Introduction
In recent years immunoactive agents have raised the
bar for cancer therapies (1,2). In addition to immune checkpoint
inhibitors [such as inhibitors of programmed cell death 1 (PD-1)
and PD-ligand 1, which predominantly target the T-cell crosstalk,
as well as inhibitors of cytotoxic T-lymphocyte-associated protein
4 (CTLA-4)] (3), chimeric antigen
receptor T cells, vaccines (4) and
plant-derived proteins have been extensively studied for their
immunomodulatory activity (5,6). The present study focuses on one of such
plant-derived proteins: A synthesized plant lectin I named
aviscumine. Aviscumine is a heterodimer, which is composed of a
toxic A-chain, representing a site-specific type-II
ribosome-inactivating N-glycosidase, and a carbohydrate-binding
subunit B, responsible for its cellular uptake (7–9). The
N-glycosidase-mediated catalytic inactivation of ribosomes leads to
a time- and dose-dependent inhibition of protein translation and
synthesis (GI50, 1 ng/ml) in various human tumor cell
lines (10–12), independently of cell cycle or
proliferation status (9,12–14). As
well as its direct cytotoxic effect, immunomodulatory activity of
aviscumine was suggested in early clinical trials in which
aviscumine demonstrated a clinical efficacy in various types of
solid tumor with tolerable toxicity profiles (15–18); this
immunomodulatory effect was presumed based on increased levels of
interleukin (IL) 1β, tumor necrosis factor a (TNF-α) and interferon
g (IFN-γ) detected in patient sera during treatment (15). Consistently, the phase I trial
(19) of subcutaneous, low-dose
(nanogram range) aviscumine application demonstrated a clinical
benefit in patients with progressive solid tumors subsequent to
standard treatment failure with a stable disease rate of 31% (8/26
patients; median duration, 17 weeks); this trial detected elevated
IL-1β, TNF-α and IFN-γ and decreased IL-6 and IL-10 levels in
patient sera during the aviscumine treatment (19). The recently published phase II trial
supports the clinical efficacy of aviscumine, as well as its
immunostimulatory activity and potential for combined use with
chemotherapeutics (17,20); however, limited data concerning its
immunological activity, particularly on the innate immune system,
are available.
It has been shown that lectins represent
pathogen-associated molecular patterns (PAMPs) and thereby activate
pattern recognition receptors (PRRs) causing the activation of the
immune system via type-I phagocytic cells (21,22).
Müthing et al (23) found a
preferential binding of lectin I to Neu5Aca2-6Galβ1-4GlcNAc
epitopes, and glycosphingolipids were also described to be
overexpressed in various tumors and associated with cellular stress
induction, causing cytokine release. A number of mechanisms,
involving PRR-like receptors on NK cells, stress induction and
crosstalk with other immune cells, may be responsible for the
immunostimulatory activity of aviscumine and warrant further
investigation.
Based on prior investigations, the present study
focused on the immunostimulatory activity of the recombinant
mistletoe lectin aviscumine on human natural killer (NK) cells via
a standardized, functional assessment. The results demonstrated a
significant and reproducible increase in NK cell antitumor activity
via degranulation.
Materials and methods
Healthy volunteers
The study was approved by the regional ethics board
(no. AN1460 294/4.15) and all healthy volunteers provided their
written informed consent. In total, 34 healthy individuals, who had
no major illness, coagulation disorders or acute infections at time
of blood withdrawal were included in the present study (median age,
30 years; age range, 22–67 years; male vs. female: 18 vs. 16).
Recombinant mistletoe lectin
Aviscumine was provided by CYTAVIS BioPharma GmbH
(Hamburg, Germany) as a pure powder. It was dissolved and diluted
according to the company's manual.
Isolation of peripheral blood
mononuclear cells (PBMCs) and NK cells
PBMC isolation from whole blood samples was
performed via density gradient centrifugation using Lymphoprep™
(Fresenius KabiNorge AS, Oslo, Norway) according to the
manufacturer's protocol. NK cells were subsequently isolated by
negative depletion with magnetic cell sorting using an NK Cell
Isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
following the manufacturer's protocol. NK cell purity was assessed
by flow cytometric analyses via quantification of
CD3/CD56+-stained cells (catalog nos., 332771 and
345811; BD Pharmingen™; BD Biosciences, Heidelberg, Germany)
following standard staining procedures, revealing a purity of ≥95%
(data not shown). The isolated NK cells were immediately subjected
to viability assessment and cellular cytotoxicity (CC) assays,
including chromium-51 (51Cr)-release and degranulation
analyses.
Viability assessment of NK cells under
aviscumine
A standard trypan blue exclusion assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to assess
the effects of different concentrations of aviscumine (0.1, 0.5, 1,
3 and 6 ng/ml) on NK cell viability with and without IL-2
stimulation (10 ng/ml). Aviscumine was added in the different
concentrations to 25,000 NK cells seeded in triplicates in 96-well
plates (n=3) and viability was assessed after 24, 36 and 72 h via
manual counting in a Neubauer plate by light microscopy. Thereby,
the appropriate aviscumine concentrations for use in the
51Cr-release and degranulation assays were
determined.
NK cell-mediated cellular
cytotoxicity
51Cr-release assay. NK
cell-mediated cellular cytotoxicity was measured with a standard
51Cr-release assay (24)
against K-562 cells (a chronic myeloid leukemia in blast crisis
cell line; Leibniz-Institute DSMZ; German Collection of
Microorganisms and Cell Cultures, Braunschweig, Germany) by two
independent investigators. In brief, two different amounts of
isolated NK cells (12,500 or 25,000 cells/well) were seeded in
96-well cell culture plates and incubated with the different
concentrations of aviscumine (0.5 and 1 ng/ml) with or without IL-2
(10 ng/ml) in complete RPMI medium with 10% fetal calf serum, 2 mM
L-glutamine and 1% penicillin/streptomycin (all from PAA
Laboratories; GE Healthcare Bio-Sciences Austria GmbH, Pasching,
Austria) at 37°C and 5% CO2 for 24 h. Subsequently,
51Cr-labeled [0.96 TBq (26.00 Ci)/mmol; 37 MBq (1
mCi)/ml; Hartmann Analytic, Braunschweig, Germany] K-562 cells
(1,000 cells/well, pre-incubated with 100 µCi at 37°C and 5%
CO2 for 1 h) were added to the pre-seeded NK cells.
After 4 h of co-incubation at 37°C, the amount of 51Cr
released into the supernatant was measured with a WIZARD 25 Wallac
Automatic Gamma Counter (PerkinElmer, Inc., Waltham, MA, USA). All
experiments were run in triplicate.
Percentage of specific lysis was calculated
according to the formula reported by Ströhlein et al
(25): Specific lysis (%) = 100 ×
(mean experimental release - mean spontaneous release)/(mean
maximal release-mean spontaneous release).
The first investigator analyzed two concentrations
of aviscumine (0.5 and 1 ng/ml) to determine
concentration-dependent effects. The second investigator extended
the experimental setting by the addition of IL-2 stimulation and
analysis of a heat-inactivated batch of aviscumine. For IL-2
stimulation 10 ng/ml IL-2 (Sigma-Aldrich; Merck KGaA) was used. The
heat inactivation of aviscumine was performed for 60 min at
90°C.
NK cell degranulation assay
NK cell function via degranulation was assesed by
measurement of CD107α expression levels (n=7) on a flow cytometer.
In short, 50,000 natural killer cells per tube were treated with or
without aviscumine (1 ng/ml) in RPMI (PAA Laboratories; GE
Healthcare Bio-Sciences Austria GmbH) overnight at 37°C in 5%
CO2. Subsequent to washing with washing buffer
[phosphate-buffered saline (PBS) + 0.5% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA) + 2 nM EDTA], 1,000 K-562 cells were
added and co-cultured for 4 h at 37°C and 5% CO2
together with 5 µl of CD107α (phycoerythrin-conjugated; catalog
no., 555801; BD Pharmingen™; BD Biosciences) diluted in 20 µl of
staining buffer [PBS + 0.5% BSA and 0.1% NaN3] for 4 h
in the dark at 37°C, with the addition of 5 µl of CD56 (fluorescein
isothiocyanate-conjugated; catalog no., 332771; BD Pharmingen™; BD
Biosciences) and CD3 (peridinin chlorophyll-Cy5.5-conjugated;
catalog no., 345811; BD Pharmingen™; BD Biosciences) for the last
25 min. This was followed by washing with the previously described
wash buffer and immediate measurement via flow cytometry
(FACSCalibur; BD Biosciences). Analyses were performed with Flowing
Software version 2.5.0 (Perttu Terho; Cell Imaging Core, Turku
Center for Biotechnology, University of Turku, Finland) based on
CD107α expression levels in histogram plots of CD3− and
CD56+ NK cells.
Statistical analyses
For statistical analyses SPSS Statistics version 20
(IBM SPSS, Armonk, NY, USA) was used. Following the assessment of
normal data distribution via Kolmogorov-Smirnov-test, paired
Student's t-tests were performed to test for significant
differences between treated and untreated (control) populations.
The statistical significance threshold was set at P<0.05;
P<0.01 was considered to indicate high significance;
0.05<P<0.1 was referred to as a non-significant trend. Graphs
show the mean values and error bars indicate one standard error of
the mean.
Results
Effect of IL-2 addition under
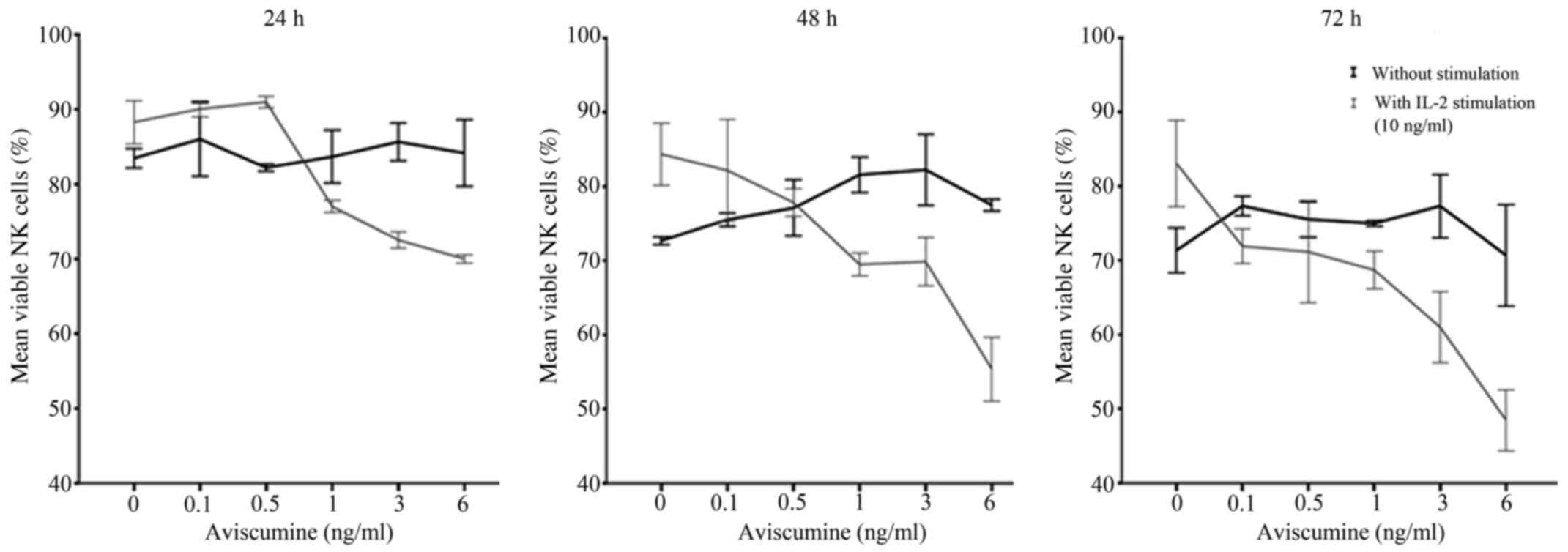
aviscumine treatment on NK cell viability
Dose-finding for subsequent immunomodulatory
activity testing was performed prior to further immunological
evaluations due to aviscumine's reported direct cytotoxic effects.
Different aviscumine concentrations (0.1–6 ng/ml) were tested on
human NK cells for various incubation times (24, 36 and 72 h) to
assess these direct toxic effects. At concentrations ≤6 ng/ml no
direct toxic effects on the NK cells by aviscumine were detected
(Fig. 1). As further immunological
testing would include IL-2 stimulation of the NK cells, viability
was also assessed under the combined use of IL-2 and aviscumine.
For the standard IL-2 concentration (10 ng/ml) no toxic effects
were observed in the experiments (Fig.
1; 0 ng aviscumine). With the combined application of IL-2 and
aviscumine a time- and concentration-dependent decrease in
viability was observed (Fig. 1).
Based on these results aviscumine was used at concentrations of 0.5
or 1 ng/ml in all subsequent functional assays for the assessment
of its immunomodulatory capacity.
Increased NK-cell mediated antitumor
cytotoxicity under aviscumine
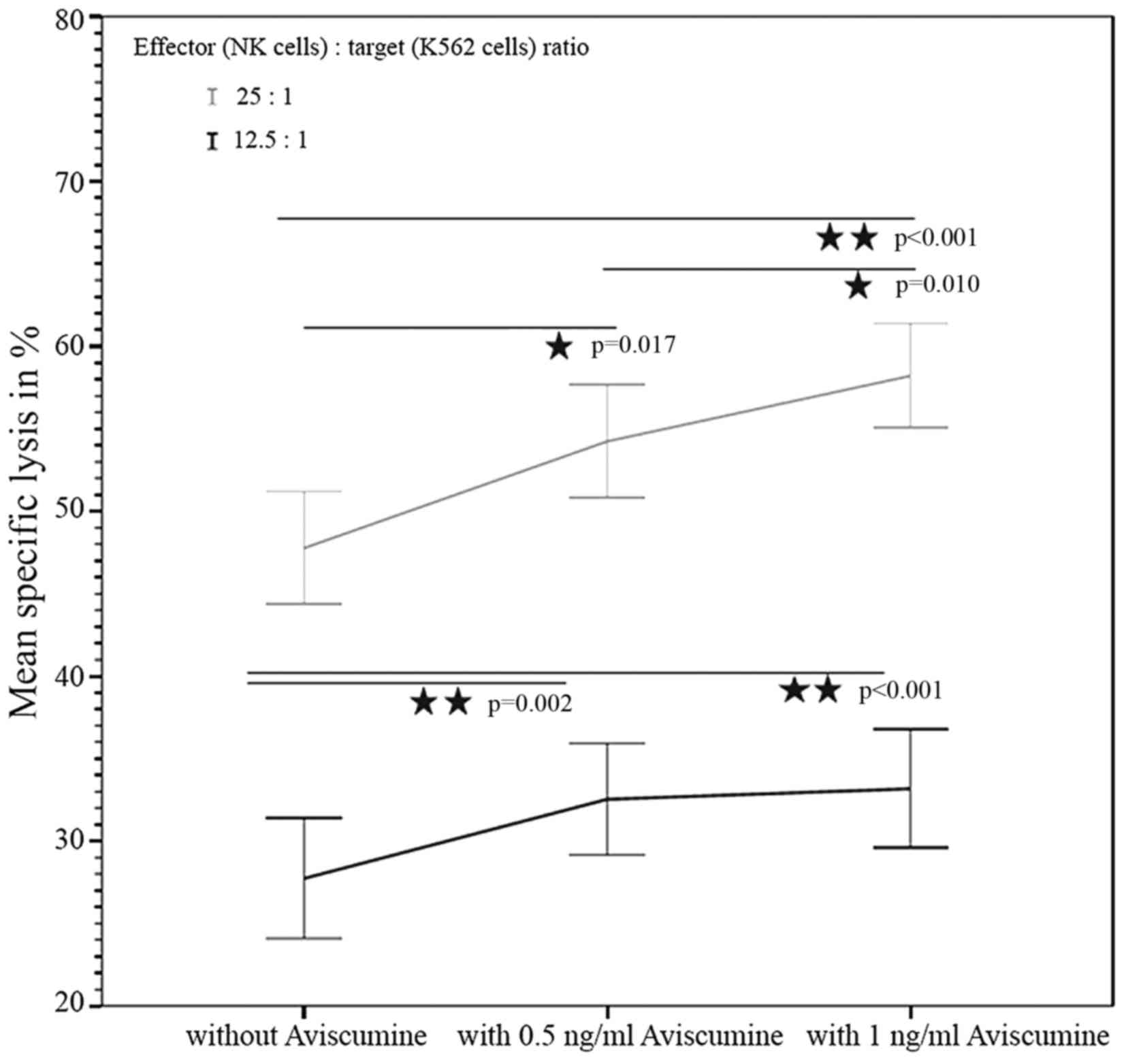
51Cr-release assay. The first
investigator assessed the concentration-dependent effect of
aviscumine on NK-cell mediated cytotoxicity (n=22) using a standard
51Cr-release assay. The test revealed a
concentration-dependent, statistically significant increase in NK
cell-mediated cytotoxicity against tumor cells following treatment
with aviscumine at the two tested concentrations (0.5 and 1 ng/ml)
and effector-to-target (NK:K-562) cell ratios (12.5:1 and 25:1).
The mean percentages of specific lysis with 0, 0.5 and 1 ng/ml
aviscumine stimulation were 27.44, 32.54 and 33.18% for the 12.5:1
effector: target ratio, and 47.76, 54.24 and 58.22% for the 25:1
effector: target ratio, respectively (Fig. 2).
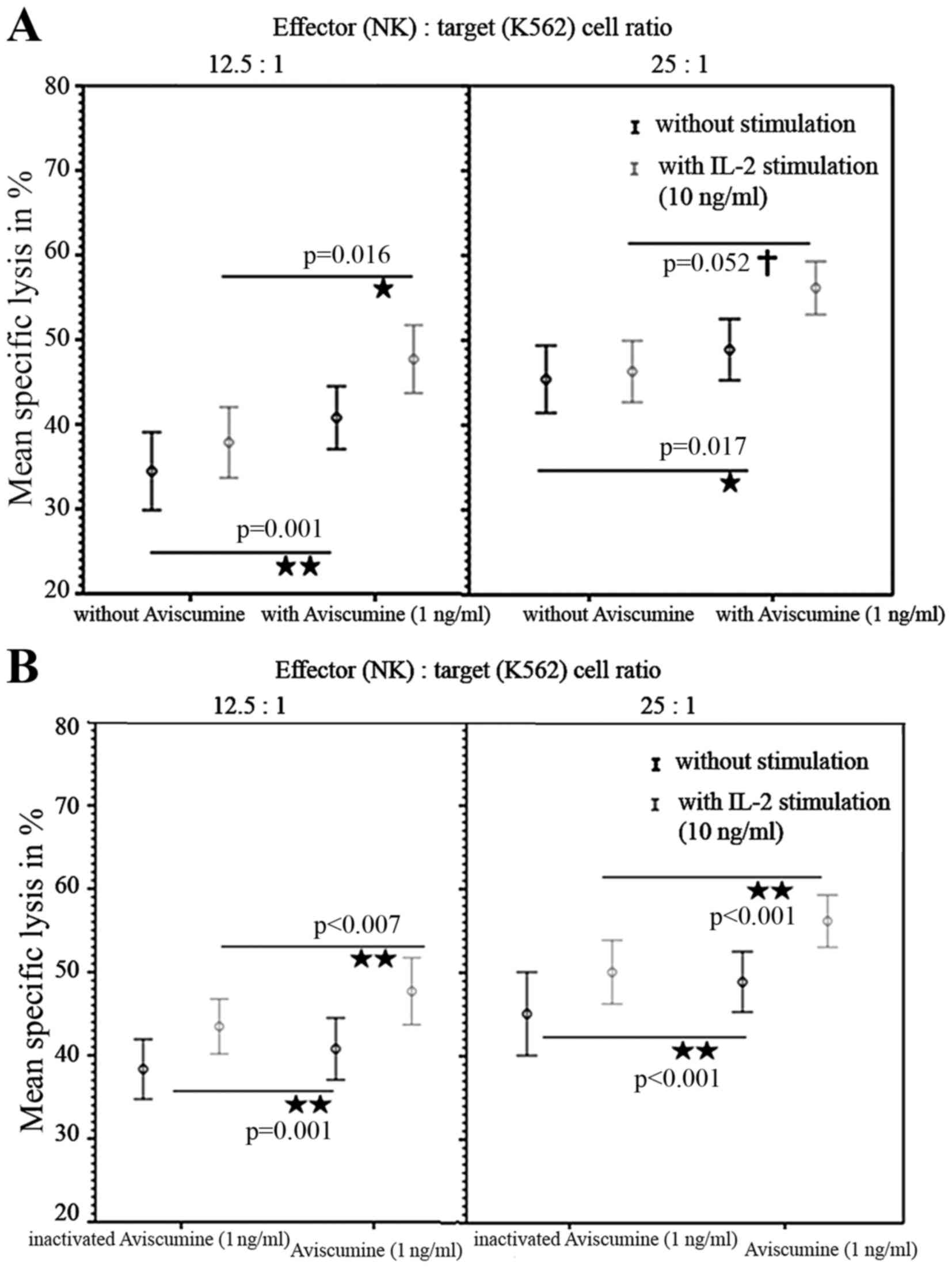
A second investigator repeated these
51Cr-release assays and confirmed the increased
cytotoxic capacity of NK-cells under 1 ng/ml aviscumine stimulation
(vs. no aviscumine) with 40.77 vs. 34.56% specific lysis for the
12.5:1 effector: target ratio and 48.9 vs. 45.4% for the 25:1
effector: target ratio, respectively (Fig. 3A, black lines). Furthermore, when IL-2
was used as an internal stimulation control, specific lysis in
cells treated with 1 ng/ml aviscumine (vs. no aviscumine) was
measured as 47.7 vs. 37.86% for the 12.5:1 ratio and 56.17 vs.
46.32% for the 25:1 ratio (Fig. 3A,
gray lines) and thus demonstrated no impairment of aviscumine
efficacy. In summary aviscumine treatment induced an increase in
specific cell lysis of 5–10%. Although this increase was moderate,
it was reproducible and reached statistical significance in various
settings (Fig 2. and Fig. 3A).
To exclude any non-specific effects of aviscumine
heat-inactivation (90°C for 30 min) was performed. Significant
differences between the effects of aviscumine vs. its
heat-inactivated form confirmed the specificity of the measured
activity with 40.78 vs. 38.35% (without IL-2) and 47.7 vs. 43.48%
(with IL-2) for the 12.5:1 effector:target ratio and 48.9 vs.
45.07% (without IL-2) and 56.17 vs. 50.07% (with IL-2) for the 25:1
effector:target ratio (Fig. 3B).
Nevertheless, these differences were less distinct than those
observed in the comparison of aviscumine with media alone (Fig. 3).
NK cell degranulation assay
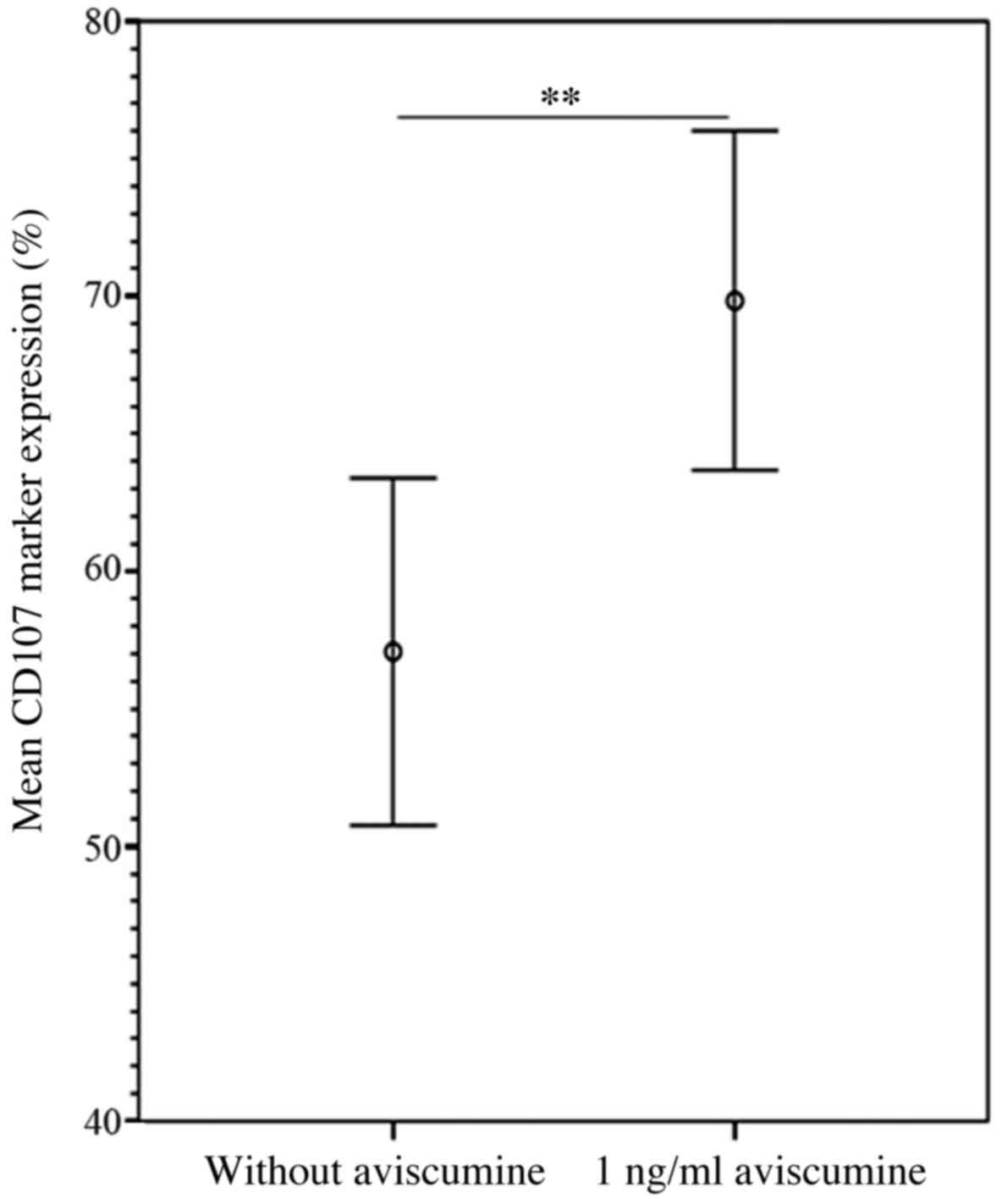
The flow cytometric analyses of the expression of
the degranulation marker CD107α confirmed the results of the
51Cr-release assay. The increase in CD107α expression
following 1 ng/ml aviscumine treatment reached statistical
significance compared with a control setting without aviscumine
(69.83 vs. 57.07%; n=7; Student's t-test, P=0.005; Fig. 4).
Discussion
NK cells serve a key role in tumor immunology
(26–28) and NK cell cytotoxicity assays have
demonstrated an impairment of NK cell activity dependent on
clinical stage in numerous types of malignancy (26). Recently, immune checkpoint inhibitors,
which predominantly target the T-cell population, such as PD-1 and
PD-L1 inhibitors but also, CTLA-4 inhibitors were found to be
capable of releasing the ‘brake’ on anticancer immunity (2). Nevertheless, with 20–30% of durable
remissions with long-term survival for various cancer entities,
such as lung cancer (29) or melanoma
(30) under these treatment
strategies, there remains a need to further improve the efficacy of
therapies. Thus, combinations with other immunostimulatory agents
may gain clinical interest with regard to the restoration of
antitumor immunity. Besides cell therapy, few immunostimulatory
agents are under clinical investigation (31). One of them is a plant-derived
recombinant lectin I, aviscumine, that has demonstrated disease
stabilizations in a number of solid tumors with tolerable toxicity
for its immunostimulatory dose range in early clinical trials
(17,19). The measured changes in patient plasma
cytokine levels (increased IL-1β, TNF-α and IFN-γ and decreased
IL-6 and IL-10 levels) indicate the activation of NK and T cells
(19). A very recent study revealed
that lectin structures represent PAMPs and thereby activate the
immune system via PRRs (21–23). By focusing on NK cells, the present
study was able to demonstrate a reproducible stimulation of NK cell
antitumor activity for a non-toxic concentration range of
aviscumine (Figs. 1–3). To the best of our knowledge, this is the
first functional study to reveal these postulated effects in a
standardized ex vivo human model and thereby support the
prior published works.
Notably, the evaluation of aviscumine's direct toxic
effects revealed a time- and concentration-dependent decrease in NK
cell viability in combination with IL-2 (Fig. 1). The underlying mechanism of this
observation is unknown. One potential mechanism may be
activation-induced cell death, wherein an IL-2-induced upregulation
of Fas ligand (an apoptosis ligand) combined with the activation of
the NK cell receptor induces apoptosis (32).
The specificity of aviscumine's effect on NK cell
stimulation was also confirmed by comparison with the effect of a
heat-inactivated aliquot (Fig. 3),
even though differences were smaller. Furthermore, the results were
reassessed by flow cytometric analysis of CD107α expression,
highlighting the capacity of aviscumine to enhance NK cell activity
via degranulation (Fig. 4).
These data, in line with the clinical findings in
early clinical trials (15,17,19),
support the potential of this plant-derived recombinant lectin I as
an anticancer agent, particularly with regard to its combined use
with immune checkpoint inhibitors or chemotherapeutics, as
postulated in case reports and clinical investigations (22,33).
Nevertheless, further studies to validate the present findings and
assess the detailed mechanisms and clinical efficacy of aviscumine
are warranted.
Acknowledgements
The present study was financially supported by the
Austrian Cancer Society Tyrol (Österreichische
Krebshilfe-Krebsgesellschaft Tirol) and TEXO (Tyrolean Association
of Experimental Oncology). The authors declare the following
conflicts of interest: Dr Heinz Zwierzina is involved in the phase
II trial of the drug as a national principal investigator; Dr Hans
Lentzen is the managing director of MELEMA Pharma GmbH, Hamburg,
Germany (and formerly of CYTAVIS BioPharma GmbH).
References
|
1
|
Brower V: Checkpoint blockade
immunotherapy for cancer comes of age. J Natl Cancer Inst. 107:pii:
djv069. 2015. View Article : Google Scholar
|
|
2
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez-Gracia JL, Labiano S, Rodriguez-Ruiz
ME, Sanmamed MF and Melero I: Orchestrating immune check-point
blockade for cancer immunotherapy in combinations. Curr Opin
Immunol. 27:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gardner TA, Elzey BD and Hahn NM:
Sipuleucel-T (Provenge) autologous vaccine approved for treatment
of men with asymptomatic or minimally symptomatic
castrate-resistant metastatic prostate cancer. Hum Vaccin
Immunother. 8:534–539. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang QL, Zhang S, Tian M, Zhang SY, Xie
T, Chen DY, Chen YJ, He J, Liu J, Ouyang L and Jiang X: Plant
lectins, from ancient sugar-binding proteins to emerging
anti-cancer drugs in apoptosis and autophagy. Cell Prolif.
48:17–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eck J, Langer M, Möckel B, Baur A, Rothe
M, Zinke H and Lentzen H: Cloning of the mistletoe lectin gene and
characterization of the recombinant A-chain. Eur J Biochem.
264:775–784. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eck J, Langer M, Möckel B, Witthohn K,
Zinke H and Lentzen H: Characterization of recombinant and
plant-derived mistletoe lectin and their B-chains. Eur J Biochem.
265:788–797. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langer M, Möckel B, Eck J, Zinke H and
Lentzen H: Site-specific mutagenesis of mistletoe lectin: The role
of RIP activity in apoptosis. Biochem Biophys Res Commun.
264:944–948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Möckel B, Burger A, Schultz RJ,
Wilhelm-Ogunbiyi K, Langer M, Zinke H, Fiebig HH and Lentzen H:
Assessing the cancerostatic potency of rViscumin towards human
tumor xenografts and cell lines in vitro. European J Cancer.
37:S122001. View Article : Google Scholar
|
|
11
|
Langer M.MB, Wilhelm-Ogunbiyi K, Witthohn
K and Lentzen H: Antitumour activity of rViscumin in vitro and in
vivo. 26:3942003.
|
|
12
|
Wilhelm-Ogunbiyi K, Möckel B, Burger A,
Langer M, Zinke H, Fiebig HH, et al: rViscumin, a novel anticancer
agent-preclinical and clinical development status. Eur J Cancer. 37
Supplement 3:S52001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abuharbeid S, Apel J, Sander M, Fiedler B,
Langer M, Zuzarte ML, Czubayko F and Aigner A: Cytotoxicity of the
novel anti-cancer drug rViscumin depends on HER-2 levels in SKOV-3
cells. Biochem Biophys Res Commun. 321:403–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hostanska K, Vuong V, Rocha S, Soengas MS,
Glanzmann C, Saller R, Bodis S and Pruschy M: Recombinant mistletoe
lectin induces p53-independent apoptosis in tumour cells and
cooperates with ionising radiation. Br J Cancer. 88:1785–1792.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schöffski P, Riggert S, Fumoleau P,
Campone M, Bolte O, Marreaud S, Lacombe D, Baron B, Herold M,
Zwierzina H, et al: Phase I trial of intravenous aviscumine
(rViscumin) in patients with solid tumors: A study of the European
Organization for Research and Treatment of Cancer New Drug
Development Group. Ann Oncol. 15:1816–1824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schöffski P, Breidenbach I, Krauter J,
Bolte O, Stadler M, Ganser A, Wilhelm-Ogunbiyi K and Lentzen H:
Weekly 24 h infusion of aviscumine (rViscumin): A phase I study in
patients with solid tumours. Eur J Cancer. 41:1431–1438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trefzer U, Gutzmer R, Wilhelm T, Schenck
F, Kähler KC, Jacobi V, Witthohn K, Lentzen H and Mohr P: Treatment
of unresectable stage IV metastatic melanoma with aviscumine after
anti-neoplastic treatment failure: A phase II, multi-centre study.
J Immunother Cancer. 2:272014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zwierzina H, Bergmann L, Fiebig H, Aamdal
S, Schöffski P, Witthohn K and Lentzen H: The preclinical and
clinical activity of aviscumine: A potential anticancer drug. Eur J
Cancer. 47:1450–1457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergmann L, Aamdal S, Marreaud S, Lacombe
D, Herold M, Yamaguchi T, Wilhelm-Ogunbiyi K, Lentzen H and
Zwierzina H: European Organisation for Research and Treatment of
Cancer: Phase I trial of r viscumin (INN: Aviscumine) given
subcutaneously in patients with advanced cancer: A study of the
European Organisation for Research and Treatment of Cancer (EORTC
protocol number 13001). Eur J Cancer. 44:1657–1662. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rose A, El-Leithy T, vom Dorp F, Zakaria
A, Eisenhardt A, Tschirdewahn S and Rübben H: Mistletoe Plant
Extract in Patients with Nonmuscle Invasive Bladder Cancer: Results
of a Phase Ib/IIa Single Group Dose Escalation Study. J Urol.
194:939–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutikhin AG and Yuzhalin AE: Editorial:
Pattern Recognition Receptors and Cancer. Frontiers in Immunology.
6:1–2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirsch A and Hajto T: Case reports of
sarcoma patients with optimized lectin-oriented mistletoe extract
therapy. J Altern Complement Med. 17:973–979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müthing J, Meisen I, Bulau P, Langer M,
Witthohn K, Lentzen H, Neumann U and Peter-Katalinić J: Mistletoe
lectin I is a sialic acid-specific lectin with strict preference to
gangliosides and glycoproteins with terminal Neu5Ac alpha 2–6Gal
beta 1–4GlcNAc residues. Biochemistry. 43:2996–3007. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiessling R, Klein E, Pross H and Wigzell
H: ‘Natural’ killer cells in the mouse. II. Cytotoxic cells with
specificity for mouse Moloney leukemia cells. Characteristics of
the killer cell. Eur J Immunol. 5:117–121. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ströhlein MA, Grützner KU, Schildberg FW
and Heiss MM: Induction of cytotoxicity against autologous tumour
cells by interleukin-12: Evidence for intrinsic anti-tumor immune
capacity in curatively resected gastrointestinal tumour patients.
Cancer Immunol Immunother. 51:505–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konjevic G, Jurisic V, Jovic V, Vuletic A,
Martinovic Mirjacic K, Radenkovic S and Spuzic I: Investigation of
NK cell function and their modulation in different malignancies.
Immunol Res. 52:139–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fregni G, Perier A, Avril MF and Caignard
A: NK cells sense tumors, course of disease and treatments:
Consequences for NK-based therapies. Oncoimmunology. 1:38–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined Nivolumab and Ipilimumab or
Monotherapy in Untreated Melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDowell KA, Hank JA, DeSantes KB,
Capitini CM, Otto M and Sondel PM: NK Cell-based immunotherapies in
pediatric oncology. J Pediatr Hematol Oncol. 37:79–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poggi A, Massaro AM, Negrini S, Contini P
and Zocchi MR: Tumor-induced apoptosis of human IL-2-activated NK
cells: Role of natural cytotoxicity receptors. J Immunol.
174:2653–2660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajto T, Baranyai L, Kirsch A, Kuzma M and
Perjési P: Can a synergistic activation of pattern recognition
receptors by plant immunomodulators enhance the effect of oncologic
therapy? Case Report of a patient with uterus and ovary sarcoma.
Clin Case Rep Rev. 1:235–238. 2015. View Article : Google Scholar
|