Introduction
The American Cancer Society stated at the 2014
American Society of Clinical Oncology annual meeting that breast,
lung and colon cancer were the most common types of cancer observed
in females; breast cancer exhibited the highest incidence (29%) and
second highest mortality rate (15%) (1). It is reported that China exhibits one of
the fastest growing incidences of breast cancer; increasing in
recent years at 3% annually, breast cancer has become the leading
cause of mortality in urban females in China (2). Despite marked progress in long-term
survival, early diagnosis and treatment of breast cancer, the
prognosis of patients with advanced cancer remains poor and
heterogeneous (3). The earlier the
diagnosis, the better the prognosis for the patient with breast
cancer. Although there have been numerous biological markers
identified to assist breast cancer diagnosis including Her2/neu,
estrogen receptor (ER) and progesterone receptor (PR) (4–6), the
identification of further biological markers is required
urgently.
Docking protein 2 (Dok2) is a member of the DOK
adaptor protein family that functions in feedback loops to modulate
tyrosine kinase signaling, involving a number of tyrosine kinase
receptors including epidermal growth factor receptor,
platelet-derived growth factor receptor, c-Kit, Tie2 and human
epidermal growth factor receptor 2 (Her2)/neu (7,8). A
previous study demonstrated the clinical significance of Dok2 in
the prognostic evaluation of patients with gastric cancer (9). A previous study demonstrated that Dok2
may potentially be used as a marker of poor prognosis in patients
with colorectal cancer following curative resection (10).
Ras p21 protein activator 1 (RASA1) is a mediator
between Ras-GTP and Ras-GDP and may decrease cellular proliferation
through the Ras/rapidly accelerated fibrosarcoma/mitogen-activated
protein kinase kinase/extracellular-signal-regulated kinase pathway
(11,12). Previous studies have identified that
RASA1 may be a potential tumor suppressor (13,14).
The aim of the present study was to assess whether
Dok2 and RASA1 are dysregulated in breast cancer using analytical
clinicopathological features and their potential value in the
prognosis of patients with breast cancer. The results of the
present study demonstrated that downregulation of Dok2 and RASA1 in
the tissues was associated with clinicopathological features,
suggesting that they may serve as independent prognostic factors
for patients following surgery.
Materials and methods
Patients
Between October 2008 and March 2013, a total of 285
patients, histopathologically diagnosed with breast cancer,
underwent surgery at Jingzhou Central Hospital (Jingzhou, China).
Following surgery, patients were followed up every 3 months and
administered appropriate clinical examinations. A total of 4 frozen
samples (N1-N4) selected from the 285 patients were analyzed using
western blotting. The average patient age was 54.8 (range, 25–87
years). The Ethics Committee of Yangtze University approved the
present study protocol and all patients provided written informed
consent.
Immunohistochemical staining
Dok2 and RASA1 were detected using
immunohistochemical staining as described previously (10). The 3.0 µm breast cancer tissue and
normal breast mucosa sections were heated at 12°C for 20 min in
EDTA-Tris buffer, pH 9.0, for antigen retrieval following
deparaffinization in xylene and dehydration in graded ethanol
solutions. Endogenous peroxidase activity was blocked by incubating
the sections with 30 ml/l H2O2 for 20 min.
Following incubation with a primary mouse anti-Dok2 (dilution
1:200, sc-17830; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or a mouse anti-RASA1 (dilution 1:200, ab-40677; Abcam, Cambridge,
UK) monoclonal antibody at 4°C overnight, staining was performed
using the labeled streptavidin-biotin method. Negative controls of
immunohistochemical reactions were established through omission of
the primary antibody. Lymphocytes were used as positive control.
Dok2 and RASA1 staining was judged to be positive when the cancer
cells in the section demonstrated immunoreactivity to Dok2 and
RASA1. All slides were assessed independently by two pathologists
and any disagreements were resolved by consensus. Pathologists were
blinded to the clinicopathological data.
Western blot analysis
Proteins of tissues were resolved by SDS-PAGE (10%
gels) and transferred onto a polyvinylidene membrane (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 3%
fat-free milk dissolved in PBS-T, and incubated with antibodies
against RASA1 (1:500 dilution, ab-40677; Abcam), Dok2 (1:500
dilution, sc-17830; Santa Cruz Biotechnology) and β-actin (1:1,000
dilution, sc-47778; Santa Cruz Biotechnology) overnight at 4°C.
Next, an appropriate secondary antibody (dilution 1:5,000, cat.
nos. BA1075 and BA1055, anti-mouse or anti-rabbit IgG,
respectively; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) was applied for 1 h at room temperature. Immunoreactivity
was detected using an enhanced chemiluminescent kit (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and analyzed with a
GS-700 Imaging Densitometer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
Associations between Dok2 and RASA1 expression and
various clinicopathological parameters were evaluated using the
χ2 and Fisher's exact probability test. Prognostic
variables were assessed using a log-rank test and disease-free
survival rate (DFS) was analyzed using the Kaplan-Meier estimator
method. In the multivariate analysis, a Cox's proportional hazard
model was employed. P<0.05 was considered to indicate a
statistically significant difference. The statistical analyses were
performed using SPSS (version 22.0; IBM Corp., Armonk, NY,
USA).
Results
Immunohistochemical tissue staining
for Dok2 and RASA1
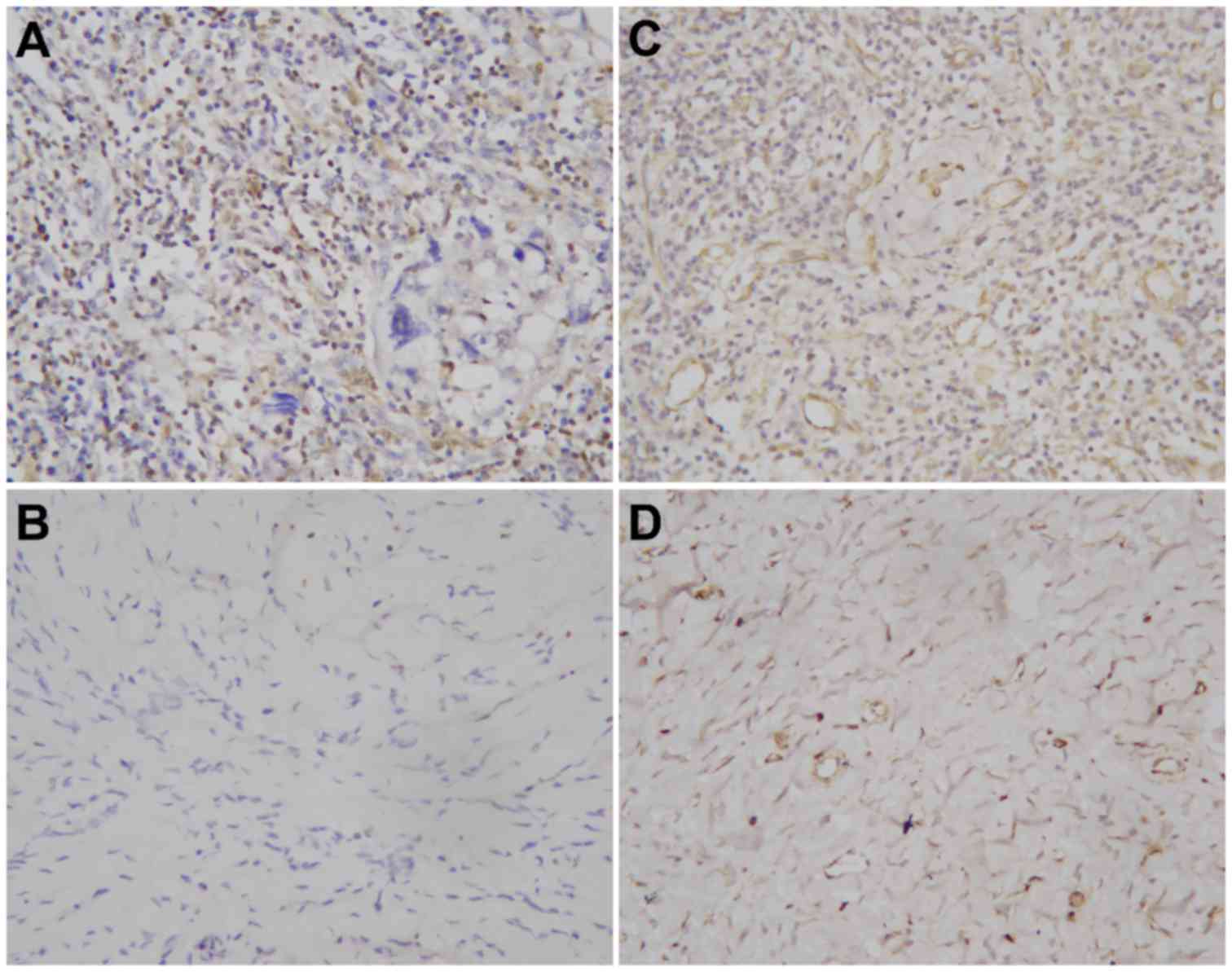
Dok2 and RASA1 staining was primarily observed in
the nuclei and cytoplasm of the breast tumor cells. Additionally,
94 (33.0%) patients exhibited positive levels of Dok2, with
decreased Dok2 immunostaining intensity observed in the breast
cancer tissue samples diagnosed as poorly differentiated
adenocarcinoma compared with the remaining moderately
differentiated adenocarcinoma samples (Fig. 1A and B). RASA1 demonstrated comparable
staining characteristics, with 89 (31.2%) of breast tumor samples
exhibiting positive levels, while presenting as markedly weaker in
poorly differentiated adenocarcinoma compared with moderately
differentiated adenocarcinoma (Fig. 1C
and D).
Expression of Dok2 and RASA1 protein
in breast cancer determined using western blot analysis
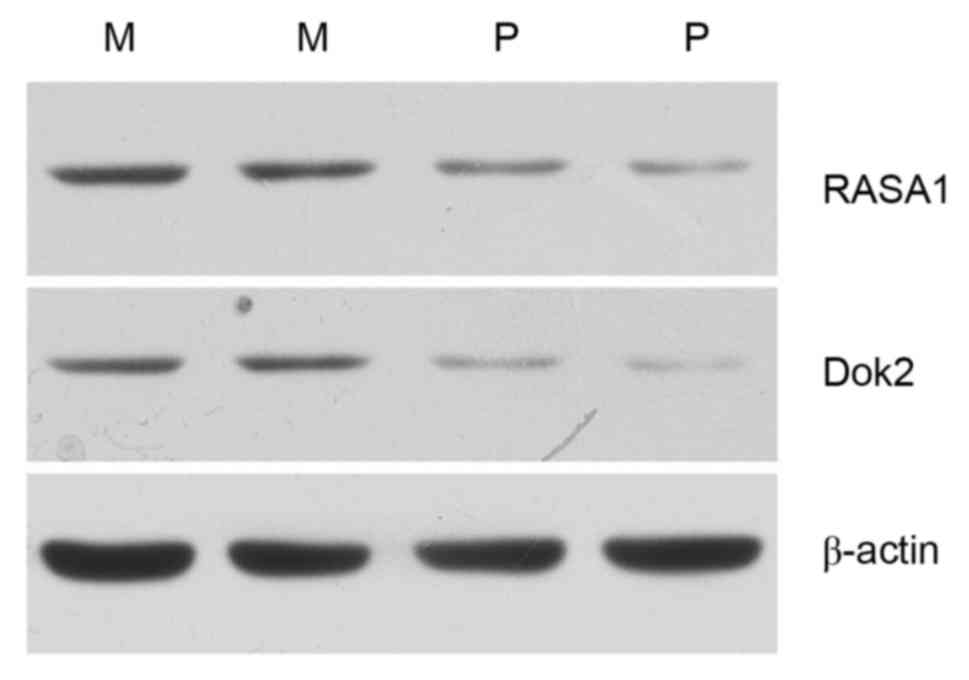
The results of the western blot analysis were
consistent with the results of the immunohistochemical staining.
Dok2 and RASA1 expression was markedly decreased in two poorly
differentiated adenocarcinoma samples compared with two moderately
differentiated adenocarcinoma samples (Fig. 2).
Association between Dok2 expression
and clinicopathological parameters
All breast cancer samples were grouped as either
Dok2-positive or -negative. Notably, patients with Dok2-negative
breast cancer exhibited poor histological differentiation and
increased tumor size. The positive group exhibited an increased
proportion of axillary lymph node metastasis, later clinical
staging and was associated with the expression of ER. No
significant differences in other clinical characteristics including
age, pathological type and expression of HER-2 were identified
(Fisher's exact test, P>0.05; Table
I).
 | Table I.Association between Dok2 expression
and various clinicopathological parameters. |
Table I.
Association between Dok2 expression
and various clinicopathological parameters.
|
| Dok2
expression |
|
|
|---|
|
|
|
|
|
|---|
| Parameter | Positive | Negative | χ2 | P-value |
|---|
| All cases | 94 | 191 |
|
|
| Age, years |
|
| 0.093 | 0.76 |
|
≤55 | 52 | 102 |
|
|
|
>55 | 42 | 89 |
|
|
| Tumor size, cm |
|
| 6.131 | 0.013 |
| ≤2 | 56 | 84 |
|
|
|
>2 | 38 | 107 |
|
|
| LN metastasis |
|
| 8.424 | 0.015 |
| No | 56 | 79 |
|
|
|
Yes | 36 | 105 |
|
|
|
Unknown | 2 |
7 |
|
|
| Histological
grade |
|
| 7.804 | 0.020 |
|
≤II | 57 | 83 |
|
|
|
>II | 33 | 100 |
|
|
|
Unknown | 4 |
8 |
|
|
| Clinical stage |
|
| 9.106 | 0.011 |
| I | 50 | 66 |
|
|
| II | 27 | 79 |
|
|
|
III | 17 | 46 |
|
|
| ER |
|
| 9.016 | 0.011 |
|
Negative | 57 | 82 |
|
|
|
Positive | 32 | 101 |
|
|
|
Unknown | 5 |
8 |
|
|
| HER-2 |
|
| 5.512 | 0.064 |
|
Negative | 33 | 75 |
|
|
|
Positive | 51 | 109 |
|
|
|
Unknown | 10 |
7 |
|
|
| Tumor type |
|
| 0.085 | 0.771 |
|
IDC | 80 | 160 |
|
|
|
Non-IDC | 14 | 31 |
|
|
| Molecular
subtype |
|
| 5.282 | 0.022 |
| Triple
negative | 17 | 59 |
|
|
|
Other | 77 | 132 |
|
|
Association between RASA1 expression
and clinicopathological parameters
The samples were grouped as RASA1-positive or
-negative. Notably, the patients with RASA1-negative breast cancer
exhibited poor histological differentiation and increased tumor
size. The RASA1-positive group exhibited an increased proportion of
axillary lymph node metastasis, later clinical staging and was
associated with the expression of ER. No significant differences in
other clinical characteristics including age, pathological type and
expression of HER-2 were identified (Fisher's exact test,
P>0.05; Table II).
 | Table II.Association between RASA1 expression
and various clinicopathological parameters. |
Table II.
Association between RASA1 expression
and various clinicopathological parameters.
|
| RASA1
expression |
|
|
|---|
|
|
|
|
|
|---|
| Parameter | Positive | Negative | χ2 | P-value |
|---|
| All cases | 89 | 196 |
|
|
| Age, years |
|
| 0.288 | 0.592 |
|
≤55 | 46 | 108 |
|
|
|
>55 | 43 | 88 |
|
|
| Tumor size, cm |
|
| 5.496 | 0.019 |
| ≤2 | 56 | 94 |
|
|
|
>2 | 33 | 102 |
|
|
| LN metastasis |
|
| 8.092 | 0.017 |
| No | 53 | 82 |
|
|
|
Yes | 33 | 102 |
|
|
|
Unknown | 3 |
9 |
|
|
| Histological
grade |
|
| 8.334 | 0.016 |
|
≤II | 55 | 85 |
|
|
|
>II | 31 | 102 |
|
|
|
Unknown | 3 |
9 |
|
|
| Clinical stage |
|
| 8.023 | 0.018 |
| I | 44 | 72 |
|
|
| II | 34 | 75 |
|
|
|
III | 11 | 52 |
|
|
| ER |
|
| 9.088 | 0.011 |
|
Negative | 53 | 86 |
|
|
|
Positive | 30 | 103 |
|
|
|
Unknown | 6 |
7 |
|
|
| HER-2 |
|
| 3.666 | 0.160 |
|
Negative | 28 | 80 |
|
|
|
Positive | 53 | 107 |
|
|
|
Unknown | 8 |
9 |
|
|
| Tumor type |
|
| 0.136 | 0.712 |
|
IDC | 76 | 164 |
|
|
|
Non-IDC | 13 | 32 |
|
|
| Molecular
subtype |
|
| 4.996 | 0.025 |
| Triple
negative | 16 | 60 |
|
|
|
Other | 73 | 136 |
|
|
| Dok2 |
|
| 8.377 | 0.004 |
|
Negative | 49 | 142 |
|
|
|
Positive | 40 | 54 |
|
|
Association between Dok2/RASA1
expression and clinical outcome
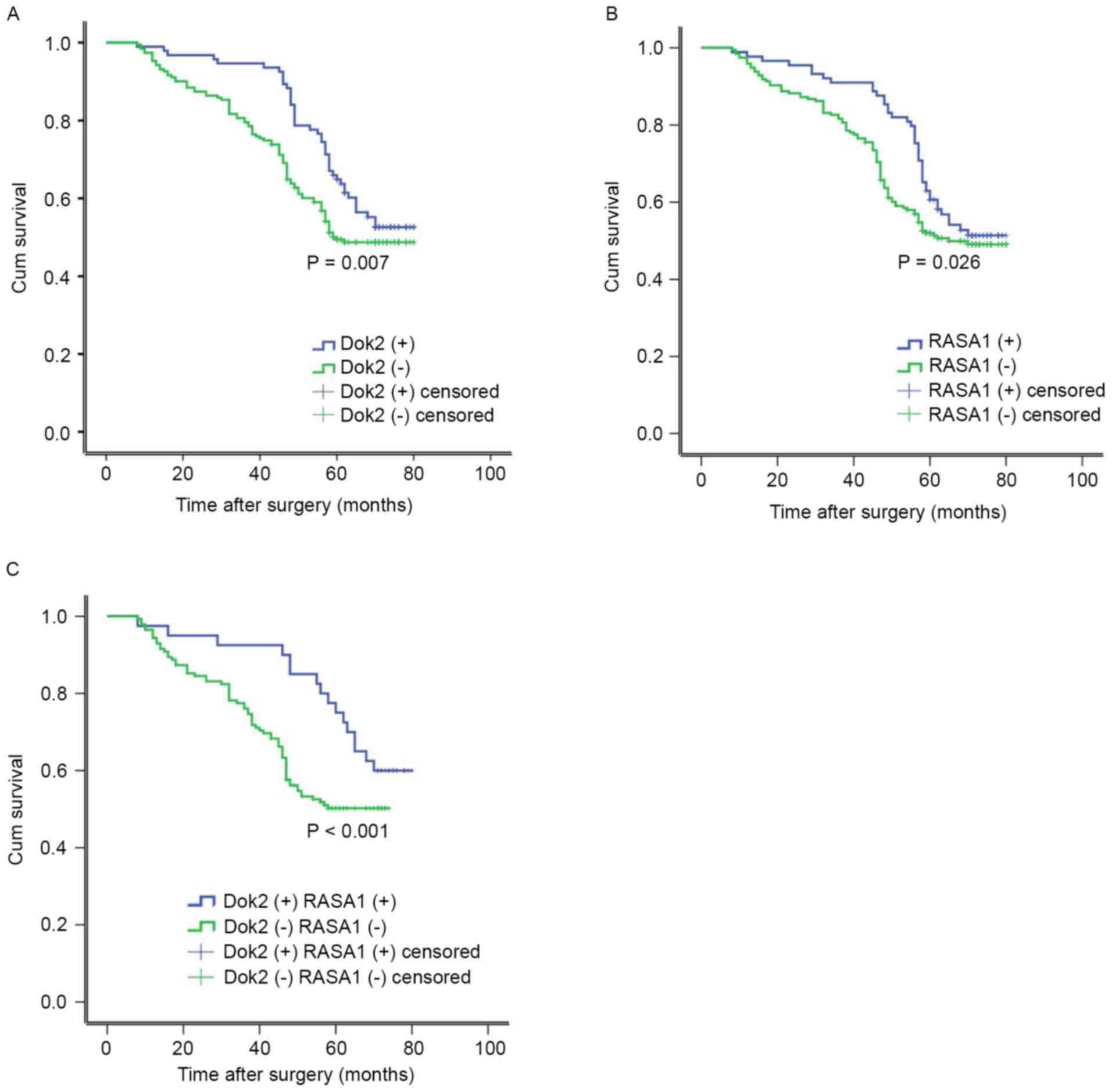
Disease relapse following surgery was diagnosed in
84/285 patients (29.5%), with a median time to relapse of 19.2
months. DFS was decreased in patients with Dok2-negative tumors
compared with Dok2-positive (P=0.007, log-rank test; Fig. 3A). Additionally, the group without
detectable RASA1 expression was markedly associated with decreased
DFS among 196 patients (P=0.026, log-rank test; Fig. 3B). Comparing the association between
Dok2 or RASA1 expression with patient outcome, DOK2 and RASA1
negative expression was associated with the poorer outcome [Dok2
(−) RASA1 (−) 78.0%, Dok2 (+) RASA1 (+) 22.0%, P<0.001, log-rank
test] (Fig. 3C). These results
indicated a statistically significant association between
Dok2/RASA1 downregulation and poorer survival rate.
Following the multivariate Cox's proportional hazard
model results, it was identified that decreased Dok2 (HR, 0.454;
95% CI, 0.297–0.735; P=0.001) and RASA1 (HR, 0.825; 95% CI,
0.584–1.216; P=0.018) expression were independent prognostic
factors for DFS in patients with breast cancer. In addition, the
proportion of axillary lymph node metastases and histological grade
were associated with the prognosis of breast cancer in which the
high node metastasis was the most effective in DFS (HR, 1.233; 95%
CI, 0.815–0.1.789; P=0.005). Although the ER and tumor size were
associated with decreased Dok2 and RASA1 expression, the
multivariate analysis indicated that neither were independent
prognostic factors in breast cancer (Table III).
 | Table III.Multivariate independent prognostic
factor analyses of overall survival in 285 patients with breast
cancer. |
Table III.
Multivariate independent prognostic
factor analyses of overall survival in 285 patients with breast
cancer.
| Parameters | HR | 95% CI | P-value |
|---|
| Tumor size (≤2
cm/>2 cm) | 0.915 | 0.645–1.328 | 0.725 |
| LN metastasis
(no/yes) | 1.233 | 0.815–1.789 | 0.005 |
| Histological grade
(≤II/>II) | 1.456 | 0.976–2.024 | 0.023 |
| ER (−/+) | 0.768 | 0.489–1.115 | 0.185 |
| Dok2 (−/+) | 0.454 | 0.297–0.735 | 0.001 |
| RASA1 (−/+) | 0.625 | 0.484–1.016 | 0.018 |
Discussion
Breast cancer is the most common type of cancer and
the second leading cause of cancer-associated mortality among
females in Asia, accounting for 39% of all breast cancers diagnosed
worldwide (15). Although marked
progress has been made in treatment strategy, the survival rate of
patients with late-stage breast cancer remains poor. Therefore,
research into appropriate tumor markers for early diagnosis of
breast cancer is urgently required.
The tumor suppressor gene Dok2 has been identified
in lung cancer (16), acute leukemias
(17), chronic myelomonocytic
leukemia (18), and gastric and
colorectal cancers (19).
Additionally, Dok2 acts as a marker of poor prognosis in patients
with colorectal cancer and gastric adenocarcinoma following
curative resection (9,10). Dok2 inhibits epidermal growth factor
receptor-mutated lung adenocarcinoma in mouse models (20). Loss of Dok2 induces chemotherapy
resistance by decreasing the level of apoptosis in response to
treatment (21). Although Dok2 was
identified as a cancer marker using the plasma antibody test in
breast cancer (22), its expression
in breast cancer and its association with clinicopathological
features require investigation.
Ras, a small GTP-binding protein that is frequently
mutated in human cancers, is regulated by Ras GTPase-activating
proteins (RasGAPs); inactivation of RasGAPs may increase the risk
of tumor development (23). RASA1 (a
GTPase-activating protein), also called p120RasGAP, was the first
RasGAP protein to be identified. In addition to numerous biological
roles including actin filament polymerization, vascular
development, cellular apoptosis and cell motility (24,25), the
role of RASA1 as a tumor suppressor has gained increased attention
and research time. RASA1 was first identified as a tumor suppressor
in the acute myelogenous tumor line HL-60 following
microarray-based comparative genome hybridization studies in 2003
(26) prior to being observed in
breast cancer (12,14,27), liver
cancer (28,29), colorectal cancer (11,13,30–32),
lung cancer (33,34), prostate cancer (35,36),
cutaneous squamous cell carcinoma (37), gastric cancer (38), acute lymphoblastic leukemia (39), spinal cancer (40), papillary thyroid carcinoma (41), gastroenteropancreatic neuroendocrine
(42) and pancreatic cancer (43) in succession. Dok2 may upregulate RASA1
expression and the two were associated with the tumor gene Ras
(44).
The present study investigated the association
between Dok2/RASA1 expression and the clinicopathological features
of breast cancer. Using immunohistochemistry and western blot
analysis, it was revealed that weak expression of Dok2/RASA1 was
associated with poorly differentiated breast adenocarcinomas.
Further results indicated that negative expression of Dok2/RASA1
was associated with increased tumor size, increased rate of lymph
node metastasis and later clinical staging. Absence of Dok2 or
RASA1 may lead to Ras/extracellular-signal-regulated kinase
signaling cascade activation, resulting in abnormal cell cycle
processes (45,46). Additionally, the negative expression
of RASA1 was associated with negative Dok2 expression
(χ2=8.377, P=0.004), indicating that RASA1 may regulate
Dok2 expression (44); however,
further studies are required to support this. Dok2 and RASA1 are
both tumor suppressors and, combined, their detection may improve
diagnosis sensitivity in breast cancer.
Survival analysis indicated that Dok2 and RASA1 may
be independent prognostic factors for DFS in patients with breast
cancer, and combined negative Dok2/RASA1 expression was the most
promising unfavorable prognostic factor in DFS, offering
therapeutic potential for diagnosis. Cox's regression analysis was
applied to identify significant prognostic factors alongside
Kaplan-Meier estimator analysis. Results of the present study
revealed that downregulation of Dok2 and RASA1 are associated with
poor outcome and relapse of breast cancer; the DFS hazard ratio for
Dok2 was 0.454 (P<0.01) and the DFS hazard ratio for RASA1 was
0.625 (P<0.05), indicating that patients with Dok2- or
RASA1-positive cancer have a 54.6 and 37.5% decreased risk of
relapse compared with patients negative for Dok2 or RASA1. The
results of the present study also revealed that lymph node
metastasis and histological grade may be the significant prognostic
factors; however, no significant association with ER was identified
(47).
In conclusion, the results of the present study
demonstrated that combined downregulation of Dok2 and RASA1 is
associated with breast cancer progression, recurrence and poor
survival rate. Therefore, Dok2/RASA1 combined detection may be an
effective predictor of prognosis and a novel therapeutic target for
patients with breast cancer.
Acknowledgements
The present study was supported by the Nature
Science Foundation of Hubei Province (grant no. 2015CFB320), the
Research Project of Hubei Provincial Education Department (grant
no. D20121204), Hubei Province Health and Family Planning
Scientific Research Project (grant no. WJ2016-Y-10), the Medical
School Youth Fund of Yangtze University (grant no. YXYQ201411) and
the Yangtze Youth Fund (grant no. 2015cqn79).
References
|
1
|
Crozier JA and Perez EA: Perspectives from
the American Society of Clinical Oncology 2014 Conference: Breast
cancer highlights. Future Oncol. 10:1897–1899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zubeda S, Kaipa PR, Shaik NA, Mohiuddin
MK, Vaidya S, Pavani B, Srinivasulu M, Latha MM and Hasan Q:
Her-2/neu status: A neglected marker of prognostication and
management of breast cancer patients in India. Asian Pac J Cancer
Prev. 14:2231–2235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clifton GT, Mittendorf EA and Peoples GE:
Adjuvant HER2/neu peptide cancer vaccines in breast cancer.
Immunotherapy. 7:1159–1168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santana AB, Gurgel MS, de Oliveira
Montanari JF, Bonini FM and de Barros-Mazon S: Serum amyloid is
associated with obesity and estrogen receptor-negative tumors in
postmenopausal women with breast cancer. Cancer Epidemiol
Biomarkers Prev. 22:270–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen T, Brandwein-Gensler M, Hameed O,
Siegal GP and Wei S: Characterization of estrogen
receptor-negative/progesterone receptor-positive breast cancer. Hum
Pathol. 46:1776–1784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shapochka DO, Zaletok SP and Gnidyuk MI:
Relationship between NF-κB, ER, PR, Her2/neu, Ki67, p53 expression
in human breast cancer. Exp Oncol. 34:358–363. 2012.PubMed/NCBI
|
|
8
|
Mashima R, Arimura S, Kajikawa S, Oda H,
Nakae S and Yamanashi Y: Dok adaptors play anti-inflammatory roles
in pulmonary homeostasis. Genes Cells. 18:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyagaki H, Yamasaki M, Takahashi T,
Kurokawa Y, Miyata H, Nakajima K, Takiguchi S, Fujiwara Y, Mori M
and Doki Y: DOK2 as a marker of poor prognosis of patients with
gastric adenocarcinoma after curative resection. Ann Surg Oncol.
19:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen X, Zhou M, Guo Y, Zhu Y, Li H, Zhang
L, Yu L, Wang X and Peng X: Expression and significance of DOK2 in
colorectal cancer. Oncol Lett. 9:241–244. 2015.PubMed/NCBI
|
|
11
|
Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J,
Zhang CY, Chen J and Zhang J: MicroRNA-31 activates the RAS pathway
and functions as an oncogenic MicroRNA in human colorectal cancer
by repressing RAS p21 GTPase activating protein 1 (RASA1). J Biol
Chem. 288:9508–9518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma SB, Lin CC, Farrugia MK, McLaughlin
SL, Ellis EJ, Brundage KM, Salkeni MA and Ruppert JM: MicroRNAs 206
and 21 cooperate to promote RAS-extracellular signal-regulated
kinase signaling by suppressing the translation of RASA1 and
SPRED1. Mol Cell Biol. 34:4143–4164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: miR-21/RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liu T, Sun Q, Niu M, Jiang Y and
Pang D: Downregulation of Ras GTPase-activating protein 1 is
associated with poor survival of breast invasive ductal carcinoma
patients. Oncol Rep. 33:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan L, Goss PE and Strasser-Weippl K:
Current status and future projections of breast cancer in Asia.
Breast Care (Basel). 10:372–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berger AH, Niki M, Morotti A, Taylor BS,
Socci ND, Viale A, Brennan C, Szoke J, Motoi N, Rothman PB, et al:
Identification of DOK genes as lung tumor suppressors. Nat Genet.
42:216–223. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MS, Chung NG, Yoo NJ and Lee SH:
Mutational analysis of DOK2 tumor suppressor gene in acute
leukemias. Leuk Res. 35:e87–e88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coppin E, Gelsi-Boyer V, Morelli X,
Cervera N, Murati A, Pandolfi PP, Birnbaum D and Nunès JA:
Mutational analysis of the DOK2 haploinsufficient tumor suppressor
gene in chronic myelomonocytic leukemia (CMML). Leukemia.
29:500–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
An CH, Kim MS, Yoo NJ and Lee SH:
Mutational and expressional analysis of a haploinsufficient tumor
suppressor gene DOK2 in gastric and colorectal cancers. APMIS.
119:562–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berger AH, Chen M, Morotti A, Janas JA,
Niki M, Bronson RT, Taylor BS, Ladanyi M, Van Aelst L, Politi K, et
al: DOK2 inhibits EGFR-mutated lung adenocarcinoma. PLoS One.
8:e795262013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lum E, Vigliotti M, Banerjee N, Cutter N,
Wrzeszczynski KO, Khan S, Kamalakaran S, Levine DA, Dimitrova N and
Lucito R: Loss of DOK2 induces carboplatin resistance in ovarian
cancer via suppression of apoptosis. Gynecol Oncol. 130:369–376.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Figueroa JD, Wallstrom G, Barker
K, Park JG, Demirkan G, Lissowska J, Anderson KS, Qiu J and LaBaer
J: Plasma autoantibodies associated with basal-like breast cancers.
Cancer Epidemiol Biomarkers Prev. 24:1332–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vigil D, Cherfils J, Rossman KL and Der
CJ: Ras superfamily GEFs and GAPs: Validated and tractable targets
for cancer therapy? Nat Rev Cancer. 10:842–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anand S, Majeti BK, Acevedo LM, Murphy EA,
Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN,
Lapinski PE, et al: MicroRNA-132-mediated loss of p120RasGAP
activates the endothelium to facilitate pathological angiogenesis.
Nat Med. 16:909–914. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pamonsinlapatham P, Hadj-Slimane R,
Lepelletier Y, Allain B, Toccafondi M, Garbay C and Raynaud F:
p120-Ras GTPase activating protein (RasGAP): A multi-interacting
protein in downstream signaling. Biochimie. 91:320–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ulger C, Toruner GA, Alkan M, Mohammed M,
Damani S, Kang J, Galante A, Aviv H, Soteropoulos P, Tolias PP, et
al: Comprehensive genome-wide comparison of DNA and RNA level scan
using microarray technology for identification of candidate
cancer-related genes in the HL-60 cell line. Cancer Genet
Cytogenet. 147:28–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu X, Stern HM, Ge L, O'Brien C, Haydu L,
Honchell CD, Haverty PM, Peters BA, Wu TD, Amler LC, et al: Genetic
alterations and oncogenic pathways associated with breast cancer
subtypes. Mol Cancer Res. 7:511–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM, Factor VM and Thorgeirsson SS: Inactivation of
Ras GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du C, Weng X, Hu W, Lv Z, Xiao H, Ding C,
Gyabaah OA, Xie H, Zhou L, Wu J and Zheng S: Hypoxia-inducible
miR-182 promotes angiogenesis by targeting RASA1 in hepatocellular
carcinoma. J Exp Clin Cancer Res. 34:672015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Organ SL, Hai J, Radulovich N, Marshall
CB, Leung L, Sasazuki T, Shirasawa S, Zhu CQ, Navab R, Ikura M and
Tsao MS: p120RasGAP is a mediator of rho pathway activation and
tumorigenicity in the DLD1 colorectal cancer cell line. PLoS One.
9:e861032014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun D, Wang C, Long S, Ma Y, Guo Y, Huang
Z, Chen X, Zhang C, Chen J and Zhang J: C/EBP-β-activated
microRNA-223 promotes tumour growth through targeting RASA1 in
human colorectal cancer. Br J Cancer. 112:1491–1500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Y, Yang H, Yuan L, Liu G, Zhang C, Hong
M, Liu Y, Zhou M, Chen F and Li X: Overexpression of miR-335
confers cell proliferation and tumour growth to colorectal
carcinoma cells. Mol Cell Biochem. 412:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu YJ, Xu B and Xia W: Hsa-mir-182
downregulates RASA1 and suppresses lung squamous cell carcinoma
cell proliferation. Clin Lab. 60:155–159. 2014.PubMed/NCBI
|
|
34
|
Liu X, Jia Y, Stoopler MB, Shen Y, Cheng
H, Chen J, Mansukhani M, Koul S, Halmos B and Borczuk AC:
Next-generation sequencing of pulmonary sarcomatoid carcinoma
reveals high frequency of actionable MET gene mutations. J Clin
Oncol. 34:794–802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sowalsky AG, Xia Z, Wang L, Zhao H, Chen
S, Bubley GJ, Balk SP and Li W: Whole transcriptome sequencing
reveals extensive unspliced mRNA in metastatic castration-resistant
prostate cancer. Mol Cancer Res. 13:98–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berndt SI, Wang Z, Yeager M, Alavanja MC,
Albanes D, Amundadottir L, Andriole G, Freeman Beane L, Campa D,
Cancel-Tassin G, et al: Two susceptibility loci identified for
prostate cancer aggressiveness. Nat Commun. 6:68892015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pickering CR, Zhou JH, Lee JJ, Drummond
JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, et
al: Mutational landscape of aggressive cutaneous squamous cell
carcinoma. Clin Cancer Res. 20:6582–6592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Li D, Zhang G, Xiong J, Jie Z, Cheng
H, Cao Y, Jiang M, Lin L, Le Z, et al: Methylation-associated
silencing of MicroRNA-335 contributes tumor cell invasion and
migration by interacting with RASA1 in gastric cancer. Am J Cancer
Res. 4:648–662. 2014.PubMed/NCBI
|
|
39
|
Lubeck BA, Lapinski PE, Oliver JA, Ksionda
O, Parada LF, Zhu Y, Maillard I, Chiang M, Roose J and King PD:
Cutting edge: Codeletion of the Ras GTPase-activating proteins
(RasGAPs) neurofibromin 1 and p120 RasGAP in T cells results in the
development of T cell acute lymphoblastic leukemia. J Immunol.
195:31–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kansal R, Li X, Shen J, Samuel D,
Laningham F, Lee H, Panigrahi GB, Shuen A, Kantarci S, Dorrani N,
et al: An infant with MLH3 variants, FOXG1-duplication and
multiple, benign cranial and spinal tumors: A clinical exome
sequencing study. Genes Chromosomes Cancer. 55:131–142. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rusinek D, Swierniak M, Chmielik E, Kowal
M, Kowalska M, Cyplinska R, Czarniecka A, Piglowski W, Korfanty J,
Chekan M, et al: BRAFV600E-associated gene expression profile:
Early changes in the transcriptome, based on a transgenic mouse
model of papillary thyroid carcinoma. PLoS One. 10:e01436882015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park C, Ha SY, Kim ST, Kim HC, Heo JS,
Park YS, Lauwers G, Lee J and Kim KM: Identification of the BRAF
V600E mutation in gastroenteropancreatic neuroendocrine tumors.
Oncotarget. 7:4024–4035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kent OA, Mendell JT and Rottapel R:
Transcriptional regulation of miR-31 by oncogenic KRAS mediates
metastatic phenotypes by repressing RASA1. Mol Cancer Res.
14:267–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mihrshahi R, Barclay AN and Brown MH:
Essential roles for Dok2 and RasGAP in CD200 receptor-mediated
regulation of human myeloid cells. J Immunol. 183:4879–4886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lapinski PE, Qiao Y, Chang CH and King PD:
A role for p120 RasGAP in thymocyte positive selection and survival
of naive T cells. J Immunol. 187:151–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Downer EJ, Johnston DG and Lynch MA:
Differential role of Dok1 and Dok2 in TLR2-induced inflammatory
signaling in glia. Mol Cell Neurosci. 56:148–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu C, Wang Z, Cui R, He H, Lin X, Sheng Y
and Zhang H: Co-expression of parathyroid hormone related protein
and TGF-beta in breast cancer predicts poor survival outcome. BMC
Cancer. 15:9252015. View Article : Google Scholar : PubMed/NCBI
|