Introduction
Breast cancer is a common cancer in women. Its
incidence has been increasing and the age of onset has been
decreasing in previous years (1).
Surgical tumor resection is frequently used to treat breast cancer,
but recurrences are common and the trauma associated with the
procedure is substantial. In addition, traditional radiotherapy can
lead to adverse reactions. It is under this context that Chinese
medicine and targeted gene therapies have become the focus of
present studies aimed at improving the treatment against breast
cancer. The main active ingredients of Stephania tetrandra
include tetrandrine and fangchinoline (FAN) (2). Studies have confirmed that FAN can
inhibit breast cancer cell proliferation; it does so by inhibiting
the proliferation of breast cancer cells, inducing cell apoptosis
mediated by mitochondrial pathways and reducing the level of
phosphorylated AKT (3,4). However, other antitumor effects of FAN
on breast cancer cells and their possible mechanisms have not been
reported. The collagenases MMP-2 and −9 are usually involved in the
metastasis of breast cancer by degrading the extracellular matrix
(ECM). High expression levels of activated NF-κβ are also
associated to metastatic breast cancer cells. This study aimed at
investigating the possible effects of FAN on the migration of
breast cancer cells and on their expression of MMP-2 and −9 and the
activation of NF-κβ, so as to provide a basis for further
clarification of the effects of FAN.
Materials and methods
Cell culture
Human breast cancer cell line MDA-MB-231 from the
Cell Bank of the Chinese Academy of Sciences (Beijing, China) was
cultured in L-15 medium supplemented with 100 U/ml penicillin G and
100 µg/ml streptomycin and containing 10% fetal bovine serum (all
from Gibco Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), the incubator was set at 37°C with 5%
CO2.
FAN (CAS: 33889-68-8, HPLC ≥98%, molecular formula:
C37H40N2O6, molecular weight: 608.20 mg) was purchased from
Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China).
It was stored at 4°C or dissolved in dimethyl sulfoxide (DMSO) to
make a stock solution; the DMSO concentration used was lower than
0.1% to avoid deleterious effects on cell growth, the same final
concentration of DMSO was also used for treatment in the control
group cells.
Methyl thiazolyl tetrazolium (MTT)
assay to detect cell proliferation
MDA-MB-231 cells were inoculated into 96-well plates
with 4–5×103 cells per well. After incubation for 24 h
different concentrations of FAN (0.25, 12.5, 25, 50, and 100 µg/ml)
were added to different wells. Next, 10 µl of a 5 mg/ml MTT
solution (Sigma-Aldrich; Merck & Co., Inc., Whitehouse Station,
NJ, USA) was added at 24 and 48 h after adding FAN. After
incubation for another 4 h (at 37°C), the supernatant was removed
and 150 µl DMSO (Sigma-Aldrich; Merck & Co., Inc.) were added
into each well, followed by incubation for 15 min. The OD value of
each well was measured at 490 nm using a microplate reader (Puyun
Biotechnology Co., Ltd., Jiangsu, China). The percentages of the OD
values of the samples to a blank control were recorded.
Hoechst 33342 fluorescent staining to
observe morphological changes of apoptotic cells
MDA-MB-231 cells were inoculated onto 6-well plates
with 5×105 cells per well. After overnight incubation,
DMSO for the blank control and 20 µg/ml FAN were added, followed by
incubation for 16 h. After washing with phosphate-buffered saline
(PBS), 10 mg/ml Hoechst 33342 staining solution was added and
incubated at 4°C for 20 min. After washing with PBS, the nuclear
morphology was observed under a fluorescent inverted microscope
(Shanghai Cai Kang Optical Instrument Factory, Shanghai,
China).
Hoechst-PI double-staining
MDA-MB-231 cells were inoculated onto 6-well culture
plates with 5×105 cells per well. DMSO for a blank
control and 20 µg/ml FAN were added and incubated for 12, 24 or 48
h. After washing with PBS 3 times, 1 ml Hoechst 333428 staining
solution was added and incubated at 20°C for 15 min, after washing
with PBS for 2 times, 50 mg/ml propidium iodide (PI) dye
(Sigma-Aldrich; Merck & Co., Inc.) were added and incubated at
20°C in the dark for 30 min. Flow cytometry was used to detect the
fluorescence intensity, and to calculate cell apoptosis rate.
Cell migration assay
A marker pen was used to draw parallel lines on the
reverse side of the 6-well culture plate so that each well was
crossed through by at least 5 lines. The MDA-MB-231 cells were
collected at logarithmic growth phase and inoculated with 2 ml of
medium with 4×106 cells per well. After incubation in a
sterile incubator for 48 h, a 200 µl tip was used to scratch the
cells along the parallel lines. The cells were washed 3 times with
PBS to remove all dislodged cells. Serum-free medium and 0, 5, 10
and 20 µg/ml FAN were added to different wells and photos were
taken 0, 6, 12 and 24 h later. The ImageJ software (version X;
Media Cybernetics, Silver Springs, MD, USA) was used to process the
images.
The detection of MMP-2 and −9
content
MDA-MB-231 cells were seeded on 6-well plates with
5×105 cells per well. After overnight incubation,
different concentrations of FAN (1, 50 and 100 µg/ml) were added to
different wells and the plates were further incubated for 24 and 48
h. After incubation, the supernatant was collected. The supernatant
was centrifuged and the levels of MMP-2 and −9 were measured by
enzyme-linked immunosorbent assay (ELISA) according to the
instructions on the kit (Dongge Biotechnology, Beijing, China). The
OD values were measured at 450 nm using a microplate reader to
calculate the concentration of MMP-2 and −9.
Western blot analysis
MDA-MB-231 cells were incubated with different
concentrations of FAN (0, 5, 10 and 20 µg/ml) for 24 h. After that,
the cells were washed thrice with pre-cooled PBS. Lysis RIPA buffer
was the added and incubated for 30 min followed by centrifugation
for 10 min at 4°C to collect the supernatant, which contained the
total soluble protein. The nuclear and cytoplasmic proteins were
extracted according to the instructions on the kit (Beijing Huaxia
Yuanyang Technology Co., Ltd., Beijing China). Protein
concentrations were measured by the Bradford method; 30 µg of each
protein sample were subjected to 8–12% polyacrylamide gel
electrophoresis, followed by tranfer to a nitrocellulose membrane.
The membrane was then blocked with 5% skim milk for 1 h followed by
incubation with primary rabbit polyclonal NF-κβ antibody (dilution,
1:500; cat. no. ab16502) and rabbit monoclonal Iκβ antibody
(dilution, 1:500; cat. no. ab32518) overnight at 4°C. After
washing, secondary goat anti-rabbit (HRP) IgG antibody (dilution,
1:2,000; cat. no. ab6721) was added and the signals were detected.
At least two batches of similar results were obtained for each
experiment and GAPDH was used as the endogenous control to
normalize quantities. All antibodies were purchased from Abcam
(Cambridge, MA, USA).
Statistical analysis
Data were processed using SPSS 19.0 (IBM Corp.,
Armonk, NY, USA) software. Measurement data are expressed as mean ±
standard deviation and processed by t-test; count data are
expressed as percentages and processed using χ2 test. A
P<0.05 was considered to indicate statistically significant
difference.
Results
The cell proliferation assays showed that, compared
with the control group, the percentages of viable cells in the
groups treated with different concentration of FAN (6.25, 12.5, 25,
50 and 100 µg/ml) for different treatment periods (24 and 48 h)
were significantly reduced in a time- and concentration-dependent
manner (Table I). The IC50 at 24 and
48 h was 14.26±1.54 µg/ml and 10.48±1.38 µg/ml, respectively.
 | Table I.Effect of FAN on the proliferation of
MDA-MB-231 cells. |
Table I.
Effect of FAN on the proliferation of
MDA-MB-231 cells.
|
|
| Cell viability |
|---|
|
|
|
|
|---|
| Groups | FAN concentrations
(µg/ml) | 24 h | 48 h |
|---|
| Control |
0 | 100.00±1.18 | 100.00±1.38 |
| FAN | 6.25 | 97.40±0.69 |
54.34±4.64a |
|
| 12.5 |
55.67±4.70a |
40.60±6.42a |
|
| 25 |
26.57±5.06a |
33.43±2.61a |
|
| 50 |
26.40±1.50a |
23.83±1.45a |
|
| 100 |
24.57±3.19a |
21.70±1.30a |
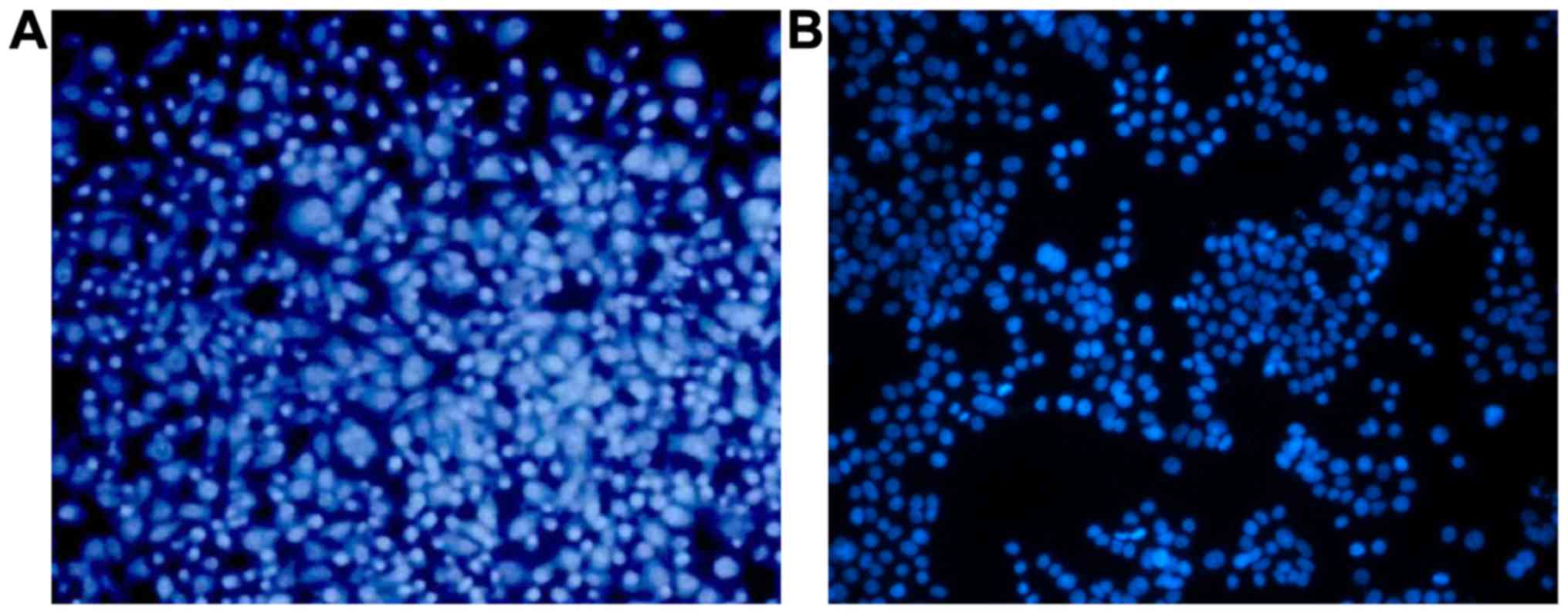
The results of MDA-MB-231 cell staining with Hoechst
33342 showed that cells in the control group were homogeneously
stained (Fig. 1A), while the cells
treated with 20 µg/ml FAN for 16 h exhibited nuclear shrinkage and
fragmentation (Fig. 1B), indicating
that FAN treatment increased the apoptosis of MDA-MB-231 cells.
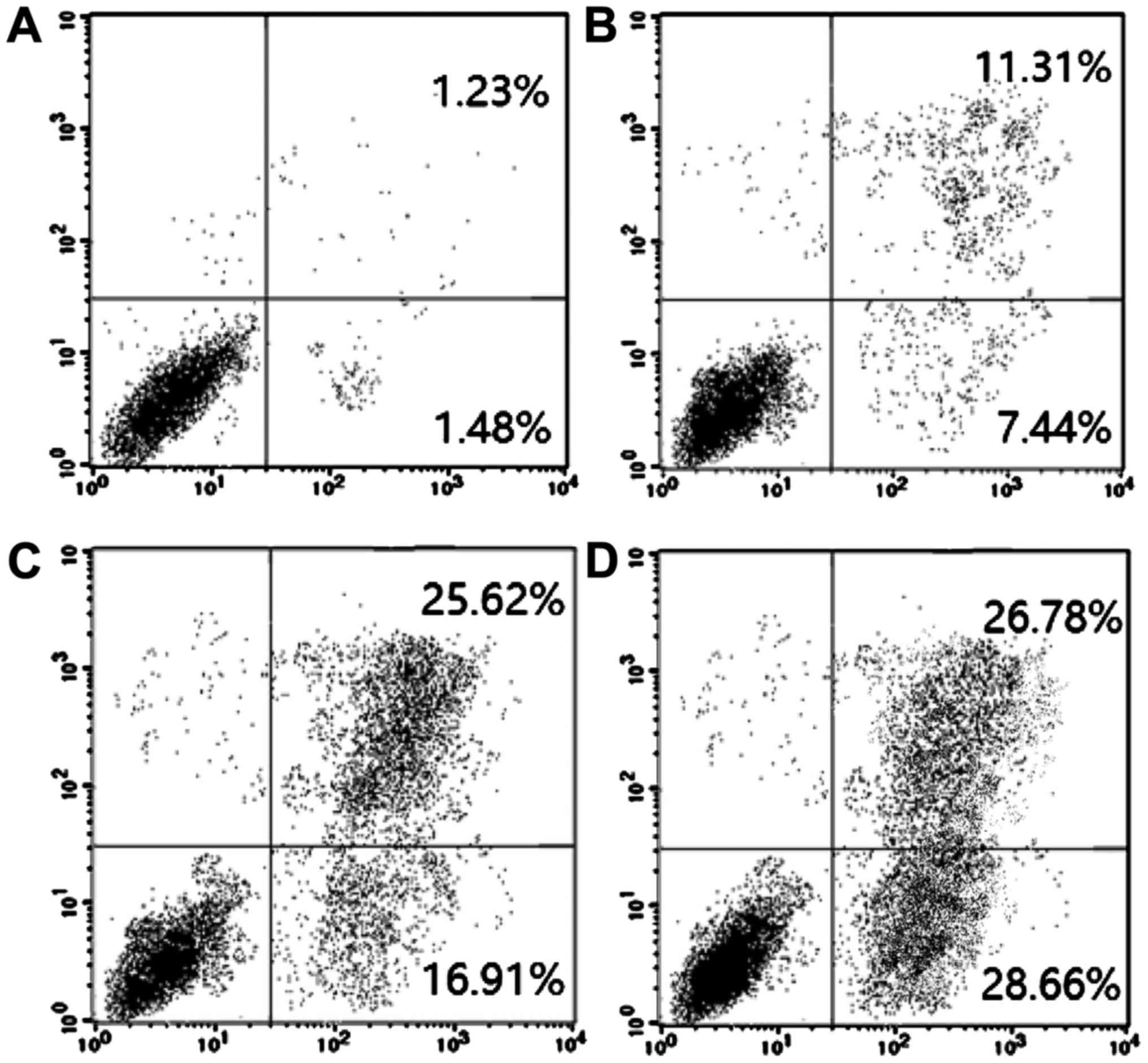
Furthermore, flow cytometry results showed that the
apoptosis rate was increased with time. The apoptosis rates of
MDA-MB-231 cells were 1.39, 7.16, 16.38 and 28.04% after 20 µg/ml
FAN treatment for 0, 12, 24 and 48 h, respectively (Fig. 2).
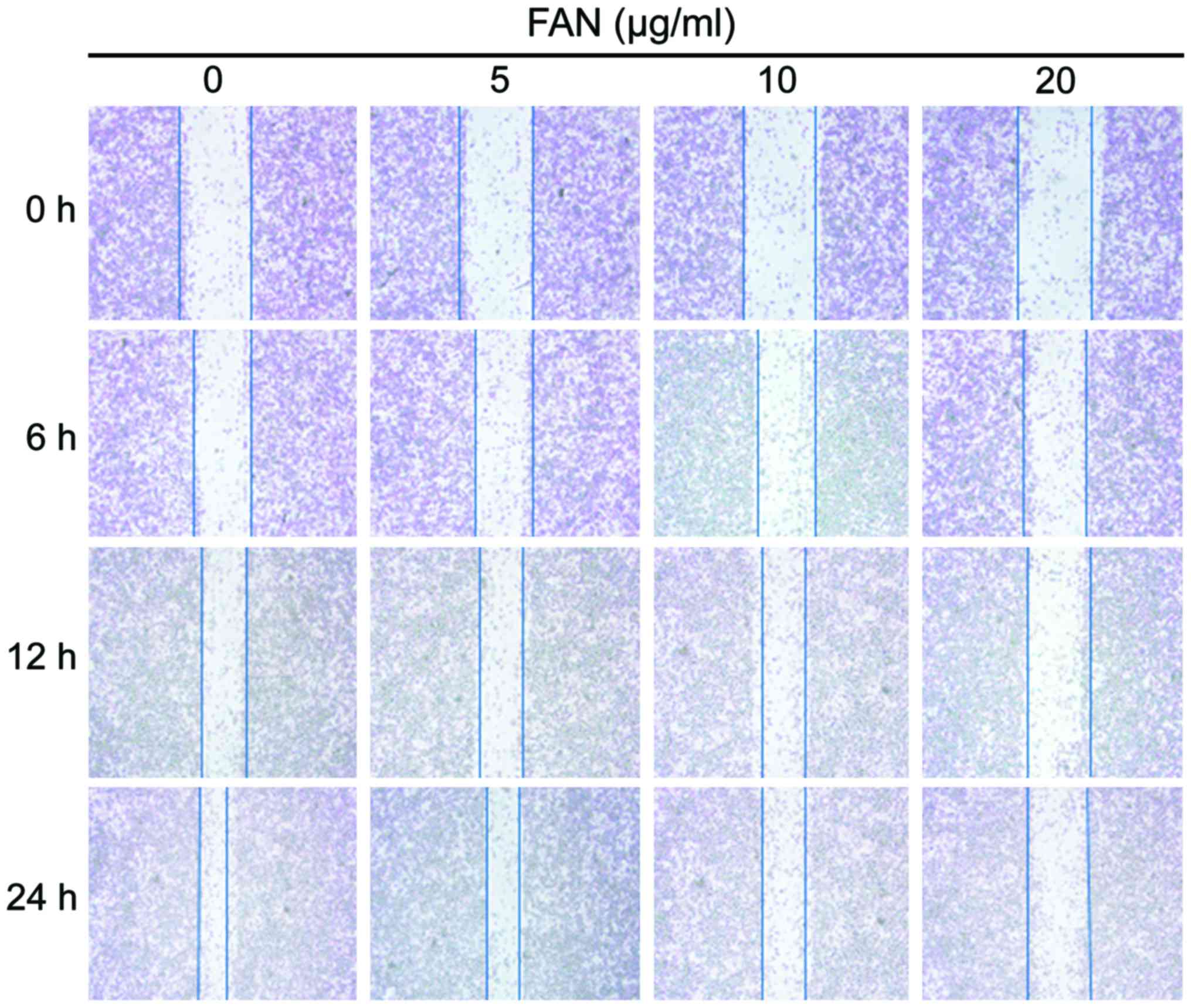
Effects of FAN on cell migration by
wound healing assay
The results of wound healing assays showed that the
inhibition of FAN on cell migration was both dose- and
time-dependent. The migration of the MDA-MB-231 cells treated with
different concentrations of FAN (0, 5, 10 and 20 µg/ml) for 24 h
was significantly inhibited. Increasing FAN concentrations led to
progressively less migration (Fig.
3).
ELISA detection of MMP-2 and −9
The effect of FAN on the expression of MMP-9 and −2
was detected at 24 and 48 h after FAN treatment using ELISA kits.
The results showed that the expression of MMP-9 and −2 decreased
significantly with increasing FAN concentrations (Table II).
 | Table II.The effect of FAN on the expression of
MMP-9 and −2 in MDA-MB-231 cells quantified by ELISA. |
Table II.
The effect of FAN on the expression of
MMP-9 and −2 in MDA-MB-231 cells quantified by ELISA.
|
|
| MMP-2 (pg/ml) | MMP-9 (pg/ml) |
|---|
|
|
|
|
|
|---|
| Groups | Concentration
(µg/ml) | 24 h | 48 h | 24 h | 48 h |
|---|
| Control |
0 | 318.76±12.58 | 317.89±12.73 | 1022.73±71.53 | 1023.16±72.53 |
| FAN |
1 |
297.48±9.19a |
284.34±9.62a |
967.40±70.39a |
951.37±64.54a |
|
| 50 |
275.63±8.73a |
262.43±7.49a |
945.67±64.57a | 938.6
±66.72a |
|
| 100 |
266.57±7.16a |
233.69±7.73a |
926.57±65.86a |
903.42±59.31a |
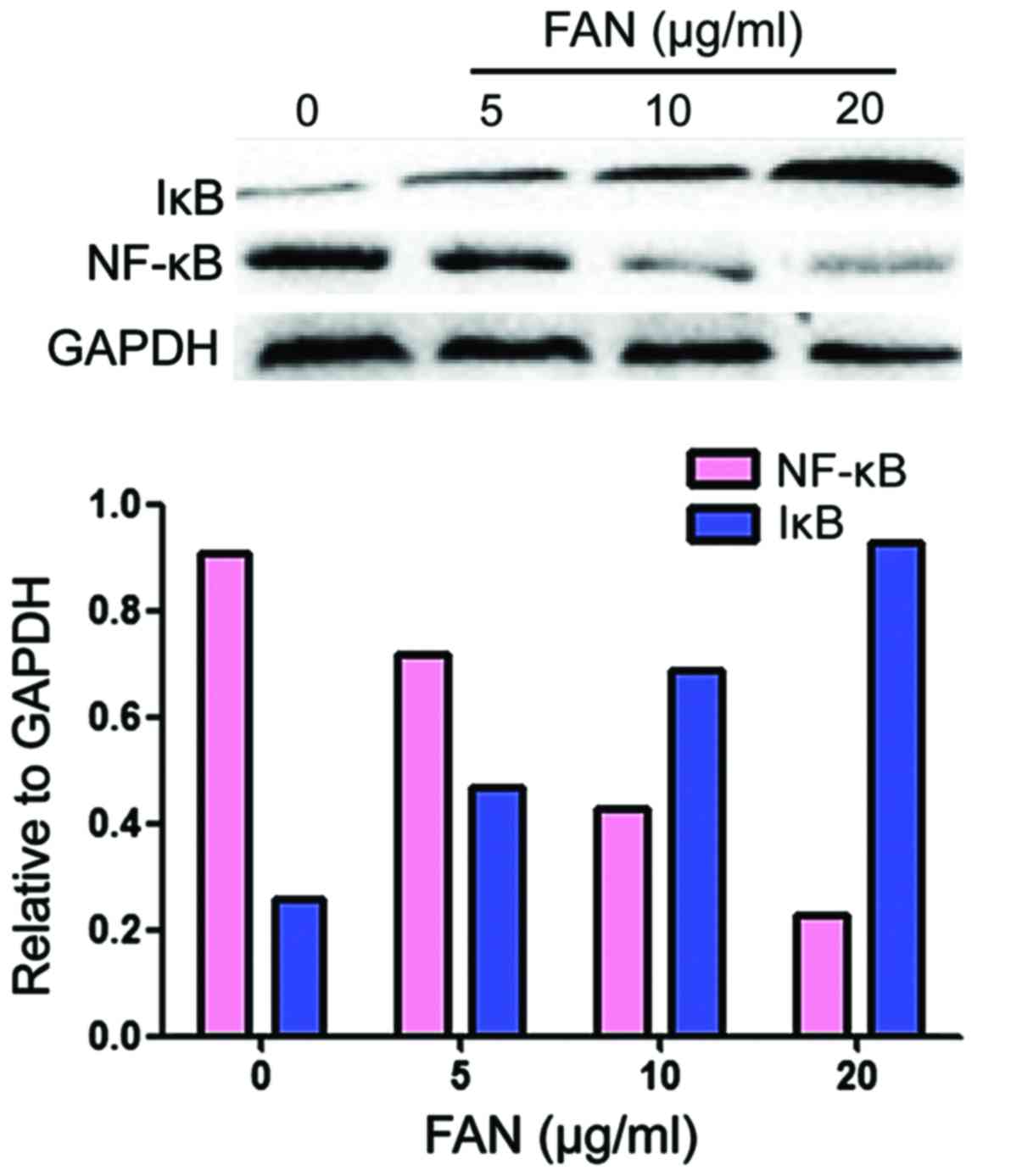
Western blot analysis to detect
expression of NF-κβ and Iκβ in MDA-MB-231 cells
The western blot analysis showed that, after
treatment with different concentrations of FAN for 24 h, the
expression levels of NF-κβ protein were reduced and the expression
levels of Iκβ were increased in a concentration-dependent manner
(Fig. 4).
Discussion
A variety of contributing factors including quality
of sleep, history of smoking and drinking, age at menarche,
gravidity, age at menopause, waist and hip ratio and the history of
breast disease can lead to the occurrence of breast cancer and
breast cancer is associated with abnormal hormonal changes
(1). The incidence of breast cancer
is relatively high in two age groups: 45–50 and 60–65 years. The
breast cancer in the first group is associated with reduced ovarian
function and increased activity of the anterior pituitary,
eventually leading the release of excessive estrogen from the
adrenal cortex. While the occurrence of breast cancer in the latter
group is associated with excessive androgen released by the adrenal
cortex (1). Early diagnosis and
treatment of breast cancer can significantly extend the survival of
breast cancer patients and improve their quality of life.
FAN, which is a type of dibenzyl-isoquinoline
alkaloid extracted from the roots of the Stephania
tetrandra, is the main active ingredient of the plant (5). FAN has showed strong anticancer
activities in a variety of tumor cell lines, including human HepG2,
human lung cancer A549, murine neuroblastoma, human hepatoblastoma,
human colon cancer cells and others (2). MDA-MB-231 cells are the most commonly
used cells in breast cancer research. FAN can inhibit the
proliferation and induce the apoptosis of MDA-MB-231 cells. FAN can
also induce cell cycle arrest of breast cancer cells and affect the
expression of cell cycle related proteins (6,7).
The results of this study show that the treatment of
MDA-MB-231 cells with FAN led to nuclear shrinkage and
fragmentation, which are the biochemical markers of apoptosis. The
MTT assays showed that MDA-MB-231 cells treated with different FAN
concentrations for up to 48 h showed a significantly reduced
percentage of viable cells in a concentration- and time-dependent
manner. The IC50 at 24 and 48 h was 14.26±1.54 µg/ml and 10.48±1.38
µg/ml, respectively, which indicated that FAN did effectively
inhibit the proliferation of breast cancer cells.
FAN is known to inhibit the growth and induce the
apoptosis of MDA-MB-231 cells, by activating the apoptosis-related
protein caspase-3 (8,9). Our results showed that the apoptosis
rates of MDA-MB-231 cells were 1.48, 7.44, 16.91 and 28.66%,
respectively, after 20 µg/ml FAN treatment for 12, 24 and 48 h. A
possible explanation is that FAN treatment can increase the
activity of intracellular caspase-3 in MDA-MB-231 cells, thereby
promoting MDA-MB-231 cell apoptosis.
Many studies have shown that degradation of matrix
metalloproteinases (MMPs) play an important role in tumor invasion
and metastasis. MMP-2 and −9, which are two members of MMPs family
secreted by macrophages, neutrophils, capillary endothelial cells
and tumor cells, are enzymes responsible for the degradation of the
ECM. MMP-2 and −9 can be involved in the invasion and metastasis of
colorectal, breast, gastric and other types of cancers by degrading
ECM and have been specially studied in the invasion and metastasis
of breast cancer (10,11). The expression of the MMP genes is
mainly regulated by transcription factors such as NF-κβ and AP-1
through PI3K/AKT pathway (12).
PI3K/AKT is a constitutive mutant gene pathway in most types of
tumors. This pathway also regulates many extracellular processes,
including tumorigenesis, cell proliferation, survival, glucose
metabolism, gene stability, metastasis and angiogenesis (13). The results of this study show that
different concentrations of FAN can significantly reduce the
concentration of MMP-2 and −9 levels, thus potentially blocking the
tumor cell metastasis.
As a universal transcription factor, NF-κβ is
usually associated with viral infection and inflammation (14). The activated NF-κβ, which can promote
the expression of angiogenesis-related factors such as VEGF, MMPs,
plasminogen activator urokinase (u-PA) and ICAM-1, is closely
related to the metastasis of tumor cells (15). Studies have shown that NF-κβ is
overexpressed in breast cancer tissues (16). NF-κβ is regulated by Iκβ in the
cytoplasm. After release, NF-κβ can enter the nucleus to promote
tumor cell proliferation, neovascularization and metastasis
(17). The activation of NF-κβ can
cause the degradation of Iκβ through PI3K/Akt pathway. Some studies
have shown that FAN can downregulate the PI3 K/AKT pathway to
induce the apoptosis and inhibit the migration of breast cancer
cells (18). The results of this
study showed that the protein levels of NF-κβ were decreased and
the protein levels of Iκβ were increased in a
concentration-dependent manner after treatment with different
concentrations of FAN for 24 h. It is therefore conceivable that
FAN can inhibit the PI3K/AKT pathway, thereby increasing the
concentration of Iκβ and that the increased in the Iκβ level can
block the NLS of NF-κβ, so as to keep the NF-κβ in the cytoplasm in
an inactive form, which would in turn inhibit the activity of NF-κβ
(19). In addition, Iκβ can regulate
the nuclear translocation of NF-κβ, thus affecting the expression
of MMP-2 in multiple types of human cells (20).
In conclusion, based on our results we propose the
following mechanisms explaining the effects of FAN on MDA-MB-231
cells: FAN inhibits the activation of AKT to increase the level of
Iκβ. The increased concentration of Iκβ in the cytoplasm will then
inhibit the activation of NF-κβ and then decrease the expression of
MMP-2 and −9, so as to inhibit migration of the cells. Further
studies are necessary to confirm the usefulness of FAN as an
effective and safe anti-breast cancer drug.
References
|
1
|
Belli P, Bufi E, Bonatesta A, Patrolecco
F, Padovano F, Giuliani M, Rinaldi P and Bonomo L: Unenhanced
breast magnetic resonance imaging: Detection of breast cancer. Eur
Rev Med Pharmacol Sci. 20:4220–4229. 2016.PubMed/NCBI
|
|
2
|
Lu X, Zhang R, Fu F, Shen J, Nian H and Wu
T: Simultaneous determination of fangchinoline and tetrandrine in
Qi-Fang-Xi-Bi-granules by RP-HPLC. J Chromatogr Sci. 53:1328–1332.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun YF and Wink M: Tetrandrine and
fangchinoline, bisbenzylisoquinoline alkaloids from Stephania
tetrandra can reverse multidrug resistance by inhibiting
P-glycoprotein activity in multidrug resistant human cancer cells.
Phytomedicine. 21:1110–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang JB, Song W, Wang YY, Liu MG, Sun MM
and Liu H: Study on correlation between PKIB and pAkt expression in
breast cancer tissues. Eur Rev Med Pharmacol Sci. 21:1264–1269.
2017.PubMed/NCBI
|
|
5
|
Li D, Lu Y, Sun P, Feng LX, Liu M, Hu LH,
Wu WY, Jiang BH, Yang M, Qu XB, et al: Inhibition on proteasome β1
subunit might contribute to the anti-cancer effects of
fangchinoline in human prostate cancer cells. PLoS One.
10:e01416812015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia M, Tong JH, Zhou ZQ, Duan ML, Xu JG,
Zeng HJ and Wang SH: Tramadol inhibits proliferation, migration and
invasion via α2-adrenoceptor signaling in breast cancer cells. Eur
Rev Med Pharmacol Sci. 20:157–165. 2016.PubMed/NCBI
|
|
7
|
Wang LP, Jin J, Lv FF, Cao J, Zhang J,
Wang BY, Shao ZM, Hu XC and Wang ZH: Norepinephrine attenuates
CXCR4 expression and the corresponding invasion of MDA-MB-231
breast cancer cells via β2-adrenergic receptors. Eur Rev Med
Pharmacol Sci. 19:1170–1181. 2015.PubMed/NCBI
|
|
8
|
Büssing A, Vervecken W, Wagner M, Wagner
B, Pfüller U and Schietzel M: Expression of mitochondrial Apo2.7
molecules and caspase-3 activation in human lymphocytes treated
with the ribosome-inhibiting mistletoe lectins and the cell
membrane permeabilizing viscotoxins. Cytometry. 37:133–139. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JD, Takahara S, Nonomura N, Ichimaru
N, Toki K, Azuma H, Matsumiya K, Okuyama A and Suzuki S: Early
induction of apoptosis in androgen-independent prostate cancer cell
line by FTY720 requires caspase-3 activation. Prostate. 40:50–55.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in
pediatric human sarcoma cell lines by cytokines, inducers and
inhibitors. Int J Oncol. 44:27–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan S, Shukla S, Sinha S, Lakra AD, Bora
HK and Meeran SM: Centchroman suppresses breast cancer metastasis
by reversing epithelial-mesenchymal transition via downregulation
of HER2/ERK1/2/MMP-9 signaling. Int J Biochem Cell Biol. 58:1–16.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cichocki M, Baer-Dubowska W, Wierzchowski
M, Murias M and Jodynis-Liebert J:
3,4,5,4-trans-tetramethoxystilbene (DMU-212) modulates the
activation of NF-κB, AP-1 and STAT3 transcription factors in rat
liver carcinogenesis induced by initiation-promotion regimen. Mol
Cell Biochem. 391:27–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Britschgi A, Andraos R, Brinkhaus H,
Klebba I, Romanet V, Müller U, Murakami M, Radimerski T and
Bentires-Alj M: JAK2/STAT5 inhibition circumvents resistance to
PI3K/mTOR blockade: A rationale for cotargeting these pathways in
metastatic breast cancer. Cancer Cell. 22:796–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Z, Boyle DL, Manning AM and Firestein
GS: AP-1 and NF-kappaB regulation in rheumatoid arthritis and
murine collagen-induced arthritis. Autoimmunity. 28:197–208. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
da Silva Facina A, Facina G, da Silva
Guerreiro IDC, Gonçalves Aparecida G, de Almeida Augusto F, de
Noronha Aparecida Alves Corrêa S, de Noronha Marcos Ribeiro S and
Nakamura Uchiyama M: Viscum album modulates apoptotic related genes
in melanoma tumor of mice. Am J Mol Biol. 4:49–58. 2014. View Article : Google Scholar
|
|
16
|
Véquaud E, Séveno C, Loussouarn D,
Engelhart L, Campone M, Juin P and Barillé-Nion S: YM155 potently
triggers cell death in breast cancer cells through an
autophagy-NF-κB network. Oncotarget. 6:13476–13486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basson MD, Sirivelu M, Downey C and Zeng
B: Mo1654 extracellular pressure-induced increases in
[Ca2+]i via T-Type CAV3.3 channels stimulate cancer cell
proliferation by enhancing activity of a PKCBeta-dependent IKK,
IKB, NF-κB pathway. Gastroenterology. 146:S–628. 2014. View Article : Google Scholar
|
|
18
|
Wang CD, Yuan CF, Bu YQ, Wu XM, Wan JY,
Zhang L, Hu N, Liu XJ, Zu Y, Liu GL, et al: Fangchinoline inhibits
cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and
induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac J
Cancer Prev. 15:769–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai CY, Li FCH, Wu CHY, Chang AYW and
Chan SHH: Sumoylation of IkB attenuates NF-kB-induced nitrosative
stress at rostral ventrolateral medulla and cardiovascular
depression in experimental brain death. J Biomed Sci. 23:652016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin T, Liu CJ, Zhang HW, Pan YF, Tang Q,
Liu JK, Wang YZ, Hu MX and Xue F: Effect of the IkBα mutant gene
delivery to mesenchymal stem cells on rat chronic pancreatitis.
Genet Mol Res. 13:371–385. 2014. View Article : Google Scholar : PubMed/NCBI
|