Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer in adults, accounting for ~3% of all adult
malignancies (1). It is the seventh
most common type of cancer in males and the ninth most common
cancer type in females, representing ~90% of all kidney
malignancies (2). The incidence and
mortality rates of RCC have increased in recent years: 65,150
patients are diagnosed and 13,680 RCC-associated mortalities occur
each year (3). Therefore, improving
current understanding of the mechanisms underlying RCC progression
and developing novel therapeutic approaches for RCC are
required.
Wnt signaling serves an important role in disease
development processes in adults (4),
triggering at least two, perhaps three, pathways that employ Wnt
receptors of the frizzled seven transmembrane classes. The first of
these is the canonical Wnt/β-catenin pathway (5), in which Wnt3a, a member of the Wnt
ligands, binds to a frizzled receptor and the corresponding
co-receptor of low-density lipoprotein receptor-related protein 6
(LRP6). This results in the stabilization of cytosolic β-catenin
and facilitates its translocation into the nucleus to activate
downstream transcription factors (6).
The second pathway involved is the planar cell polarity (PCP)
pathway, which does not involve β-catenin but recruits small
GTPases of the Rho/Cdc42 family to activate c-Jun N-terminal kinase
(JNK); in vertebrates, this appears to be triggered by Wnts
(7). The final possible pathway, the
Wnt/Ca2+ cascade, is still largely controversial and may
overlap in part with the PCP pathway (8).
Dickkopf (DKK) genes comprise an evolutionary
conserved gene family of four members (DKK 1–4) and exhibit
distinct cross-overlapping expression patterns in humans and mice
(9). Exogenous DKK 1, 2 and 4 are
reported to possess similar functions in suppressing the activity
of Wnt/β-catenin (canonical) pathway by binding to the co-receptor
LRP5/6 with high affinity (10,11).
However, the suppressive role of exogenous DKK-3 in the
Wnt/β-catenin signaling cascade has been controversial. DKK-3, the
most divergent member of the DKK family in terms of DNA sequence,
function and evolution, alternatively known as reduced expression
in immortalized cells (REIC), does not appear to bind to LRP5/6
(12) or inhibit the Wnt/β-catenin
signaling pathway, as has been reported in numerous studies
(9,11,13,14).
The recombinant protein of full length DKK-3 was
prepared in the current study in order to investigate the exogenous
effects of the protein in vitro in KPK1 human renal cell
carcinoma cells. The anti-proliferative effects of the DKK-3
protein were assessed to determine a possible therapeutic use for
the protein. To clarify whether exogenous DKK-3 inhibits
Wnt/β-catenin signaling, the effects of recombinant DKK-3 protein
on the levels of phosphorylated (p-) and non-p-β-catenin in KPK1
cells were investigated. The involvement of the LRP6 transmembrane
receptor in the extracellular actions of DKK-3 protein and in the
Wnt/β-catenin signaling pathway was also evaluated.
Materials and methods
Cell culture
The KPK1 human renal cell carcinoma cell line was
kindly provided by Professor S. Naito (Department of Urology,
University of Kyushu, Fukuoka, Japan) (15). The PC3 human prostate cancer cell line
and the 211H human malignant pleural biphasic mesothelioma cell
line were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were grown in Dulbecco's modified
Eagle's medium (DMEM) (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) supplemented with 10% (v/v) fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
penicillin (100 IU/ml) and streptomycin (100 µg/ml). The cells were
cultured at 37°C in an atmosphere containing 5% CO2 and
air, and split at a ratio of 1:5 every three days.
Preparation of the recombinant DKK-3
protein
The recombinant protein of human full length DKK-3
was transiently expressed in FreeStyle™ 293-F cells (Thermo Fisher
Scientific, Inc.) using Freestyle 293 Expression Medium and the
293Fectin transfection reagent (Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's instructions. Briefly,
exponentially growing cells (1×106 cells/ml) with 180 ml
medium were prepared in a 500-ml flask. Following transfection with
180 µg each of the expression plasmid DNA and 293Fectin complex,
the cells were cultivated using an orbital shaker (125 rpm) at 37°C
in the presence of 8% CO2 for four days. The secreted
proteins in the culture medium were concentrated using Amicon
Ultra-15 Centrifugal Filters (EMD Millipore, Billerica, MA, USA),
then purified and stored as previously described (16).
Western blot analysis
The cells were treated with the recombinant DKK-3
protein at a final concentration of 10 µg/ml in the culture medium,
and the cell lysate was obtained prior to treatment and at 1, 6,
24, 48 and 72 h after treatment. The cells were collected in
ice-cold lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM
Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM
β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin] for 15 min on
ice (17). The cell fragments were
removed by centrifugation (21,500 × g) at 4°C for 10 min and the
supernatant lysate was immediately stored at −80°C. The protein
concentrations were determined using the Pierce BCA protein assay
reagent kit (Thermo Fisher Scientific, Inc.). After adding 5X
loading buffer [450 mM Tris (pH 6.8), 45% sucrose, 10%
β-mercaptoethanol, 15% SDS and bromophenol blue], the extracted
cell proteins (10 µg) were separated using a
Mini-Protean® TGX™ gels kit (10% 15-well comb; 15 µl;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The proteins on the
gel were then transferred onto a PVDF (Thermo Fisher Scientific,
Inc.) membrane using a Trans-Blot Turbo Transfer System (Bio-Rad
Laboratories, Inc.). Following the transfer, the membrane was
blocked in 5% non-fat milk (cat. no. 1706404XTU; Bio-Rad
Laboratories, Inc.) diluted with TBST buffer (Bio-Rad Laboratories,
Inc.; cat. no. 1610734) and then incubated with the following
primary antibodies (all used as supplied) overnight at 4°C:
p-β-catenin (cat. no. 9561), non-P-β-catenin (cat. no. 8814),
p-glycogen synthase kinase 3 (GSK-3)β (cat. no. 9323),
transcription factor 1 (TCF1; cat. no. 2203), c-Myc (cat. no.
9402), Met (cat. no. 4560), low-density lipoprotein
receptor-related protein 6 (LRP6; cat. no. 3395), Wnt3a (cat. no.
2721) and β-actin (cat. no. 4967) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA). After washing the membrane in
TBST buffer three times (5 min each), the membrane was incubated
with the anti-rabbit (cat. no. 9670531) or anti-mouse (cat. no.
9471754) secondary antibodies (GE Healthcare Life Sciences, Little
Chalfont, UK) with 5% BSA in TBST (1:5,000) buffer for 1 h at room
temperature. The protein-antibody complexes were visualized using
the enhanced chemiluminescence detection method (ECL Prime Western
Blotting Detection Reagent; GE Healthcare Life Sciences) and
medical X-ray film.
Cell proliferation assay
KPK1 cells were plated in 96-well plates at a final
volume of 100 µl culture medium (1,000 cells/well) at 37°C with
6.5% CO2. Following a 12-h incubation, the cells were
treated with DKK-3 protein at final concentrations of 10 or 50
µg/ml in culture medium. For the control treatment, an equal volume
of PBS was added. Cell viability was assessed at the indicated days
(days 1, 2, 3 and 4) following treatment using a Cell Proliferation
kit II (XTT; Roche Diagnostics GmbH, Mannheim, Germany), in
accordance with the manufacturer's instructions. The conversion of
the orange formazan dye was measured using a microplate reader
(model 550; Bio-Rad Laboratories, Inc.) at an absorbance wavelength
of 492–690 nm.
Small interfering (si)RNA transfection
for LRP6 knockdown
KPK1 cells (2×105 cells per well in
6-well plates) were incubated with 2 ml antibiotic-free culture
medium supplemented with 10% FBS for 18–24 h until the cells were
60–80% confluent. The siRNA reagent [LRP6 siRNA (h); #sc-37233;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA] and matched
control reagent were transfected into the KPK1 cells for 6 h at
37°C in a 5% CO2 incubator. Following transfection, the
medium was changed to complete medium and further incubated for 24
h. The cells were then treated with the recombinant DKK-3 protein
at a final concentration of 10 µg/ml in culture medium, and the
cell lysates were obtained for western blot analysis at the
indicated time points.
Statistical analysis
The data are presented as the means ± standard
error. Unpaired Student's t-tests were performed to analyze the
statistical significance of differences between two groups.
Statistical analyses were performed using StatView version 4.5
software (Abacus Concepts, Piscataway, NJ, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Exogenous DKK-3 inhibits Wnt/β-catenin
signaling in a time-dependent manner in KPK1 cells
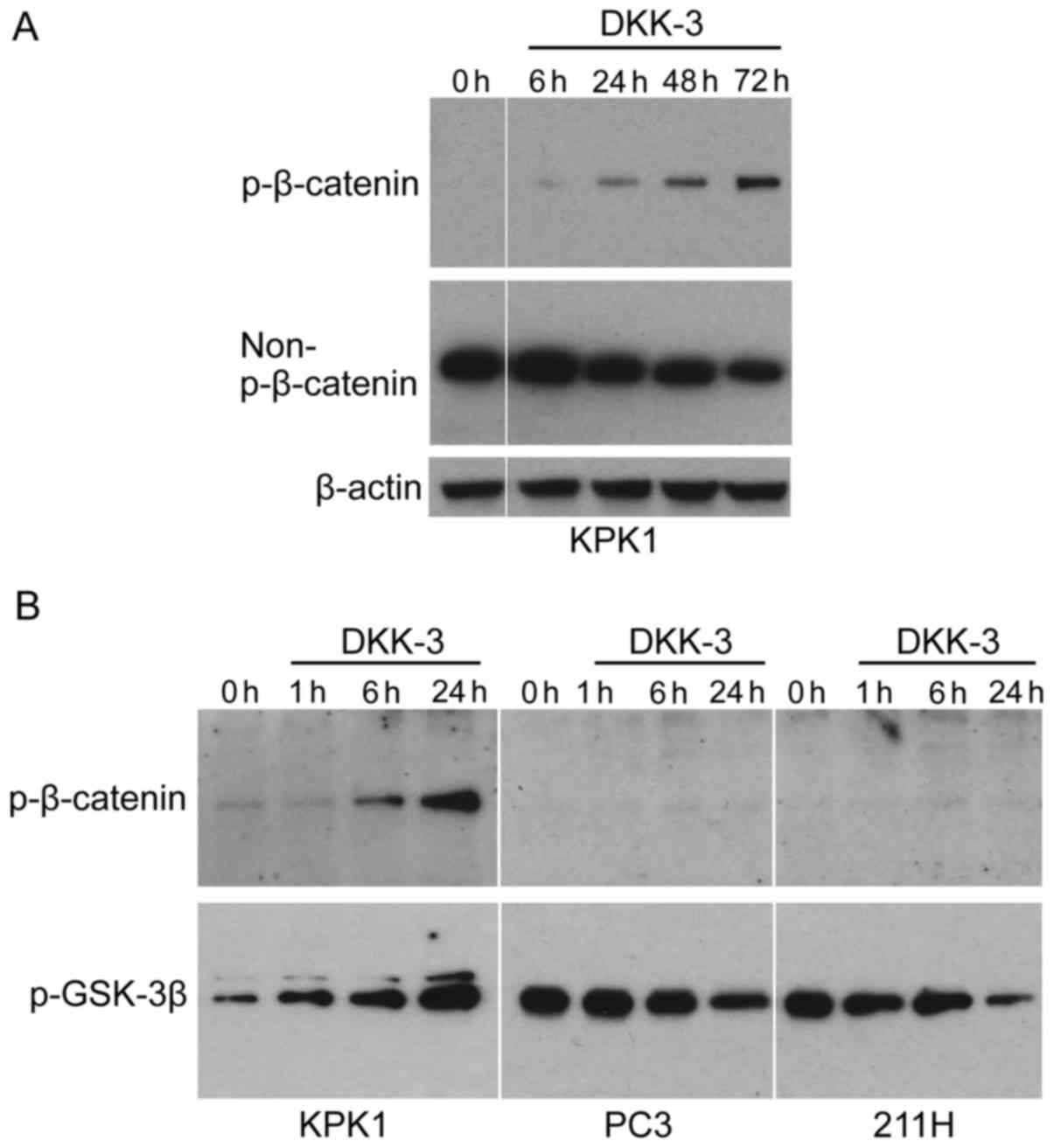
KPK1 cells were treated with the recombinant DKK-3
protein, following which the expression of p-β-catenin (inactive
form of β-catenin) and non-p-β-catenin (activated form) was
examined by western blot analysis (Fig.
1A). The p-β-catenin was gradually increased in KPK1 cells in a
time-dependent manner. The level of non-p-β-catenin was
correspondingly reduced, indicating that exogenous DKK-3 protein
inhibits Wnt/β-catenin signaling in a time-dependent manner. To
further elucidate the inhibitory mechanism in Wnt/β-catenin
signaling effected by the exogenous DKK-3 protein, the p-level of
glycogen synthase kinase-3β (GSK-3β) was analyzed, which has been
demonstrated to phosphorylate β-catenin, thus targeting it for
degradation (5,6). In KPK1 cells, the level of p-GSK-3β was
gradually increased as the p-β-catenin was increased (Fig. 1B). By contrast, in PC3 and 211H cells
the levels of p-β-catenin were low and did not increase during the
course of treatment with DKK-3 protein. In these cells, contrary to
KPK1 cells, the level of p-GSK-3β was gradually decreased.
Exogenous DKK-3 inhibits the
downstream targets of Wnt/β-catenin signaling
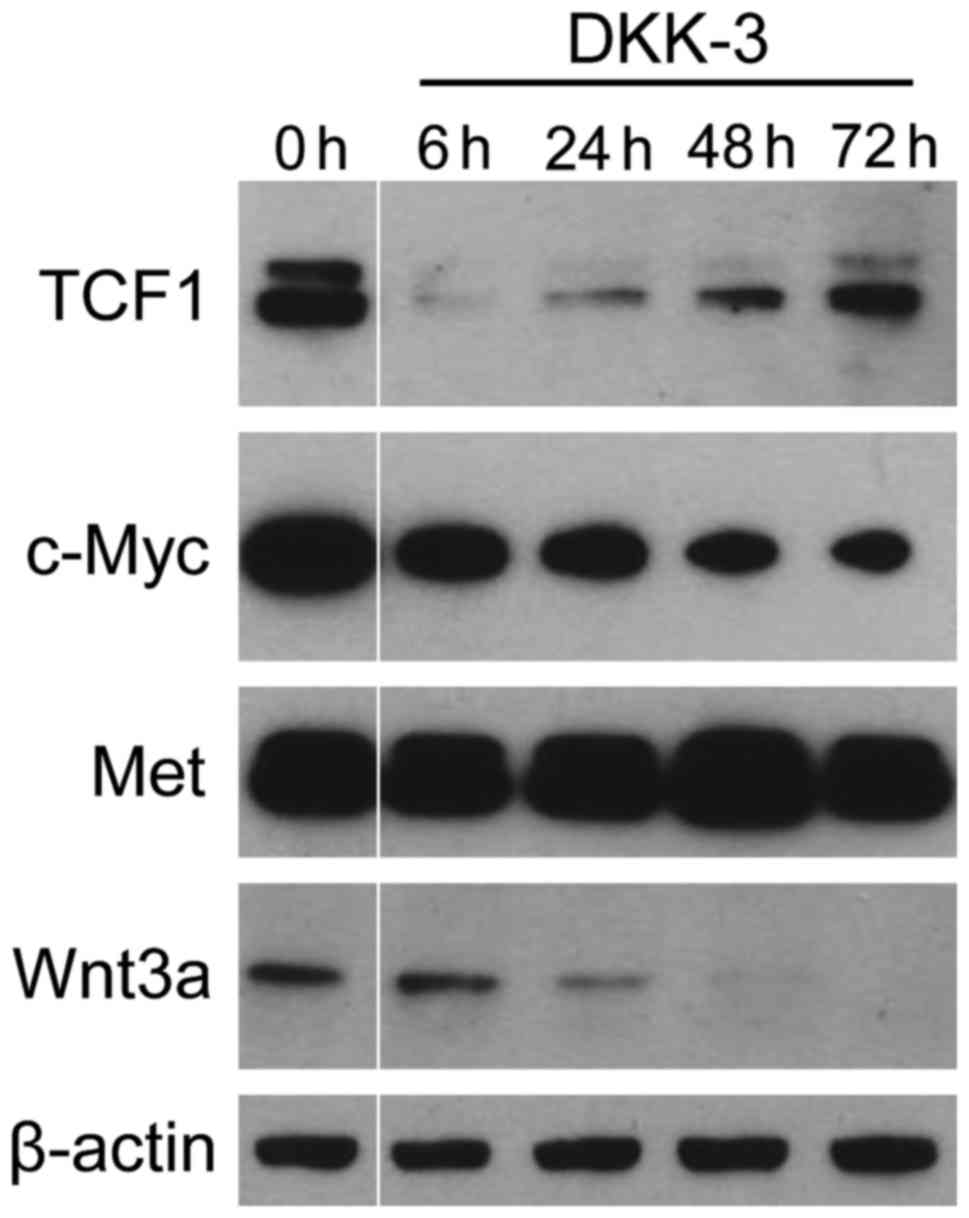
It was investigated whether DKK-3 protein suppresses
the expression of TCF1 and c-Myc, the downstream key effectors of
Wnt/β-catenin signaling (Fig. 2). The
levels of two key targets, (known as tumor progression factors),
which have an important role in the cell cycle, apoptosis and
cellular transformation (4,6), were significantly suppressed following
treatment. TCF1 expression was markedly reduced during the early
phase following DKK-3 treatment and subsequently restored. With
regard to c-Myc, the expression level was gradually reduced after
treatment. The expression of Wnt3a was also decreased in a
time-dependent manner. By contrast, no significant changes were
observed in the expression of Met, the level of which was regulated
by signaling pathways other than Wnt/β-catenin (18,19). These
findings indicate that exogenous DKK-3 protein downregulates the
Wnt/β-catenin-targeted molecules of TCF1 and c-Myc.
Exogenous DKK-3 exhibits
anti-proliferative effects in KPK1 cells
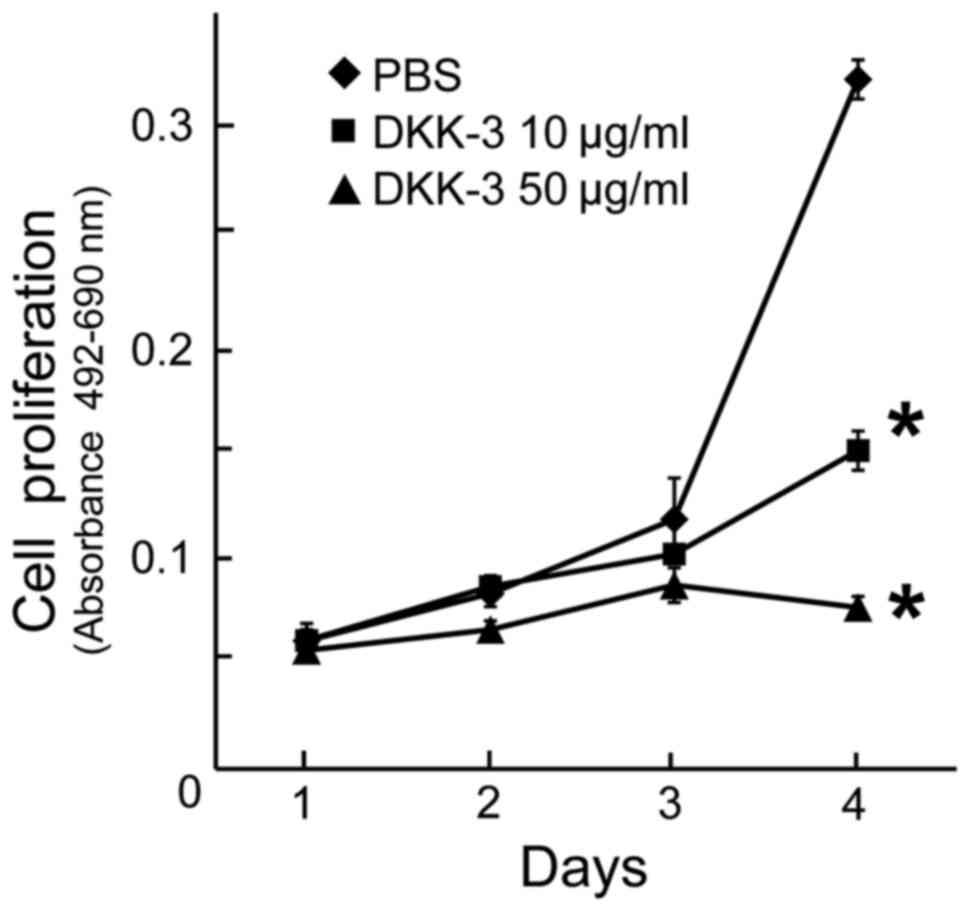
To examine the anti-proliferative effects of
exogenous DKK-3 protein, the viability of KPK1 cells post-treatment
was assayed. The viability of cells treated with the final
concentrations of 10 or 50 µg/ml DKK-3 protein was evaluated, and a
significant inhibition of cell proliferation was observed in each
case at day 4 (Fig. 3).
Dose-dependency was observed in the inhibitory effects.
LRP6 knockdown elevated the base level
of the Wnt/β-catenin signaling but did not influence the
upregulation course or pattern
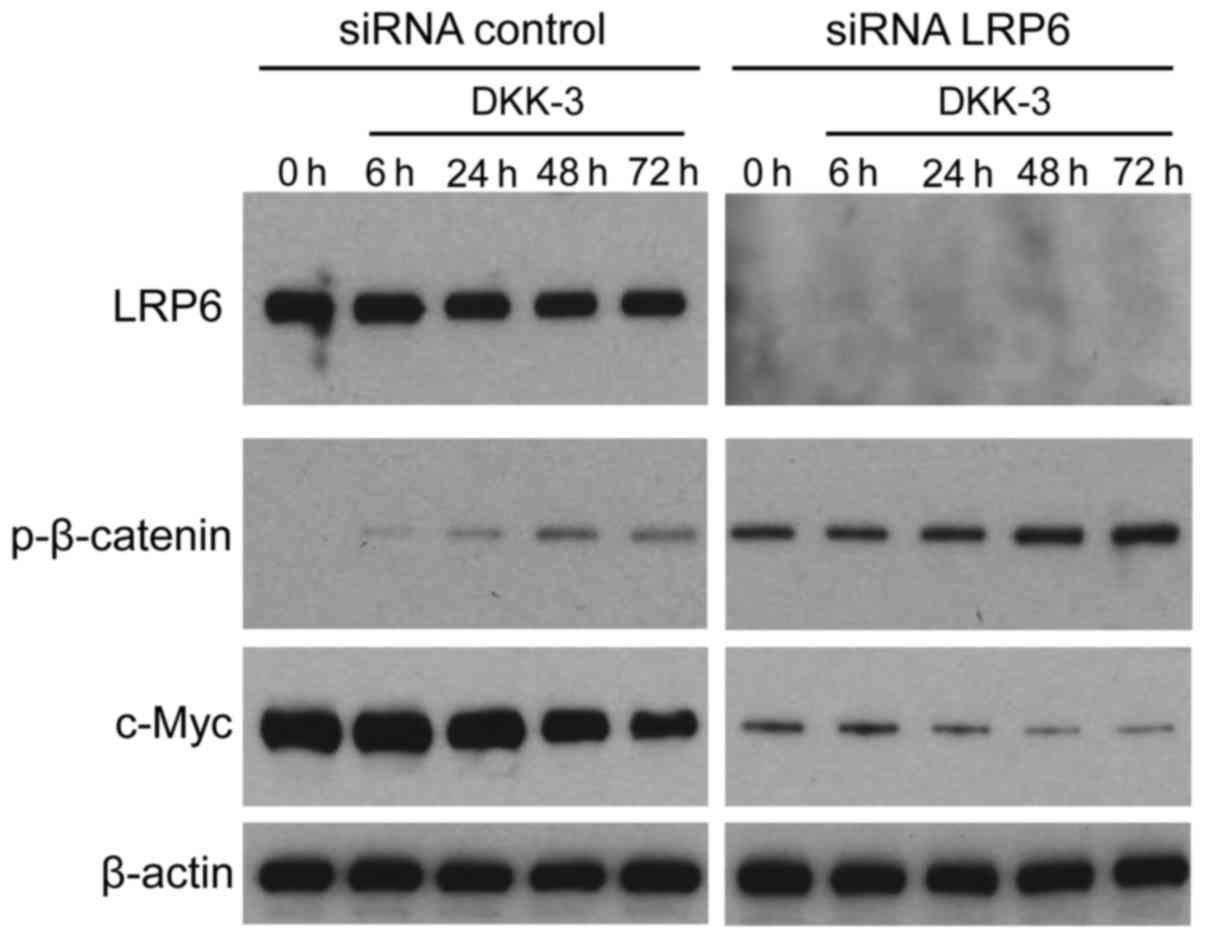
It was investigated whether exogenous DKK-3 protein
affects LRP6-mediated Wnt/β-catenin signaling in KPK1 cells. The
LRP6 gene was silenced using specific siRNA, subsequently
facilitating verification of expression depletion via western blot
analysis (Fig. 4). Under a lack of
LRP6 expression, the effects of DKK-3 were analyzed with respect to
the time course of p-β-catenin and c-Myc expression. However, LRP6
depletion upregulated the basal expression levels of p-β-catenin,
thus maintaining its upregulation course and pattern of control.
p-β-catenin expression in the knocked LRP6 group was strongly
upregulated compared with the un-knocked LRP6. In addition, the
basal expression of p-β-catenin was also enhanced by DKK-3 without
LRP6. For c-Myc downstream of β-catenin, the base level was
attenuated, maintaining its downregulation course and pattern.
Depletion of the Wnt pathway receptor LRP6 exerted no significant
effects on the extracellular DKK-3-induced course of Wnt/β-catenin
signaling, indicating that DKK-3 blocks the signaling in a
LRP6-independent manner in KPK1 cells.
Discussion
DKK-3 is a tumor suppressor in a variety of cancer
cells; however, the role and mechanism of exogenous DKK-3 protein
in the Wnt/β-catenin signaling pathway has remained unclear
(9). In addition, it is unclear
whether or not exogenous DKK-3 (or DKK-1/2) inhibits Wnt signaling
(13). In the present study, it was
demonstrated that the levels of p-β-catenin were increased in KPK1
cells treated with exogenous DKK-3 protein. The non-p-β-catenin was
consistently decreased, indicating that the DKK-3 protein functions
as an inhibitor of Wnt/β-catenin on the cell surface.
Few prior studies have detailed the involvement of
extracellular DKK-3 protein in Wnt/β-catenin signaling (9–11). In
DKK-3-transfected human glioma cells, a decrease in non-p-β-catenin
(activated form of β-catenin) was observed in parallel with an
increase in the levels of intra- and extracellular DKK-3 protein
(20). The authors suggested that
extracellular DKK-3 inhibited the Wnt/β-catenin signaling pathway
via binding to transmembrane receptors. The findings of previous
studies demonstrating a lack of modulation by extracellular DKK-3
protein on the Wnt/β-catenin pathway (20), as well as the results of the current
study, suggest that the role of DKK-3 may be dependent upon the
cell type and characteristics.
It was revealed that the expression of TCF1 and
c-Myc, the downstream transcription factors of Wnt/β-catenin
signaling in cancer transcription cascades (4–6), is
downregulated following treatment with recombinant DKK-3 protein.
These results not only support the suppressive effects of exogenous
DKK-3 protein on Wnt/β-catenin signaling, but also suggest an
inhibitory role of DKK-3 in the cell proliferation promoted by TCF1
and c-Myc. Indeed, a cancer cell viability assay confirmed the
anti-proliferative effects of exogenous DKK-3 protein in KPK1
cells, as presented in Fig. 3,
possibly due to the suppressed levels of TCF1 and c-Myc in the
Wnt/β-catenin signaling cascade (21). Notably, the significant downregulation
of TCF1 was observed in the early stages (6 h) following treatment,
and the expression of c-Myc gradually decreased, as depicted in
Fig. 2. As TCF1 is a transcription
factor for c-Myc expression (4,5,21), the downregulation of TCF1 likely
induced the gradual decrease in c-Myc levels. The protein
expression levels of Met were also examined; Met is regulated by
transcription factors other than TCF1 (18,19). There
were no significant changes recorded in Met expression following
treatment with recombinant DKK-3 protein, suggesting that its
regulation is independent of exogenous DKK-3-associated
modifications of Wnt/β-catenin signaling. With regard to the
reduced expression of Wnt3a following DKK-3 treatment,
modifications to the activity of β-catenin/TCF1 target genes may
have suppressed its transcription (6,21). The
downregulation of these molecules may be due to transcriptional
suppression via the Wnt/β-catenin signaling pathway effected by
exogenous DKK-3 protein.
As LRP6 is a cell-surface receptor for extracellular
DKK-1 and DKK-2 proteins, and these interactions inhibit the
Wnt/β-catenin signaling pathway (11,14), it
was examined whether or not exogenous DKK-3 protein affects the
LRP6-mediated Wnt/β-catenin signaling in KPK1 cells. The LRP6 gene
was silenced and the effects of DKK-3 on the time course of the
upregulation of p-β-catenin expression subsequently analyzed.
Notably, LRP6 depletion elevated the basal level of p-β-catenin;
however, there was no significant effect on its upregulation course
or pattern. With regard to downstream c-Myc, the basal level
declined but maintained its downregulation course and pattern. The
involvement of extracellular DKK-3 in the Wnt/β-catenin signaling
pathway may function as an alternative mechanism rather than as an
antagonist blocking LRP6 in KPK1 cells. A previous study reported
that DKK-3 interacts with the Wnt pathway receptors Kremen1 (Krm1)
and Krm2, and not with LRP6 in biochemical assays (22), it is worth further exploring the
modification of Wnt/β-catenin signaling in terms of the interaction
of DKK-3 with Krm receptors on the cell surface.
In order to analyze cell types other than KPK1, the
exogenous DKK-3-associated modifications of Wnt/β-catenin signaling
were examined in PC3 human prostate cancer cells and 211H human
malignant mesothelioma cells. Treatment with recombinant DKK-3
protein did not lead to elevation of p-β-catenin levels in PC3
cells or 211H cells. The results indicated that the inhibitory
effect of DKK-3 on Wnt/β-catenin signaling could be absent or
masked in these cell types, possibly due to cross-talk signaling
occurring in untested pathways. The effect of exogenous DKK-3
protein on the level of p-GSK-3β, the possible upstream regulator
of p-β-catenin in Wnt/β-catenin signaling, was also investigated.
However, the effect on p-GSK-3β levels was contrary between the
KPK1 human kidney cancer cells and the other two cell types.
Further studies may enable elucidation of the molecular mechanisms
by which exogenous DKK-3 protein modifies p-β-catenin levels in the
GSK-3β/β-catenin axis.
In conclusion, it was demonstrated for the first
time that exogenous treatment with DKK-3 protein exerts
anti-proliferative effects in KPK1 cells and inhibits the
Wnt/β-catenin signaling pathway in an LRP6-independent manner.
Exogenous DKK-3 protein appears to inhibit KPK1 cell proliferation
by inactivating Wnt/β-catenin signaling through the upregulation of
p-β-catenin. Although the binding partner of DKK-3 on the cell
surface remains to be elucidated, these findings assist in
clarifying the anti-cancer mechanisms of extracellular DKK-3. The
Wnt signaling pathway serves an important role in the
carcinogenesis and the progression of renal cell carcinoma
(23), and the in vivo
tumor-suppressive effects of the recombinant full length DKK-3
protein were previously demonstrated in the RENCa tumor model of
murine renal carcinoma (24).
Therefore, recombinant DKK-3 protein may be a promising agent for
treating renal cell carcinoma.
Acknowledgements
This study was supported by the Ministry of
Education, Culture, Sports, Science and Technology of Japan, Takeda
Science Foundation and Novartis Pharma Research Grants (JSPS
KAKENHI, grant nos. JP15H04297, JP15H04974, JP15K10590 and
JP16K11004) and the China Scholarship Council (grant no.
201308210161). The authors thank Ms. Fusaka Oonari (Okayama
University) for her valuable assistance.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cadigan KM and Liu YI: Wnt signaling:
Complexity at the surface. J Cell Sci. 119:395–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tada M, Concha ML and Heisenberg CP:
Non-canonical Wnt signalling and regulation of gastrulation
movements. Semin Cell Dev Biol. 13:251–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohn AD and Moon RT: Wnt and calcium
signaling: Beta-catenin-independent pathways. Cell Calcium.
38:439–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brott BK and Sokol SY: Regulation of
Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol
Cell Biol. 22:6100–6110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glinka A, Wu W, Delius H, Monaghan AP,
Blumenstock C and Niehrs C: Dickkopf-1 is a member of a new family
of secreted proteins and functions in head induction. Nature.
391:357–362. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujii Y, Hoshino T and Kumon H: Molecular
simulation analysis of the structure complex of C2 domains of DKK
family members and β-propeller domains of LRP5/6: Explaining why
DKK3 does not bind to LRP5/6. Acta Med Okayama. 68:63–78.
2014.PubMed/NCBI
|
|
13
|
Krupnik VE, Sharp JD, Jiang C, Robison K,
Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et
al: Functional and structural diversity of the human Dickkopf gene
family. Gene. 238:301–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao B, Wu W, Li Y, Hoppe D, Stannek P,
Glinka A and Niehrs C: LDL-receptor-related protein 6 is a receptor
for Dickkopf proteins. Nature. 411:321–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naito S, Kanamori T, Hisano S, Tanaka K,
Momose S and Kamata N: Human renal cell carcinoma: Establishment
and characterization of two new cell lines. J Urol. 128:1117–1121.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakaguchi M, Sadahira T, Ueki H, Kinoshita
R, Murata H, Yamamoto KI, Futami J, Nasu Y, Ochiai K, Kumon H, et
al: Robust cancer-specific gene expression by a novel cassette with
hTERT and CMV promoter elements. Oncol Rep. Jun 12–2017.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abounader R, Ranganathan S, Kim BY,
Nichols C and Laterra J: Signaling pathways in the induction of
c-met receptor expression by its ligand scatter factor/hepatocyte
growth factor in human glioblastoma. J Neurochem. 76:1497–1508.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morozov VM, Massoll NA, Vladimirova OV,
Maul GG and Ishov AM: Regulation of c-met expression by
transcription repressor Daxx. Oncogene. 27:2177–2186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizobuchi Y, Matsuzaki K, Kuwayama K,
Kitazato K, Mure H, Kageji T and Nagahiro S: REIC/Dkk-3 induces
cell death in human malignant glioma. Neuro Oncol. 10:244–253.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
22
|
Nakamura RE and Hackam AS: Analysis of
Dickkopf3 interactions with Wnt signaling receptors. Growth
Factors. 28:232–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Q, Krause M, Samoylenko A and Vainio S:
Wnt signaling in renal cell carcinoma. Cancers (Basel). 8:pii: E57.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinoshita R, Watanabe M, Huang P, Li SA,
Sakaguchi M, Kumon H and Futami J: The cysteine-rich core domain of
REIC/Dkk-3 is critical for its effect on monocyte differentiation
and tumor regression. Oncol Rep. 33:2908–2914. 2015. View Article : Google Scholar : PubMed/NCBI
|