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simons MJ: The origin of genetic risk for
nasopharyngeal carcinoma: A commentary on: Is nasopharyngeal cancer
really a ‘Cantonese cancer’? Chin J Cancer. 29:527–537. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Mao YP, Xie FY, Liu LZ, Sun Y,
Tian L, Tang LL, Lin AH, Li L and Ma J: The seventh edition of the
UICC/AJCC staging system for nasopharyngeal carcinoma is
prognostically useful for patients treated with intensity-modulated
radiotherapy from an endemic area in China. Radiother Oncol.
104:331–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ludwig JA and Weinstein JN: Biomarkers in
cancer staging, prognosis and treatment selection. Nat Rev Cancer.
5:845–856. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weissleder R: Molecular imaging in cancer.
Science. 312:1168–1171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohandas A, Marcus C, Kang H, Truong MT
and Subramaniam RM: FDG PET/CT in the management of nasopharyngeal
carcinoma. AJR Am J Roentgenol. 203:W146–W157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura H, Hirata T, Kitamura H and
Nishikawa J: Correlation of the standardized uptake value in
FDG-PET with the expression level of cell-cycle-related molecular
biomarkers in resected non-small cell lung cancers. Ann Thorac
Cardiovasc Surg. 15:304–310. 2009.PubMed/NCBI
|
|
9
|
Imamura Y, Azuma K, Kurata S, Hattori S,
Sasada T, Kinoshita T, Okamoto M, Kawayama T, Kaida H, Ishibashi M
and Aizawa H: Prognostic value of SUVmax measurements obtained by
FDG-PET in patients with non-small cell lung cancer receiving
chemotherapy. Lung Cancer. 71:49–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao W, Xu A, Han F, Lin X, Lu L, Shen G,
Huang S, Fan W, Deng X and Zhao C: Positron emission
tomography-computed tomography before treatment is highly
prognostic of distant metastasis in nasopharyngeal carcinoma
patients after intensity-modulated radiotherapy treatment: A
prospective study with long-term follow-up. Oral Oncol. 51:363–369.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hung TM, Wang HM, Kang CJ, Huang SF, Liao
CT, Chan SC, Ng SH, Chen IH, Lin CY, Fan KH and Chang JT:
Pretreatment (18)F-FDG PET standardized uptake value of primary
tumor and neck lymph nodes as a predictor of distant metastasis for
patients with nasopharyngeal carcinoma. Oral Oncol. 49:169–174.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan SC, Chang JT, Lin CY, Ng SH, Wang HM,
Liao CT, Chang CJ, Lin SY and Yen TC: Clinical utility of 18F-FDG
PET parameters in patients with advanced nasopharyngeal carcinoma:
Predictive role for different survival endpoints and impact on
prognostic stratification. Nucl Med Commun. 32:989–996. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore MM, Chua W, Charles KA and Clarke
SJ: Inflammation and cancer: Causes and consequences. Clin
Pharmacol Ther. 87:504–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmidt H, Suciu S, Punt CJ, Gore M, Kruit
W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U,
et al: Pretreatment levels of peripheral neutrophils and leukocytes
as independent predictors of overall survival in patients with
American Joint Committee on Cancer Stage IV Melanoma: Results of
the EORTC 18951 Biochemotherapy Trial. J Clin Oncol. 25:1562–1569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
multinational trial organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology-Basel. 73:215–220. 2007.
View Article : Google Scholar
|
|
20
|
Shankar A, Wang JJ, Rochtchina E, Yu MC,
Kefford R and Mitchell P: Association between circulating white
blood cell count and cancer mortality: A population-based cohort
study. Arch Intern Med. 166:188–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He JR, Shen GP, Ren ZF, Qin H, Cui C,
Zhang Y, Zeng YX and Jia WH: Pretreatment levels of peripheral
neutrophils and lymphocytes as independent prognostic factors in
patients with nasopharyngeal carcinoma. Head Neck. 34:1769–1776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun W, Zhang L, Luo M and Hu G, Mei Q, Liu
D, Long G and Hu G: Pretreatment hematologic markers as prognostic
factors in patients with nasopharyngeal carcinoma:
Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head
Neck. 38 Suppl 1:E1332–E1340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kubota R, Yamada S, Kubota K, Ishiwata K,
Tamahashi N and Ido T: Intratumoral distribution of
fluorine-18-fluorodeoxyglucose in vivo: High accumulation in
macrophages and granulation tissues studied by
microautoradiography. J Nucl Med. 33:1972–1980. 1992.PubMed/NCBI
|
|
24
|
Brown RS, Leung JY, Fisher SJ, Frey KA,
Ethier SP and Wahl RL: Intratumoral distribution of tritiated
fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells
important? J Nucl Med. 36:1854–1861. 1995.PubMed/NCBI
|
|
25
|
Cottone L, Valtorta S, Capobianco A,
Belloli S, Rovere-Querini P, Fazio F, Manfredi AA and Moresco RM:
Evaluation of the role of tumor-associated macrophages in an
experimental model of peritoneal carcinomatosis using (18)F-FDG
PET. J Nucl Med. 52:1770–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delbeke D, Coleman RE, Guiberteau MJ,
Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA,
Hubner K, et al: Procedure guideline for tumor imaging with 18F-FDG
PET/CT 1.0. J Nucl Med. 47:885–895. 2006.PubMed/NCBI
|
|
27
|
Ren YY, Li YC, Wu HB, Wang QS, Han YJ,
Zhou WL and Li HS: Whole-body 18F-FDG PET/CT for M staging in the
patient with newly diagnosed nasopharyngeal carcinoma: Who needs?
Eur J Radiol. 89:200–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Pierce LA II, Alessio AM and
Kinahan PE: The impact of respiratory motion on tumor
quantification and delineation in static PET/CT imaging. Phys Med
Biol. 54:7345–7362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chua ML, Wee JT, Hui EP and Chan AT:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Neill LA and Hardie DG: Metabolism of
inflammation limited by AMPK and pseudo-starvation. Nature.
493:346–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mochizuki T, Tsukamoto E, Kuge Y, Kanegae
K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M and Tamaki N: FDG
uptake and glucose transporter subtype expressions in experimental
tumor and inflammation models. J Nucl Med. 42:1551–1555.
2001.PubMed/NCBI
|
|
32
|
Wu C, Li F, Niu G and Chen X: PET imaging
of inflammation biomarkers. Theranostics. 3:448–466. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vaidyanathan S, Patel CN, Scarsbrook AF
and Chowdhury FU: FDG PET/CT in infection and inflammation-current
and emerging clinical applications. Clin Radiol. 70:787–800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan WK, Kwong DL, Yeung DW, Huang B and
Khong PL: Prognostic impact of standardized uptake value of F-18
FDG PET/CT in nasopharyngeal carcinoma. Clin Nucl Med.
36:1007–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inokuchi H, Kodaira T, Tachibana H,
Nakamura T, Tomita N, Nakahara R, Takada A, Mizoguchi N, Tamaki T
and Fuwa N: Clinical usefulness of [18F] fluoro-2-deoxy-D-glucose
uptake in 178 head-and-neck cancer patients with nodal metastasis
treated with definitive chemoradiotherapy: Consideration of its
prognostic value and ability to provide guidance for optimal
selection of patients for planned neck dissection. Int J Radiat
Oncol Biol Phys. 79:747–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao CT, Wang HM, Chang JT, Lin CY, Ng SH,
Huang SF, Chen IH, Hsueh C, Lee LY, Lin CH, et al: Influence of
pathological nodal status and maximal standardized uptake value of
the primary tumor and regional lymph nodes on treatment plans in
patients with advanced oral cavity squamous cell carcinoma. Int J
Radiat Oncol Biol Phys. 77:421–429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kubicek GJ, Champ C, Fogh S, Wang F, Reddy
E, Intenzo C, Dusing RW and Machtay M: FDG-PET staging and
importance of lymph node SUV in head and neck cancer. Head Neck
Oncol. 2:192010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fondevila C, Metges JP, Fuster J, Grau JJ,
Palacín A, Castells A, Volant A and Pera M: p53 and VEGF expression
are independent predictors of tumour recurrence and survival
following curative resection of gastric cancer. Br J Cancer.
90:206–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshizaki T, Kondo S, Murono S, Endo K,
Tsuji A, Nakanishi Y, Nakanishi S, Sugimoto H, Hatano M, Ueno T and
Wakisaka N: Progress and controversy for the role of chemotherapy
in nasopharyngeal carcinoma. Jpn J Clin Oncol. 45:244–247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015. View Article : Google Scholar : PubMed/NCBI
|