Introduction
Positron emission tomography (PET) is widely used
for cancer detection, staging and monitoring the response to
therapy. [18F] fluorodeoxyglucose (18F-FDG)
and 3′-deoxy-3′-[18F] fluorothymidine
(18F-FLT) are commonly used PET tracers for imaging
glucose metabolism and cell proliferation, respectively (1–12). In a
mouse model of human lung cancer, it has been previously
demonstrated that intratumoral distribution between
18F-FLT and 18F-FDG was mutually exclusive.
18F-FLT accumulated primarily in proliferating cancer
cells, whereas 18F-FDG accumulated in hypoxic cancer
cells that are less proliferative (13–16). To
the best of our knowledge, intratumoral distribution of
18F-FLT and 18F-FDG in patients with lung
cancer has not been previously reported.
Differential diagnosis of malignant pulmonary
lesions may be challenging. Computed tomography (CT) is the method
of choice for the diagnosis of pulmonary lesions. PET/CT imaging
reflects the biological and metabolic aspects of pulmonary lesions
(17). 18F-FDG PET/CT has
been widely used for the diagnosis of pulmonary lesions; however,
false-negative as well as false-positive results are frequently
observed (17,18). 18F-FLT is a positron
radioactive tracer that reflects cancer cell proliferation.
Therefore, 18F-FLT may be a useful tool for the
diagnosis of pulmonary lesions (19).
In the present study, it was hypothesized that the
mutually exclusive distribution pattern between 18F-FLT
and 18F-FDG described in animal tumor models may apply
to patients with lung malignancies as well. To examine this
hypothesis, patients with pulmonary lesions that initially
underwent a 18F-FDG PET/CT scan and subsequently a
18F-FLT PET/CT scan were studied.
Materials and methods
Patients
The present study was approved by the Institutional
Review Boards of the Inner Mongolia Medical University (Hohhot,
China) and the Soochow Medical University (Jiangsu, China). Written
informed consent was obtained from all patients prior to
participation. The Institutional Review Board of the University of
Louisville (Louisville, KY, USA) approved data transfer and use.
From June 2013 to August 2015, a total of 55 patients (Table I) with pretreated lung lesions were
recruited to the present study (31 males and 24 females; age range,
17–68 years). Histological examination of the lesions was performed
in every patient. The diameter of the lesions ranged between 8 and
50 mm.
 | Table I.Patients' clinical data and PET/CT
results. |
Table I.
Patients' clinical data and PET/CT
results.
| Patient no. | Age/sex | SUVmax
FDG/FLT | Pathological
diagnosis | FDG/FLT PET/CT
SUVmax |
|---|
| 1 | 55/F | 5.3/2.7 | Adenocarcinoma | Mismatch |
| 2 | 48/F | 3.5/1.4 | Tuberculoma | Match |
| 3 | 61/F | 4.2/2.1 | Squamous
carcinoma | Match |
| 4 | 65/F | 2.8/1.1 | Tuberculoma | Mismatch |
| 5 | 59/F | 2.3/1.0 | Organizing
pneumonia | Match |
| 6 | 63/F | 5.8/2.1 | Adenocarcinoma | Mismatch |
| 7 | 60/F | 2.3/1.2 | Tuberculoma | Mismatch |
| 8 | 62/F | 4.8/2.2 | Adenocarcinoma | Mismatch |
| 9 | 64/F | 3.2/2.0 | Adenocarcinoma | Mismatch |
| 10 | 64/F | 1.6/0.9 | Tuberculoma | Mismatch |
| 11 | 67/F | 1.9/0.9 | Hamartoma | Match |
| 12 | 49/F | 2.1/1.5 | Tuberculoma | Match |
| 13 | 57/F | 3.8/2.8 | Adenocarcinoma | Mismatch |
| 14 | 59/F | 4.1/2.6 | Adenocarcinoma | Mismatch |
| 15 | 47/F | 2.0/1.4 | Tuberculoma | Match |
| 16 | 49/M | 2.6/1.6 | Tuberculoma | Mismatch |
| 17 | 53/M | 3.7/2.4 | Adenocarcinoma | Mismatch |
| 18 | 60/M | 7.9/2.8 | Squamous
carcinoma | Match |
| 19 | 65/M | 1.5/0.9 | Organizing
pneumonia | Match |
| 20 | 67/M | 3.5/2.0 | Inflammatory
pseudotumor | Mismatch |
| 21 | 57/M | 6.8/2.4 | Squamous
carcinoma | Match |
| 22 | 60/M | 3.7/2.6 | Squamous
carcinoma | Match |
| 23 | 58/M | 4.2/2.0 | Squamous
carcinoma | Mismatch |
| 24 | 62/M | 3.6/2.5 | Adenocarcinoma | Mismatch |
| 25 | 63/M | 3.4/2.6 | Adenocarcinoma | Mismatch |
| 26 | 66/M | 1.8/1.0 | Inflammatory
pseudotumor | Mismatch |
| 27 | 45/M | 1.6/1.3 | Tuberculoma | Mismatch |
| 28 | 59/M | 2.6/1.2 | Tuberculoma | Match |
| 29 | 17/M | 2.9/1.5 | Tuberculoma | Match |
| 30 | 48/M | 3.0/1.8 | Squamous
carcinoma | Mismatch |
| 31 | 62/M | 1.1/1.0 | Tuberculoma | Match |
| 32 | 62/M | 2.4/1.6 | Tuberculoma | Match |
| 33 | 62/M | 2.1/0.8 | Organizing
pneumonia | Mismatch |
| 34 | 50/M | 3.1/0.9 | Tuberculoma | Match |
| 35 | 52/M | 1.5/1.0 | Hamartoma | Match |
| 36 | 57/M | 3.6/2.2 | Adenocarcinoma | Mismatch |
| 37 | 54/M | 3.2/1.9 | Adenocarcinoma | Mismatch |
| 38 | 52/M | 1.6/0.7 | Tuberculoma | Match |
| 39 | 44/M | 1.0/0.7 | Hamartoma | Mismatch |
| 40 | 49/M | 5.4/1.8 | Squamous
carcinoma | Mismatch |
| 41 | 62/M | 1.5/0.7 | Tuberculoma | Match |
| 42 | 68/M | 3.5/2.0 | Adenocarcinoma | Mismatch |
| 43 | 47/F | 4.1/1.1 | Tuberculoma | Match |
| 44 | 49/M | 3.1/1.4 | Tuberculoma | Match |
| 45 | 58/M | 6.8/2.4 | Squamous
carcinoma | Mismatch |
| 46 | 60/M | 2.6/1.1 | Tuberculoma | Mismatch |
| 47 | 67/M | 8.2/3.5 | Adenocarcinoma | Mismatch |
| 48 | 55/F | 3.2/1.8 | Adenocarcinoma | Match |
| 49 | 48/F | 1.5/1.0 | Organizing
pneumonia | Mismatch |
| 50 | 61/F | 5.8/2.5 | Adenocarcinoma | Mismatch |
| 51 | 65/F | 2.7/2.1 | Adenocarcinoma | Mismatch |
| 52 | 59/F | 1.9/0.8 | Inflammatory
pseudotumor | Match |
| 53 | 63/F | 1.6/0.8 | Hamartoma | Mismatch |
| 54 | 60/F | 1.8/1.1 | Inflammatory
pseudotumor | Match |
| 55 | 62/F | 1.5/0.9 | Hamartoma | Match |
Radiopharmaceuticals
[18F] fluoride was generated in-house
using a cyclotron. 18F-FDG and 18F-FLT were
synthesized automatically using FX-FN conventional modules at the
PET/CT facility of the Inner Mongolia Medical University (Hohhot,
China). 18F-FDG and 18F-FLT were pyrogen-free
and qualified for clinical use, with radiochemical purity
>98%.
PET/CT imaging protocol
PET/CT images were obtained using a GE Discovery ST
PET/CT scanner. Prior to 18F-FDG PET scanning, patients
were instructed to fast for >6 h and their blood glucose levels
were determined to be <6 mmol/l. Whole body 18F-FDG
PET/CT scans were performed 1 h after intravenous administration of
3.7 MBq/kg 18F-FDG. Subsequently, 3 days after
18F-FDG imaging, local thoracic 18F-FLT
PET/CT scans were performed, 1 h after the injection of
18F-FLT (3.7 MBq/kg). Spiral CT scans (voltage, 120 kV;
current, 160–220 mA) were conducted for attenuation correction and
anatomy referral.
A board of three certified physicians in nuclear
medicine assessed the PET/CT images. Visual analysis to score
lesion radioactivity uptake of each tracer was performed (20). The maximal standardized uptake value
(SUVmax) was used to spatially compare the intralesional
distribution of 18F-FDG and 18F-FLT.
Histological examination of the lesions was
performed for all patients by board-certified pathologists at the
Department of Pathology (Affiliated Hospital of Inner Mongolian
Medical University). Routine hematoxylin and eosin (H&E)
staining was performed. Briefly, slides containing 5 µm paraffin
sections were placed on a slide holder, deparaffinized and
rehydrated. Sections were treated with hematoxylin solution, dipped
8–12 times in acid ethanol to destain, and stained for 30 sec with
eosin. H&E stain imaging was developed with a light microscope
at ×100 magnification.
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used to analyze the data using a χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The clinical information and PET/CT results of the
patient cohort are summarized in Table
I. Among the 55 cases, 24 lesions were confirmed as primary
lung malignancies (16 cases with adenocarcinoma, 8 cases with
squamous cell carcinoma) and 31 lesions were benign (18 cases with
tuberculosis, 5 with hamartoma, 4 with inflammatory pseudo-tumor
and 4 with organizing pneumonia).
Spatial intratumoral distribution of
18F-FLT and 18F-FDG mismatched in 19/24
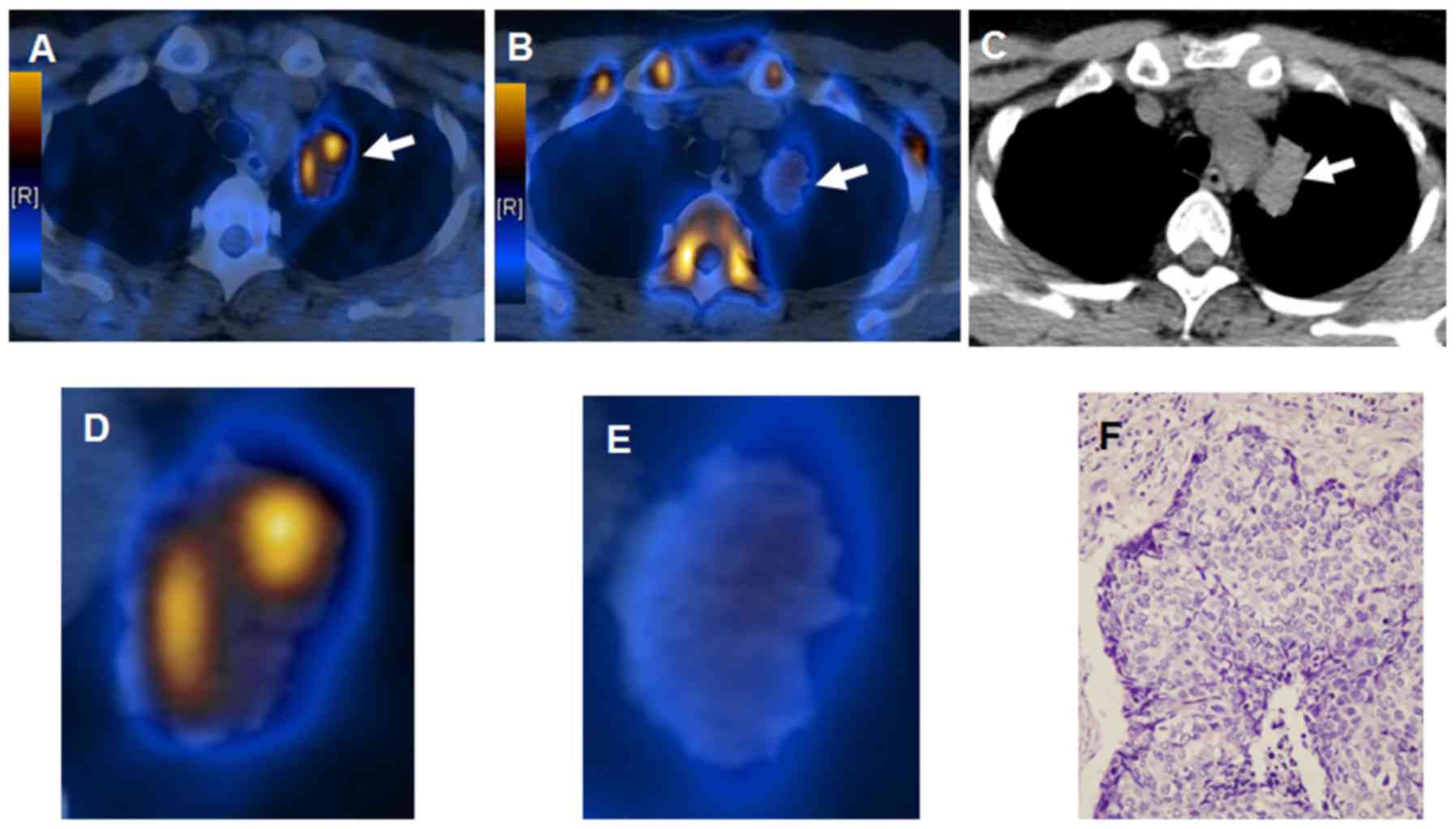
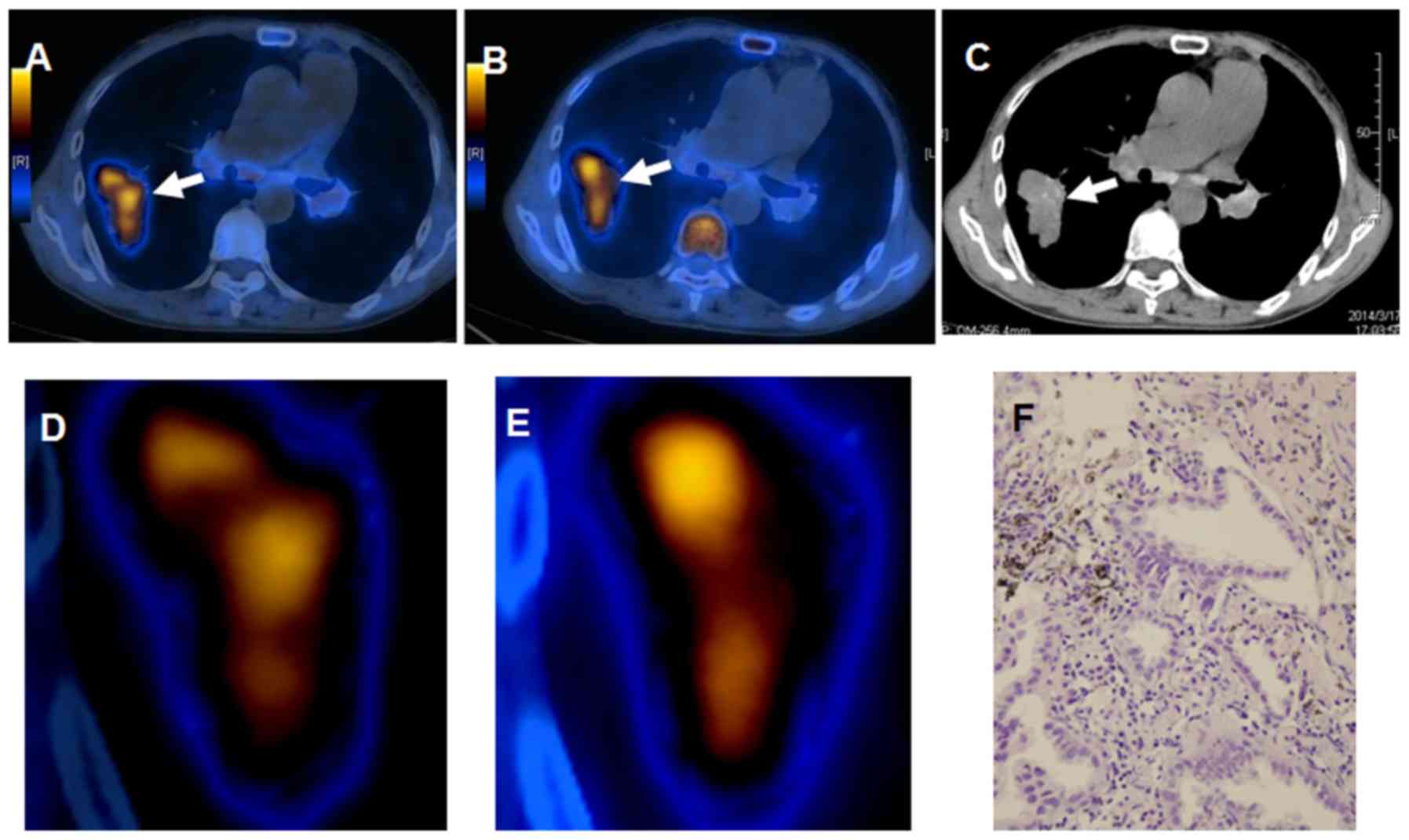
malignant lesions (79%) and matched in 5 (21%). Fig. 1 presents an apparent mismatch in
intratumoral distribution of 18F-FLT and
18F-FDG in a 67-year-old male patient with pretreated
lung adenocarcinoma. Increased 18F-FDG uptake combined
with decreased 18F-FLT accumulation in a patient with
squamous carcinoma is presented in Fig.
2. Intratumoral distribution of 18F-FLT and
18F-FDG in lung malignancies was identified to be mainly
heterogeneous and mutually excluded.
Regarding the 31 benign lesions, intralesional
mismatched distribution of 18F-FLT and
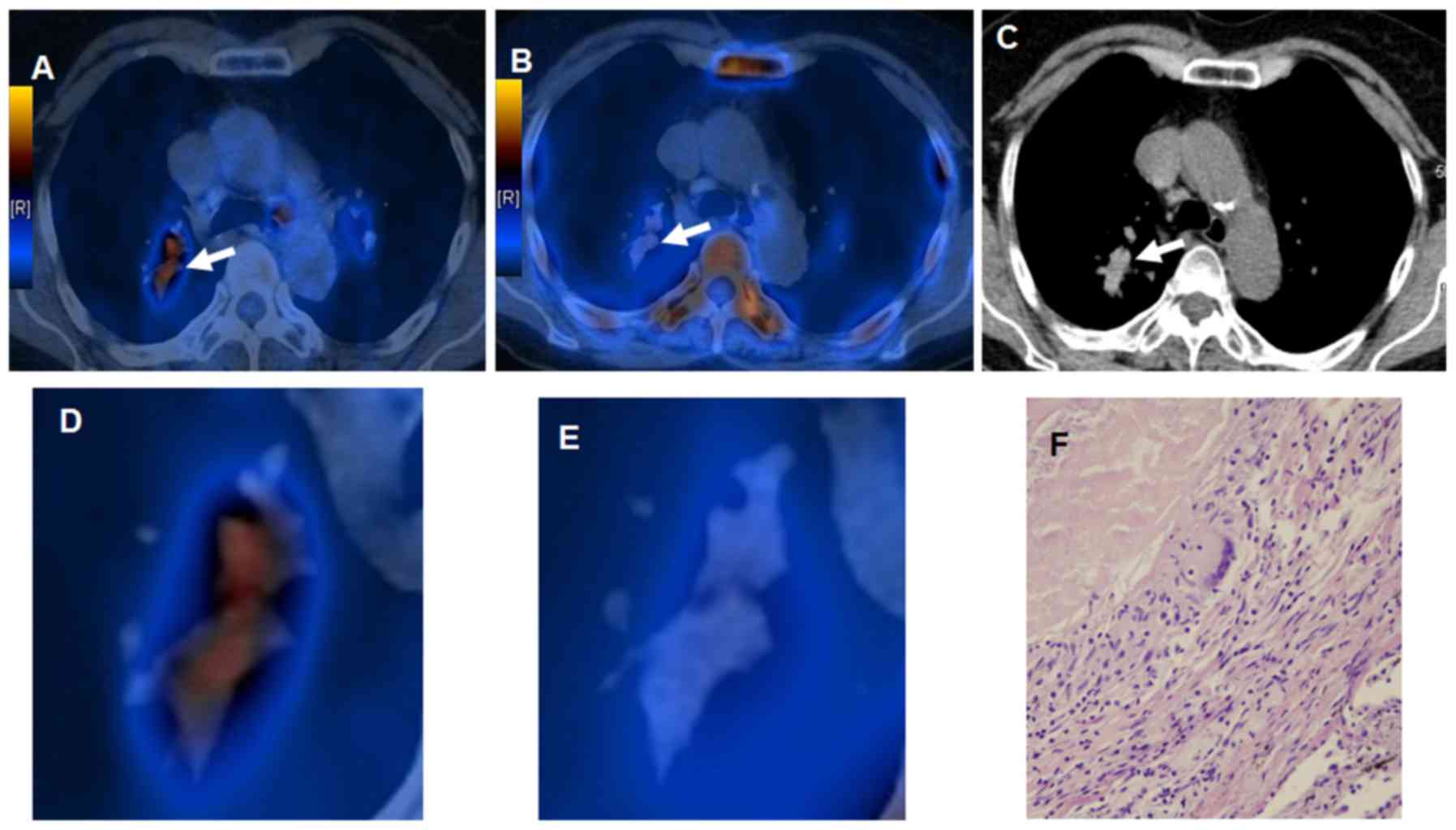
18F-FDG was observed in 12 cases (39%). Fig. 3 presents scan images of mismatched
18F-FLT and 18F-FDG intralesional
distribution in a 49-year-old male patient with lung tuberculoma.
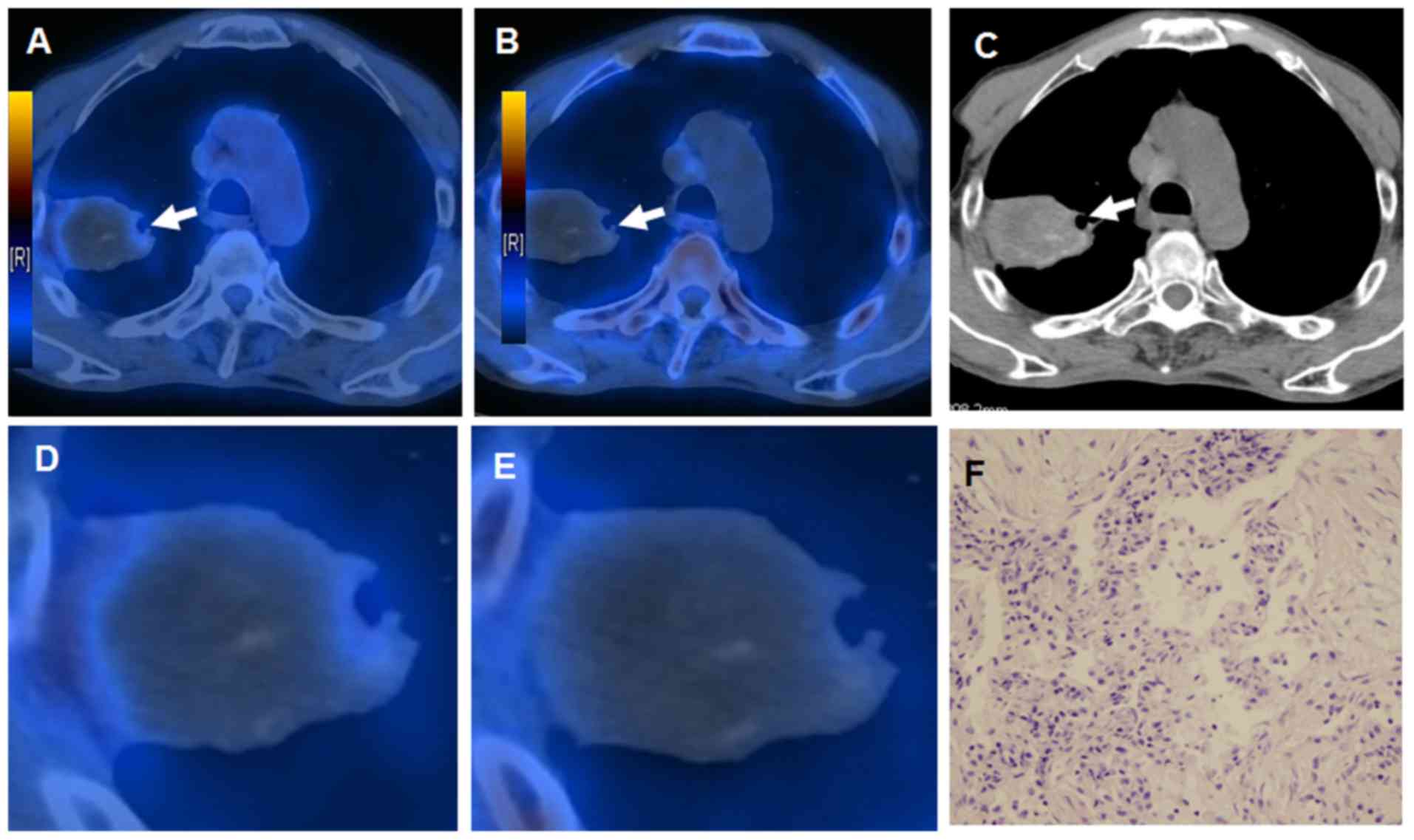
Matched intralesional distribution of 18F-FLT and
18F-FDG was observed in 19/31 benign lesions (61%). An
indicative example of a patient with an inflammatory pseudotumor
demonstrating negative 18F-FLT and positive
18F-FDG PET scans is presented in Fig. 4.
These results indicate that mismatched intralesional
accumulation of 18F-FLT and 18F-FDG was more
frequently observed in malignant compared with benign lung lesions.
The difference in intralesional distribution of 18F-FLT
and 18F-FDG between malignant and benign lesions was
statistically significant (P<0.05).
Discussion
It has previously been reported based on studies
using mouse non-small cell lung cancer models that
18F-FDG accumulates in hypoxic regions, whereas
18F-FLT accumulates in well-oxygenated proliferating
cells. Additionally, it has been demonstrated that the intratumoral
distribution of 18F-FDG and 18F-FLT is
mutually exclusive (13,14). In the present study, the association
between 18F-FDG and 18F-FLT uptake was
further elucidated in patients with lung cancer.
In the present study, it was demonstrated that
intratumoral 18F-FDG and 18F-FLT accumulation
is mutually exclusive. It was observed that regions with increased
18F-FDG accumulation were mainly associated with
decreased 18F-FLT uptake. This is consistent with
previous preclinical results in mouse lung cancer models (13–16).
Intratumoral heterogeneity of 18F-FDG and
18F-FLT accumulation reflected the heterogeneous
distribution of hypoxic (increased 18F-FDG uptake) and
highly proliferative (increased 18F-FLT uptake) cancer
cells; in agreement with previously reported preclinical results
(13–16).
18F-FDG PET/CT is widely used in clinical
practice for the detection of malignancies. However, it is not a
cancer-specific tracer as it accumulates in hypoxic tissues
regardless of malignant phenotype (10,13,15). Even
though benign lesions present mainly low 18F-FDG uptake,
in certain cases increased 18F-FDG accumulation is
observed in inflammatory diseases including tuberculosis. Activated
macrophages and other inflammatory cells may result in enhanced
18F-FDG accumulation in benign conditions including
pneumonia, bronchiectasis, pulmonary tuberculosis, fungal
infections, sarcoidosis, histoplasmosis and granuloma (21,22).
Macrophages and other inflammatory cells, frequently observed in
necrotic regions of inflammatory lesions, accumulate increased
levels of 18F-FDG possibly due to the hypoxic
microenvironment (23).
In the present study, 18F-FDG and
18F-FLT PET/CT scan uptake, performed with a 3-day
interval, were compared in patients with lung lesions. A mismatch
in the intralesional 18F-FLT and 18F-FDG
accumulation was observed particularly in lung malignancies
compared with benign lesions (Table
I). Therefore, on the basis of the results of the present
study, it is suggested that this mismatch may serve as an indicator
of lung malignancy.
In well-differentiated slow-growing tumors,
including bronchiole alveolar carcinomas, false-negative
18F-FDG PET results have been reported (20,24). This
may be attributed to to the absence of hypoxic microenvironment of
slow-growing malignancies“18F-FDG is mainly considered
as a hypoxia-specific rather than a tumor avid tracer (13,15,16). This
explains why 18F-FDG exhibited relatively low
specificity in distinguishing malignant from benign lesions.
It has been demonstrated that the combination of
18F-FLT and 18F-FDG, either as separate PET
scans performed on subsequent days or as one scan using a
18F-FLT and 18F-FDG cocktail, may be superior
to an 18F-FDG scan for accurate disease detection
(14). 18F-FDG mainly
accumulates in hypoxic regions, whereas 18F-FLT
accumulates in highly proliferating cells (6,7,13,14). The
use of 18F-FLT and 18F-FDG cocktail PET may
have an advantage compared with individual tracer PET. A clinical
trial for 18F-FLT and 18F-FDG cocktail PET
scanning for cancer detection and management is currently underway
(25).
The results of the present study demonstrate that
mismatched intratumoral distribution of 18F-FLT and
18F-FDG is a common feature of patients with lung cancer
and may serve as an indicator of lung malignancy.
Acknowledgements
The authors would like to thank Dr Cheng Wang, Mr.
Baoliang Bao and Dr Chunmei Wang (Department of Nuclear Medicine,
Affiliated Hospital of Inner Mongolian Medical University, Hohhot,
China) for their technical assistance. The present study was
supported by the Natural Science Foundation of Inner Mongolia
(grant no. 2013MS1188), the Scientific Research Project of the
Affiliated Hospital of Inner Mongolian Medical University (grant
no. 2014NYFYYB008), the Inner Mongolian Major Basic Science
Research Program (grant no. 201503001) and the Inner Mongolian
Science and Technology Innovation Project (grant no.
2015cztcxyd03). Part of the present study was presented at The
Society of Nuclear Medicine and Molecular Imaging 2015 Annual
Meeting (Baltimore, MD, USA; 4–10 June 2015) (26).
References
|
1
|
Herrmann K, Erkan M, Dobritz M, Schuster
T, Siveke JT, Beer AJ, Wester HJ, Schmid RM, Friess H, Schwaiger M,
et al: Comparison of 3′-deoxy-3′-[18F]fluorothymidine positron
emission tomography (FLT PET) and FDG PET/CT for the detection and
characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging.
39:846–851. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakajo M, Nakajo M, Kajiya Y, Jinguji M,
Mori S, Aridome K, Suenaga T and Tanaka S: High FDG and low FLT
Uptake in a thyroid papillary carcinoma incidentally discovered by
FDG PET/CT. Clin Nucl Med. 37:607–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zander T, Scheffler M, Nogova L, Kobe C,
Engel-Riedel W, Hellmich M, Papachristou I, Toepelt K, Draube A,
Heukamp L, et al: Early prediction of nonprogression in advanced
non-small-cell lung cancer treated with erlotinib by using
[(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron
emission tomography. J Clin Oncol. 29:1701–1708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frings V, de Langen AJ, Smit EF, van
Velden FH, Hoekstra OS, van Tinteren H and Boellaard R:
Repeatability of metabolically active volume measurements with
18F-FDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med.
51:1870–1877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang W, Zhang Y, Fu Z, Yu J, Sun X, Mu D
and Han A: Imaging of proliferation with 18F-FLT PET/CT versus
18F-FDG PET/CT in non-small-cell lung cancer. Eur J Nucl Med Mol
Imaging. 37:1291–1299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto Y, Nishiyama Y, Ishikawa S,
Nakano J, Chang SS, Bandoh S, Kanaji N, Haba R, Kushida Y and
Ohkawa M: Correlation of 18F-FLT and 18F-FDG uptake on PET with
Ki-67 immunohistochemistry in non-small cell lung cancer. Eur J
Nucl Med Mol Imaging. 34:1610–1616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buck AK, Halter G, Schirrmeister H,
Kotzerke J, Wurziger I, Glatting G, Mattfeldt T, Neumaier B, Reske
SN and Hetzel M: Imaging proliferation in lung tumors with PET:
18F-FLT versus 18F-FDG. J Nucl Med. 44:1426–1431. 2003.PubMed/NCBI
|
|
8
|
Burgman P, O'Donoghue JA, Humm JL and Ling
CC: Hypoxia-induced increase in FDG uptake in MCF7 cells. J Nucl
Med. 42:170–175. 2001.PubMed/NCBI
|
|
9
|
Pugachev A, Ruan S, Carlin S, Larson SM,
Campa J, Ling CC and Humm JL: Dependence of FDG uptake on tumor
microenvironment. Int J Radiat Oncol Biol Phys. 62:545–553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XF, Ma Y, Sun X, Humm JL, Ling CC and
O'Donoghue JA: High 18F-FDG uptake in microscopic peritoneal tumors
requires physiologic hypoxia. J Nucl Med. 51:632–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dence CS, Ponde DE, Welch MJ and Lewis JS:
Autoradiographic and small-animal PET comparisons between
(18)F-FMISO, (18)F-FDG, (18)F-FLT and the hypoxic selective
(64)Cu-ATSM in a rodent model of cancer. Nucl Med Biol. 35:713–720.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mudd SR, Holich KD, Voorbach MJ, Cole TB,
Reuter DR, Tapang P, Bukofzer G, Chakravartty A, Donawho CK, Palma
JP, et al: Pharmacodynamic evaluation of irinotecan therapy by FDG
and FLT PET/CT imaging in a colorectal cancer xenograft model. Mol
Imaging Biol. 14:617–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang T, Civelek AC, Li J, Jiang H, Ng CK,
Postel GC, Shen B and Li XF: Tumor microenvironment-dependent
18F-FDG, 18F-fluorothymidine, and 18F-misonidazole uptake: A pilot
study in mouse models of human non-small cell lung cancer. J Nucl
Med. 53:1262–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XF, Huang T, Jiang H, Wang X, Shen B,
Wang X, Ng CK, Postel GC and Civelek AC: Combined injection of
(18)F-fluorodeoxyglucose and 3′-Deoxy-3′-[(18)F]fluorothymidine PET
achieves more complete identification of viable lung cancer cells
in mice and patients than individual radiopharmaceutical: A
proof-of-concept study. Transl Oncol. 6:775–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XF, Du Y, Ma Y, Postel GC and Civelek
AC: (18)F-fluorodeoxyglucose uptake and tumor hypoxia: Revisit
(18)F-fluorodeoxyglucose in oncology application. Transl Oncol.
7:240–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang G, Li J, Wang X, Ma Y, Yin X, Wang
F, Zheng H, Duan X, Postel GC and Li XF: The reverse Warburg effect
and 18F-FDG uptake in non-small cell lung cancer A549 in mice: A
pilot study. J Nucl Med. 56:607–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bunyaviroch T and Coleman RE: PET
evaluation of lung cancer. J Nucl Med. 47:451–469. 2006.PubMed/NCBI
|
|
18
|
Graves EE, Maity A and Le QT: The tumor
microenvironment in non-small-cell lung cancer. Semin Radiat Oncol.
20:156–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vesselle H, Grierson J, Muzi M, Pugsley
JM, Schmidt RA, Rabinowitz P, Peterson LM, Vallières E and Wood DE:
In vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine
([(18)F]FLT) as a proliferation imaging tracer in humans:
Correlation of [(18)F]FLT uptake by positron emission tomography
with Ki-67 immunohistochemistry and flow cytometry in human lung
tumors. Clin Cancer Res. 8:3315–3323. 2002.PubMed/NCBI
|
|
20
|
Tian J, Yang X, Yu L, Chen P, Xin J, Ma L,
Feng H, Tan Y, Zhao Z and Wu W: A multicenter clinical trial on the
diagnostic value of dual-tracer PET/CT in pulmonary lesions using
3′-deoxy-3′-18F-fluorothymidine and 18F-FDG. J Nucl Med.
49:186–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Waarde A, Cobben DC, Suurmeijer AJ,
Maas B, Vaalburg W, de Vries EF, Jager PL, Hoekstra HJ and Elsinga
PH: Selectivity of 18F-FLT and 18F-FDG for differentiating tumor
from inflammation in a rodent model. J Nucl Med. 45:695–700.
2004.PubMed/NCBI
|
|
22
|
Grierson JR and Shields AF: Radiosynthesis
of 3′-deoxy-3′-[(18)F]fluorothymidine: [(18)F]FLT for imaging of
cellular proliferation in vivo. Nucl Med Biol. 27:143–156. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarkin JM, Joshi FR and Rudd JH: PET
imaging of inflammation in atherosclerosis. Nat Rev Cardiol.
11:443–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi
M, Noguchi T, Taniguchi M, Tonami H, Okimura T and Yamamoto I:
Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung
carcinoma. J Nucl Med. 39:1016–1020. 1998.PubMed/NCBI
|
|
25
|
Kurdziel K, Ravizzini G, Croft B, Tatum J,
Choyke P and Kobayashi H: The evolving role of nuclear molecular
imaging in cancer. Expert Opin Med Diagn. 2:829–842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Wang X, Zhao Z and Li XF: The
mismatched intratumoral distribution of 18F-FLT and
18F-FDG may be a better indicator of malignancy: A
PET/CT study in patients with solitary pulmonary nodule. J Nucl
Med. 56 Suppl 3:S1242015.
|