Introduction
The two major characteristics of malignant tumors
are invasion and metastasis. These characteristics involve complex
processes that includes multiple genes. The underlying mechanisms
of gene regulation are precise and complicated in their control and
have yet to be fully resolved. At present, research view points are
based on Paget's ‘seed and soil hypothesis’. Previous studies have
focused on the ‘seed’, cancer cells, and emphasized the impact of
the molecular pathological changes of cancer cells on biological
behaviors, including proliferation, invasion and metastasis
(1–5).
However, no specific biological markers that are able to predict
the invasion and metastasis of tumors have been identified. The
role of the ‘soil’, the tumor microenvironment, in the development
of tumors has been the focus of a number of studies investigating
the expression and function of relevant genes, developing a novel
approach for the basic research of tumor development (6,7).
Mesenchymal-specific genes bridge the ‘seed’ and ‘soil’ aspects of
research and are also important in the tumor regulatory process
(8,9).
The expression of mesenchymal-specific genes
produces proteins with a variety of structures and functions,
including secreted proteins and extracellular matrix (ECM) protein.
Periostin, a newly identified mesenchymal-specific gene, was
originally cloned from a mouse osteoblastic cell line (10). Previous studies revealed that the
periostin gene was associated with the formation of bones (11) and teeth (12), as well as the maintenance of their
structures. At present, studies on periostin mainly focus on two
areas: i) The facilitation of the growth and development of heart
valves and its involvement in the pathophysiological processes of
various ischemic heart diseases, including myocardial infarction
and heart failure (13); ii) the
variations between the expression levels of periostin mRNA and
protein in normal and tumor tissues, as this is implicated to be
closely associated with the occurrence, development and prognosis
of malignant tumors (14).
Periostin, also termed osteoblast-specific factor 2,
is an ECM secreted protein that is able to improve the
proliferation and differentiation of osteoblasts, and the
aggregation and adhesion of periosteal osteoblast precursor cells
(15). It is expressed in various
normal human tissues that are not only involved in numerous normal
physiological processes, but are also closely associated with the
pathological processes involved in the occurrence and development
of cardiovascular diseases, asthma and tumors (16). Currently, the majority of studies
indicate that the overexpression of periostin is associated with
the malignancy grade of a cancer; however, other studies have
reported that periostin may inhibit the invasion and metastasis of
bladder cancer (17,18). Kanno et al (19) identified that periostin has the dual
effect of promoting and inhibiting pancreatic cancer. Collectively,
the results of these studies suggested that the variable biological
effects of periostin are observed in distinct tissues, and further
research is required to examine its complex and multifaceted
functions (20). The current review
focuses on the progression of periostin research for the diagnosis
and treatment of malignant tumors, and its underlying mechanisms of
action.
Structures and features of periostin
The human periostin gene is located on chromosome
13q13.3, with two isoforms identified through studies on human
placenta and osteosarcoma cDNA libraries (10). One of the variants is a 779-amino acid
protein with a molecular weight of 87 kDa and the other is an
836-amino acid protein with a molecular weight of 93.3 kDa
(10). The mouse periostin gene is
located on chromosome 3, and the coding region is 30 kbp in length,
producing an 811-amino acid protein with a molecular weight of 90.2
kDa. Periostin is highly conserved, and has 89.2% protein sequence
homology between humans and mice. Compared with other regions of
periostin, the C-terminus is less conserved, with 85.5% sequence
homology (10). Periostin also shares
homology with the adhesion molecule of the insect embryonic central
nervous system, fasciclin I (10).
Periostin is in the same protein family as transforming growth
factor β-induced, stabilin I and II, myelin basic protein-70 and
algal-cell adhesion molecule (10).
Periostin has an N-terminal signal peptide, a
cysteine-rich region, four internal homology domains and a carboxyl
terminal region (21). There is a
typical signal sequence at the N-terminus suggesting that it has
the potential to be a secreted protein (21). The cysteine-rich region contains ~75
amino acids and may be associated with protein-protein interactions
or protein polymerization (21). The
four internal homology domains are homologous with the insect
protein fasciclin I and contain the binding sites for integrin and
glucosamine (21). This allows
periostin to interact with integrin to mediate the
epithelial-mesenchymal transition (EMT) (21). The EMT enables tumor cells to
phenotypically resemble mesenchymal cells, thus mediating the
migration and adhesion of cells and enabling tumor cells to acquire
stronger metastasis potential (22–24). The
C-terminus of periostin is hydrophilic and is able to undergo
alternative splicing at the transcriptional level to form splice
variants or isomers of periostin (25). Periostin has eight types of homologous
isomers in human tissues that are considered to be associated with
the occurrence of specific tumors (26).
Periostin in the diagnosis of malignant
tumors
Periostin is highly expressed in multiple solid
tumor tissues, including head and neck, lung, breast, colorectal,
ovarian and liver cancer (27).
Periostin protein is secreted from tumor cells and the surrounding
stromal cells and this continuously destroys the surrounding matrix
during the infiltration process of tumors (28), leading to the release of periostin
into the blood circulation. Previous studies have identified that
there is a high level of periostin expression in the blood serum of
patients with head and neck, breast, colorectal and non-small cell
lung carcinoma, and that the potential for infiltration and
metastasis was higher in those with a raised level of serum
periostin (29,30). Periostin is able to facilitate the
survival, invasion and angiogenesis of tumor cells, and enhance
their tolerance to hypoxia and chemicals, suggesting it has a close
association with the grade of malignancy, metastasis and prognosis
of malignant tumors (31,32). Following further study of periostin,
it may present an ideal marker for the diagnosis and prognosis of
tumors.
Kudo et al (33) demonstrated that periostin expression
correlates with vascular endothelial growth factor C (VEGF-C)
expression levels in tissue and serum from patients with head and
neck squamous cell carcinoma (HNSCC). Periostin and
periostin-induced upregulation of VEGF-C may promote
lymphangiogenesis, and this has been reported to be mediated by Src
and Akt activity (33). Periostin has
the potential to be a marker for the prediction of malignant
behaviors and a potential therapeutic target to obstruct lymphatic
invasion and lymphangiogenesis in patients with HNSCC.
Wang et al (34) reported that periostin was frequently
highly expressed in esophageal squamous cancers and was associated
with lymphatic metastasis, tumor staging, vascular invasion and
tumor node metastasis (TNM) staging. High levels of periostin
expression are associated with the progression of tumors,
angiogenesis and poor prognosis, indicating that it may be an
independent prognostic factor for esophageal squamous cancer.
Heidari et al (35)
demonstrated that specific imaging of ECM periostin in esophageal
squamous cell carcinoma is feasible using a targeted positron
emission tomography tracer. Detection of periostin in the tumor
microenvironment may aid early detection, post-surgical follow up,
and in situ characterization of primary and metastatic
lesions.
Soltermann et al (36) examined the expression levels of
periostin in the tumor tissue sections of 533 patients with
non-small cell lung carcinoma (NSCLC). The results revealed that
periostin was expressed in epithelial and mesenchymal tumor tissues
and that its expression levels exhibited a positive correlation
with the size of tumor, the tumor staging and progression-free
survival, as well as being elevated in male patients, and with a
higher postoperative recurrence rate in patients with high levels
of periostin expression in the tumor mesenchymal tissues (36). This previous study also indicated that
the abnormal expression of periostin in patients with lung cancer
serves an important role in the occurrence and development of the
tumor, affecting patient prognosis (36).
Zhang et al (37) demonstrated that, compared with benign
breast tumors and normal breast tissue, the levels of periostin
mRNA and proteins were higher in breast cancer tissues and
thisassociation was positively correlated with TNM stage. Puglisi
et al (38) examined tumor
tissue sections of 189 patients with breast cancer and revealed
that periostin was mainly located in tumor mesenchymal tissues and
the cytoplasm of tumor cells. The expression level of periostin in
the cytoplasm was associated with the size of the tumor, and its
progesterone receptor and VEGF receptor status, suggesting
periostin has an effect on the growth of tumors and angiogenesis
(38). Contié et al (39) transplanted the MDA-B02 human breast
cancer cell lineinto nude mice and identified that high levels of
periostin were expressed mainly in the matrix of cells surrounding
the tumor, compared with the breast cancer cells.
The expression level of periostin is abnormally
increased in the tumor tissues and blood serum of patients with
colorectal cancer (40). Using ELISA,
Ben et al (40) revealed that
the serum level of periostin in patients with colorectal cancer was
significantly higher compared with healthy individuals and patients
with colorectal polyps or colorectal adenoma, and that this was
closely associated with distant metastasis, tumor staging and
prognosis. This may be of clinical value in the determination of
whether patients with colorectal cancer have a high risk of tumor
invasion and metastasis. Using reverse transcription-polymerase
chain reaction, it was also revealed that the expression of
periostin mRNA was significantly increased in colorectal cancer
tissues compared with normal tissues, but periostin mRNA was not
detected in four cultured colon cancer cell lines suggesting that
periostin was secreted by the surrounding matrix cells and not
colorectal cancer cells (40).
Kikuchi et al (6) used
immunohistochemistry (IHC) and immunoelectron microscopy to detect
periostin in the blood serum of patients with colon cancer, which
was secreted by the fibroblasts that surroundcolon glands and
tumor-associated fibroblasts. Li et al (41) performed IHC staining of periostin on
tissue samples from 115 patients with colorectal cancer during the
follow-up period, revealing positive periostin expression. The
levels of periostin expression in colorectal cancer cells (59.13%,
68/115) were significantly higher compared withthe adjacent normal
colon mucosa (0.47%, 11/109) (41).
In colorectal cancer, the overexpression of periostin was
positively correlated with tumor size, serosal invasion,
differentiation, five-year survival rates, lymph node metastasis
andclinical stage (41,42). Those with early-stage colorectal
cancer and low periostin expression levels had higher survival
rates compared with patients with advanced-stage colorectal cancer
and high levels of periostin expression. These findings suggest
that periostin may be important in the progression of various types
of colorectal cancers.
A previous study examined the expression of
periostin in intrahepatic cholangiocarcinoma using IHC, revealing
that periostin was expressed only in the interstitial fibroblasts
and not in cancer cells and immune cells (6). The survival time of patients with
cholangiocarcinoma with a high level of periostin expression was
shorter compared with those with low levels of periostin expression
(6). The multivariate analysis
revealed high expression level of periostin and lymphatic
metastasis may be independent prognostic factors for
cholangiocarcinoma (6). In
vitro analysis of recombinant periostin also indicated that
periostin is able to induce the proliferation and invasion of
cholangiocarcinoma cells (43).
Preoperative serum levels of periostin are of limited value for the
diagnosis of benign and malignant hepatic disease, but may be used
as an independent prognostic indicator for liver cancer (44).
Periostin in the treatment of malignant
tumors
As aforementioned, the expression of periostin and
changes to the extracellular matrix may alter the adhesion and
migration of tumor cells, characteristics that are closely
associated with the treatment of several types of malignant tumor
(45). Periostin is able to
facilitate the survival, migration and invasion of tumor cells, as
well as promoting angiogenesis, enhancing the metastatic potential
of tumors (46). Therefore, periostin
may be a novel target for tumor treatment.
The periostin-integrin signaling pathway regulates
the development of breast cancer and the tumor microenvironment at
multiple levels, and the periostin-binding DNA aptamer is reported
to be a potential target for inhibiting the development of breast
cancer (45). Choi et al
(46) demonstrated that recombinant
periostin is able to stimulate the adhesion and invasion of the
SK-OV-3 human ovarian adenocarcinoma cell line and induce the
expression of matrix metalloproteinase-2. Zhu et al
(47) constructed periostin
expression vectors and inserted them into the OVCAR-3 and OV2008
ovarian cancer cell lines. The results revealed that the
overexpression of periostin did not alter the in vitro
growth speed of tumor cells, whereas it greatly enhanced the growth
of peritoneal metastatic tumors in immunodeficient mice (47). This growth enhancement was primarily
associated with increased angiogenesis and decreased tumor-cell
apoptosis (47). Purified periostin
has been reported to facilitate the in vitro adhesion,
migration and invasion of ovarian cancer cells and human umbilical
vein endothelial cells (HUVEC) (47).
Gillan et al (14) identified
that the purification of recombinant periostin facilitated the
adhesion of ovarian epithelial cells where as antibodies against
αvβ3 and αvβ5 inhibited this adhesion, suggesting that αvβ3 and
αvβ5 are the receptors of periostin on the ovarian cancer cell
membrane. It was hypothesized that periostin serves an important
role in the angiogenesis and distant metastasis of ovarian cancer,
which mayprovide a novel target for the treatment of ovarian cancer
(14).
The 293T human renal epithelial cell line exhibits
high levels of periostin expression and has increased migratory and
invasive potential compared with normal cells (48). However, this effect may be blocked by
αvβ5 antibody or an epidermal growth factor receptor (EGFR) kinase
inhibitor, and cell movement and invasion mayalso be promoted by
the increased expression of EMT associated genes (elastic protein
genes and fibrin genes) and the activation of matrix
metalloproteinase-9 (MMP-9) (48). It
has been suggested that periostin may enhance the invasion and
metastasis of cancer cells through integrin and the EGF signaling
pathway, and periostin may be an effective therapeutic target for
human epithelial renal cell cancer (48).
A previous study demonstrated that the expression of
periostin increased and promoted the survival of A549 cells that
were placed in a chemically-simulated hypoxic tumor
microenvironment via activation of the phosphatidylinositol
3-kinase/protein kinase B (PI3K/Akt) signaling pathway (49). Hong et al (50) constructed a plasmid vector that
expressed periostin and inserted it into A549 cells. The expression
of vimentin and neural-cadherin were induced and simultaneously the
expression of epithelial-cadherin was inhibited suggesting that the
enhancement of the epithelial-mesenchymal transition may facilitate
the proliferation and migration of A549 cells (50). This demonstrated that periostin was
closely associated with the invasion and migration of NSCLC and it
may be a potential therapeutic target for the treatment of NSCLC
(29,50).
An in vitro study demonstrated that
recombinant periostin induces the proliferation and invasion of
cholangiocarcinoma cells (43). It
has also been reported that the upregulation of periostin
expression enhanced the invasion of prostatic cancer, suggesting
that it may be a potential target for the treatment of primary and
metastatic prostatic cancer (51).
Tai et al (52) identified
that periostin antibodies promote the apoptosis of cancer cells and
enhance the efficacy of 5-fluorouracilin the treatment of
colorectal cancer. Periostin serves an important role in the
facilitation of cancer cell survival; therefore, periostin
antibodies may be an effective therapeuticfor colorectal cancer
(52).
Regulatory factors of periostin
Yang et al (53) demonstrated that histamine induced the
production of periostin and collagen, by activating the H1
receptor-mediated extracellular signal-regulated kinase (ERK) 1/2
pathway. Tai et al (52)
identified that, in colon cancer cells, transforming growth factor
(TGF)-β1 induced the production of periostin. Fibroblast growth
factor (FGF)-1 and angiotensin II enhanced the expression of
periostin in pulmonary arterial smooth muscle cells (54). Bone morphogenetic protein,
platelet-derived growth factors and acidic and basic FGF were all
potential factors promoting pancreas stellate cells to secrete
periostin (55). In a hypoxic
environment, TGF-α and basic FGF are able to increase the
expression of periostin in A549 lung cancer cells by activating the
PI3K/Akt signaling pathway (49). In
human ovarian cancer tissues, periostin was not expressed in the
cancer cells, but in the cancer-associated mesenchymal cells
(56). Lysophosphatidic acid (LPA) is
able to induce the secretion of periostin by mesenchymal cells and
periostin expression may be effectively inhibited using viral
delivery of short hairpin RNA to silence LPA receptor 1 (46). In addition, previous studieshave
demonstrated that Twist and interleukin-4 and −13 are also able to
induce the expression and secretion of periostin (57,58).
Fig. 1 summarizes the aforementioned
regulatory factors of periostin.
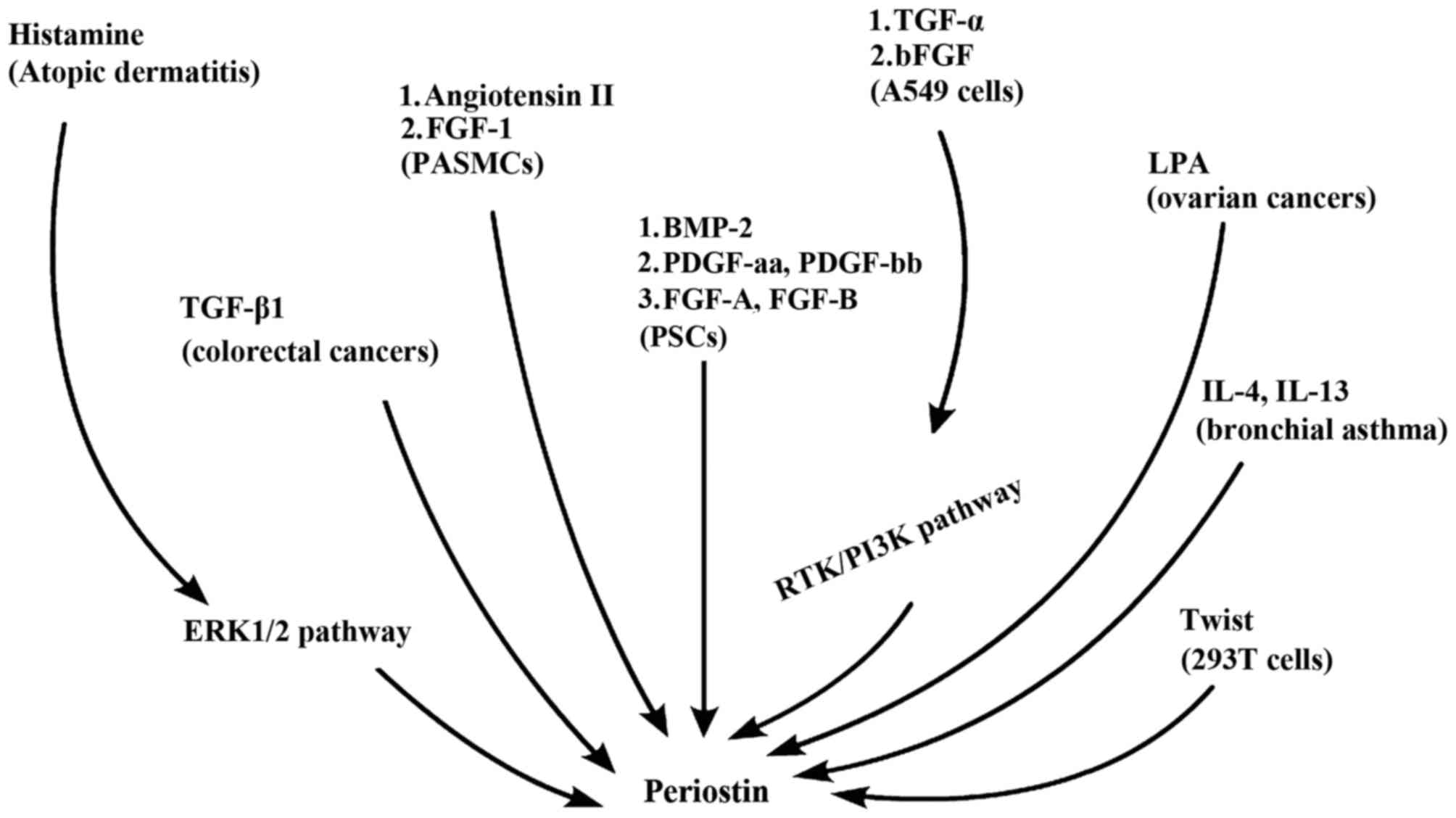 | Figure 1.Regulatory factors of periostin.
Previous studies in eight diseases have revealed more than twelve
factors might regulate periostin. TGF, transforming growth factor;
ERK1/2, extracellular signal-regulated kinase 1/2; FGF, fibroblast
growth factor; PASMCs, pulmonary artery smooth muscle cells; BMP-2,
bone morphogenetic protein 2; PDGF, platelet-derived growth factor;
PSC, pancreatic stellate cells; LPA, lysophosphatidic acid;
RTK/PI3K, receptor tyrosine kinase/phosphatidylinositol 3-kinase;
IL, interleukin. |
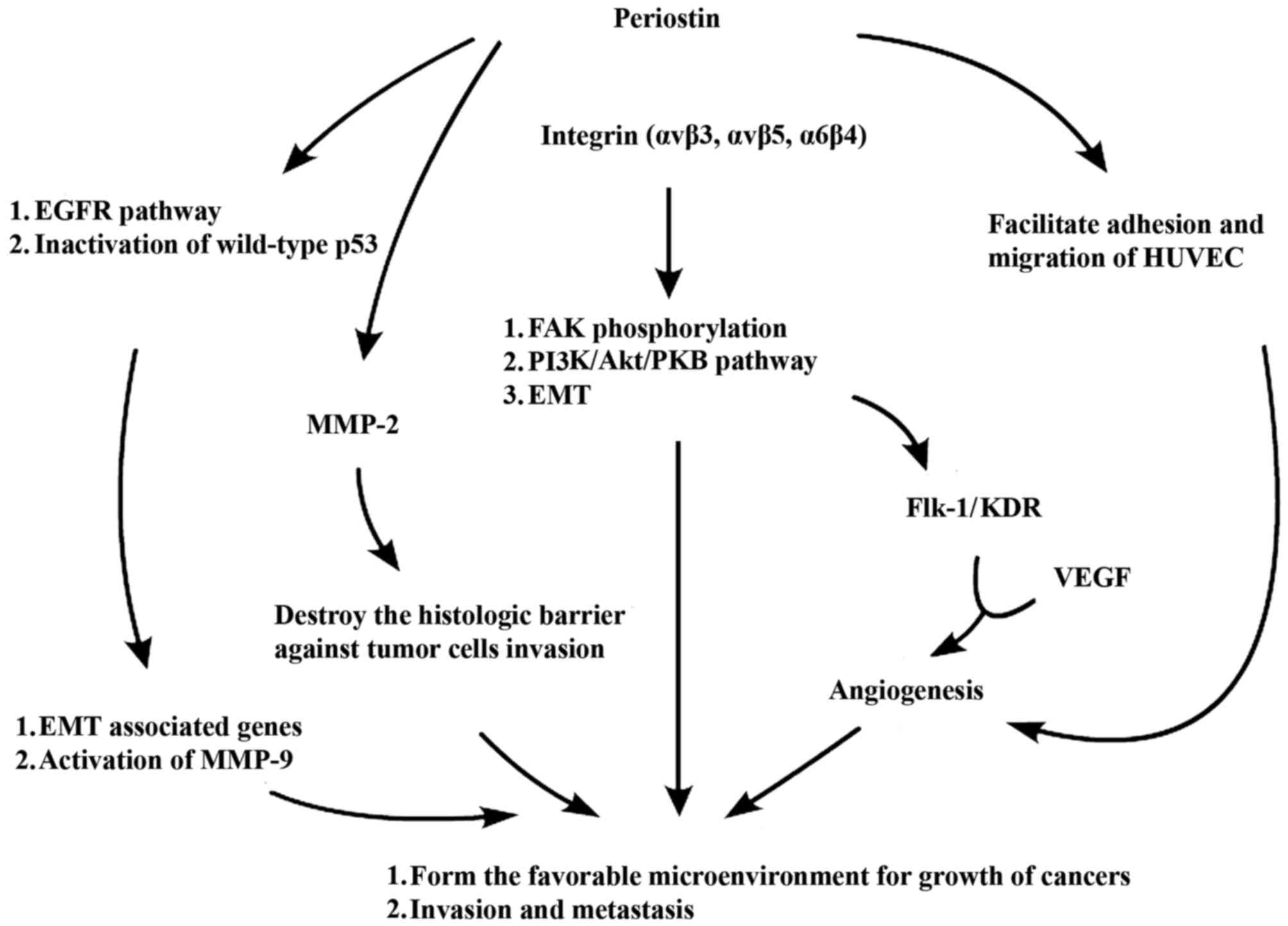
Mechanisms underlying periostin action
The activation of relevant signaling pathways,
including the EGFR pathway and PI3K/Akt pathway, is able to enhance
the proliferation and migration of vascular endothelial cells,
inhibit the apoptosis of endothelial cells, reduce damage to the
cells in hypoxic and low nutrition environments and maintain normal
cell functions (49,59). These processes are beneficial for the
angiogenesis of tumor tissues and enhancement of tumor invasion,
thus facilitating the formation of metastatic tumors. Due to the
biological structures and features of periostin, it is able to bind
to various subtypes of integrin receptor on the surface of the cell
membrane, including αvβ3, αvβ5 and α6β4, and trigger relevant
signal transduction pathways, including PI3-K/Akt and focal
adhesion kinase (FAK) phosphorylation. Periostin may also bind to
EGFR andthis serves an important role in the occurrence and
development of tumors through the enhancement of tumor cell
survival, angiogenesis, and the invasion and metastasis of tumors
(15,49). The potential underlying mechanisms of
periostin action are summarized in Fig.
2.
Periostin is able to bind to several cell surface
receptors, and particularly interacts with integrin receptors on
the cell surface. Integrin is a transmembrane receptor heterodimer
that is involved in cell-cell and cell-ECM interactions, which
promote the EMT, enhance the invasion of cells and inhibit
ECM-integrin interaction via periostin-integrin interaction
(56,59). This triggers relevant signaling
transduction pathways, including the PI3-K/Akt signaling pathway,
and may alter the microenvironment to favor cancer cell survival
and growth (20). Baril et al
(47,56) demonstrated that periostin facilitated
the invasion of tumor cells by enhancing the activity of pancreatic
cancer cells, and enhanced the survival of cancer cells under
hypoxic conditions. At the molecular level, periostin is able to
activate the PI3K/Akt signaling pathway by binding to integrin α6β4
receptor, thus promoting FAK phosphorylation rather than expression
of MMP-9, to exert biological effects (56). Zhu et al (47) revealed that the interaction between
periostin and ovarian cancer cells and HUVEC cells may facilitate
the migration and invasion of ovarian cancer cells andthe adhesion
and migration of HUVEC cells, as well as improving the angiogenesis
of tumors, allowing the invasion and metastasis of tumors. Another
previous study demonstrated that purified recombinant periostin is
able to enhance the adhesion of ovarian epithelial cells, a process
that is inhibited by αvβ3 and αvβ5 antibodies but not αvβ1
antibody. The selection of integrin occurred according to the
distinct subtypes of integrin receptors on the cell membrane that
are expressed in various cell types, suggesting that αvβ3 and αvβ5
are the receptors of periostin on the ovarian cancer cell membrane,
and that periostin functions by binding to them (47,56). Yan
et al (48) identified that
periostin-transfected cells exhibited morphological changes and
increased expression of the matrix markerselastic protein and
fibrin. 293T cells, which express a high level of periostin, had
stronger migratory and invasive potential compared with normal
cells. However, this effect was able to be blocked by αvβ5
antibodies or EGFR kinase inhibitors, suggesting that periostin may
improve the invasion and metastasis of tumor cells via the integrin
and EGFR signaling pathways. The expression of EMT associated genes
(elastic protein and fibrin genes) and activation of MMP-9 may also
enhance the migratory and invasive potential of cancer cells
(48,56).
Growth of new capillaries to provide nutrient
substance and oxygen is essential when solid tumors grow to a
diameter of 2–3 mm, and this requires the proliferation and
migration of vascular endothelial cells and the formation of
capillary lumens. It has been identified that VEGF and its
receptor, fetal liver kinase-1/kinase insert domain receptor
(Flk-1/KDR), serve an important role in this process (59). Tumor cells and mesenchymal cells
secrete VEGF to act on the vascular endothelial cell receptors but
VEGF also facilitates the adhesion and migration of endothelial
cells by activating the receptors Flk-1/KDR and integrin αvβ3. Shao
et al (59) identified that,
in breast cancer, periostin upregulates the VEGF receptor Flk-1/KDR
through the integrin αvβ3-FAK-mediatedsignaling transduction
pathway. This has an important impact on tumor angiogenesis and the
growth of metastatic tumors (59).
The regulation of growth in rectal cancer is implemented through
the αvβ3-PI3 K/Akt signaling pathway; however in pancreatic cancer,
this occurs through the α6β4-PI3K-Akt and FAK signaling pathways
(59). Therefore, tumor cells secrete
periostin in a paracrine manner, which allows it to bind to
integrin receptors that facilitate the survival of endothelial
cells and the formation of new blood vessels. Thus, periostin has
an important role in the development of tumor vessels and the
growth of metastatic tumors. Despite differing cell origins, the
regulatory effects of periostin on signaling pathways are generally
consistent across various studies and all of these pathways are
common angiogenesis regulators (59).
In human ovarian cancer tissues, periostin was not
expressed in cancer cells, but in the cancer-associated mesenchymal
cells. Recombinant periostin is able to stimulate the adhesion and
invasion of SK-OV-3 human ovarian adenocarcinoma cells and induce
cancer cells to express MMP-2, which may destroy the histological
barrier of the basement membrane and extracellular matrix against
tumor cell invasion, thus serving an important role in the invasion
and metastasis of tumors (46).
Using three-dimensional tissue culture of esophageal
squamous cancer cells, Michaylira et al (60) identified that periostin is a cell
adhesion molecule that is highly expressed by tumor cells and may
be a novel molecular marker for tumor invasion. Inhibition of the
EGFR signaling transduction pathway and restoration of the function
of wild type p53 weakened the effect of periostin suggesting an
interdependent association exists between these two common genetic
alterations and the functions of periostin (60).
Using in situ hybridization, Kikuchi et
al (61) revealed that periostin
was generated by gastric cancer-associated fibroblasts rather than
cancer cells, and thismay form a favorable microenvironment for the
growth of gastric cancers by activating ERK, thuspromoting the
progression of tumors. Genome-wide analysis of gene expression
identified significantly higher levels of periostin expression in
stage II–IV gastric cancer tissuescompared with normal tissues. In
parallel with the activation of ERK, periostin may enhance the
in vitro growth of diffuse gastric cancer cell lines,
including OCUM-2MLN and OCUM-12 (61).
Conclusion
Notable progress has been achieved on investigating
the involvement of periostin in the occurrence and development of
tumors. This hasprovided novel approaches for tumor research
through providing a potentialdiagnostic marker and therapeutic
target for tumors. Previous studies reveal that periostin is
primarily produced by the mesenchymal cells surrounding cancer
cells, and cancer cells may stimulate its expression and secretion
by mesenchymal cells (8,12,49). This
may occur through interactions with receptor molecules on the cell
surface, including integrin (20),
triggering relevant signaling transduction pathways that alter the
microenvironment in favor of cancer cell survival and growth.
At present, there remain numerous problems that
require addressing to elucidate the role of periostin in tumor
development. These include: i) investigating the mechanisms
underlying periostin action on the survival, invasion, angiogenesis
and metastasis of tumor cells; ii) identifying the factors that
regulate the expression of periostin by mesenchymal cells, and
whether the regulatory factors of periostin differ in the varying
types of cancer; iii) determining the receptor molecules that
interact with periostin and the tumor factors that may be regulated
to produce effects including facilitation of survival, invasion and
angiogenesis of tumor cells and enhance tolerance to hypoxia and
chemicals; iv) identifying the factors that cause periostin to
exhibit varied effects among differing tumor cells and v) detecting
whether multiple types of tumor cells express or secrete periostin
individually. Continuous in-depth research may aid further
understanding of the role of periostin in the occurrence and
development of tumors, and provide novel evidence for the
application of periostin in the clinical diagnosis and treatment of
tumors.
Acknowledgements
The present review was supported by grants from the
Scientific Innovation Team Project of Ningbo (grant no.
2012B82019), the Ningbo Social Developmental Key Research Project
(grant no. 2012C5015), the Natural Science Foundation of Ningbo
(grant no. 2013A610217) and the Medical and Health Training Project
of Zhejiang Province (grant no. 2015RCB025).
References
|
1
|
Chin AR and Wang SE: Cancer-derived
extracellular vesicles: The ‘soil conditioner’ in breast cancer
metastasis? Cancer Metastasis Rev. 35:669–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lazcano-Ponce EC, Miquel JF, Munoz N,
Herrero R, Ferrecio C, Wistuba II, de Ruiz Alonso P, Urista Aristi
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Zhang CS and Zhang Y: Molecular
aspects of prostate cancer with neuroendocrine differentiation.
Chin J Cancer Res. 28:122–129. 2016.PubMed/NCBI
|
|
4
|
Sabnis AJ and Bivona TG: HSP70 dependence
in rhabdomyosarcoma: Seed or soil? Cell cycle. 16:147–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von der Heide EK, Neumann M, Vosberg S,
James AR, Schroeder MP, Ortiz-Tanchez J, Isaakidis K, Schlee C,
Luther M, Jöhrens K, et al: Molecular alterations in bone marrow
mesenchymal stromal cells derived from acute myeloid leukemia
patients. Leukemia. 31:1069–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kikuchi Y, Kashima TG, Nishiyama T,
Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo
A and Fukayama M: Periostin is expressed in pericryptal fibroblasts
and cancer-associated fibroblasts in the colon. J Histochem
Cytochem. 56:753–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Li F, Gao F, Xing L, Qin P, Liang
X, Zhang J, Qiao X, Lin L, Zhao Q and Du L: Periostin promotes
tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling.
Oncotarget. 7:40148–40159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chun HW and Hong R: Significance of the
hedgehog pathway-associated proteins Gli-1 and Gli-2 and the
epithelial-mesenchymal transition-associated proteins Twist and
E-cadherin in hepatocellular carcinoma. Oncol Lett. 12:1753–1762.
2016.PubMed/NCBI
|
|
9
|
Zeng G, Xun W, Wei K, Yang Y and Shen H:
MicroRNA-27a-3p regulates epithelial to mesenchymal transition via
targeting YAP1 in oral squamous cell carcinoma cells. Oncology
Reports. 36:1475–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Litvin J, Selim AH, Montgomery MO, Lehmann
K, Rico MC, Devlin H, Bednarik DP and Safadi FF: Expression and
function of periostin-isoforms in bone. J Cell Biochem.
92:1044–1061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kruzynska-Frejtag A, Wang J, Maeda M,
Rogers R, Krug E, Hoffman S, Markwald RR and Conway SJ: Periostin
is expressed within the developing teeth at the sites of
epithelial-mesenchymal interaction. Dev Dyn. 229:857–868. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kühn B, del Monte F, Hajjar RJ, Chang YS,
Lebeche D, Arab S and Keating MT: Periostin induces proliferation
of differentiated cardiomyocytes and promotes cardiac repair. Nat
Med. 13:962–969. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
15
|
Contié S, Voorzanger-Rousselot N, Litvin
J, Bonnet N, Ferrari S, Clézardin P and Garnero P: Development of a
new ELISA for serum periostin: Evaluation of growth-related changes
and bisphosphonate treatment in mice. Calcif Tissue Int.
87:341–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanabe H, Takayama I, Nishiyama T,
Shimazaki M, Kii S, Li M, Amizuka N, Katsuba Ki and Kudo A:
Periostin associates with Notch1 precursor to maintain Notch1
expression under a stress condition in mouse cells. PLoS One.
5:e122342010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isono T, Kim CJ, Ando Y, Sakurai H, Okada
Y and Inoue H: Suppression of cell invasiveness by periostin via
TAB1/TAK1. Int J Oncol. 35:425–432. 2009.PubMed/NCBI
|
|
18
|
Kim CJ, Yoshioka N, Tambe Y, Kushima R,
Okada Y and Inoue H: Periostin is down-regulated in high grade
human bladder cancers and suppresses in vitro cell invasiveness and
in vivo metastasis of cancer cells. Int J Cancer. 117:51–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanno A, Satoh K, Masamune A, Hirota M,
Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F, et al:
Periostin, secreted from stromal cells, has biphasic effect on cell
migration and correlates with the epithelial to mesenchymal
transition of human pancreatic cancer cells. Int J Cancer.
122:2707–2718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morra L and Moch H: Periostin expression
and epithelial-mesenchymal transition in cancer: A review and an
update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coutu DL, Wu JH, Monette A, Rivard GE,
Blostein MD and Galipeau J: Periostin, a member of a novel family
of vitamin K-dependent proteins, is expressed by mesenchymal
stromal cells. J Biol Chem. 283:17991–18001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishioka R, Itoh S, Gui T, Gai Z, Oikawa
K, Kawai M, Tani M, Yamaue H and Muragaki Y: SNAIL induces
epithelial-to-mesenchymal transition in a human pancreatic cancer
cell line (BxPC3) and promotes distant metastasis and invasiveness
in vivo. Exp Mol Pathol. 89:149–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoersch S and Andrade-Navarro MA:
Periostin shows increased evolutionary plasticity in its
alternatively spliced region. BMC Evol Biol. 10:302010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tilman G, Mattiussi M, Brasseur F, van
Baren N and Decottignies A: Human periostin gene expression in
normal tissues, tumors and melanoma: Evidences for periostin
production by both stromal and melanoma cells. Mol Cancer.
6:802007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blanchard C, Mingler MK, McBride M, Putnam
PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD and
Rothenberg ME: Periostin facilitates eosinophil tissue infiltration
in allergic lung and esophageal responses. Mucosal Immunol.
1:289–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sasaki H, Yu CY, Dai M, Tam C, Loda M,
Auclair D, Chen LB and Elias A: Elevated serum periostin levels in
patients with bone metastases from breast but not lung cancer.
Breast Cancer Res Treat. 77:245–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimamatsu S, Okamoto T, Haro A, Kitahara
H, Kohno M, Morodomi Y, Tagawa T, Okano S, Oda Y and Maehara Y:
Prognostic significance of expression of the epithelial-mesenchymal
transition-related factor brachyury in intrathoracic lymphatic
spread of non-small cell lung cancer. Ann Surg Oncol. 23 Suppl
5:1012–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kudo Y, Iizuka S, Yoshida M, Nguyen PT,
Siriwardena SB, Tsunematsu T, Ohbayashi M, Ando T, Hatakeyama D,
Shibata T, et al: Periostin directly and indirectly promotes tumor
lymphangiogenesis of head and neck cancer. PloS One. 7:e444882012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Sun QK, He YF, Ma DC, Xie MR, Ji
CS and Hu B: Overexpression of periostin is significantly
correlated to the tumor angiogenesis and poor prognosis in patients
with esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
7:593–601. 2014.PubMed/NCBI
|
|
35
|
Heidari P, Esfahani SA, Turker NS, Wong G,
Wang TC, Rustgi AK and Mahmood U: Imaging of secreted extracellular
periostin, an important marker of invasion in the tumor
microenvironment in esophageal cancer. J Nucl Med. 56:1246–1251.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soltermann A, Tischler V, Arbogast S,
Braun J, Probst-Hensch N, Weder W, Moch H and Kristiansen G:
Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W:
The expression analysis of periostin in human breast cancer. J Surg
Res. 160:102–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Puglisi F, Puppin C, Pegolo E, Andreetta
C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante
G and Di Loreto C: Expression of periostin in human breast cancer.
J Clin Pathol. 61:494–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Contié S, Voorzanger-Rousselot N, Litvin
J, Clézardin P and Garnero P: Increased expression and serum levels
of the stromal cell-secreted protein periostin in breast cancer
bone metastases. Int J Cancer. 128:352–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F and
Yuan YZ: Circulating levels of periostin may help identify patients
with more aggressive colorectal cancer. Int J Oncol. 34:821–828.
2009.PubMed/NCBI
|
|
41
|
Li Z, Zhang X, Yang Y, Yang S, Dong Z, Du
L, Wang L and Wang C: Periostin expression and its prognostic value
for colorectal cancer. Int J Mol Sci. 16:12108–12118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu X, Chang W, Yuan J, Han X, Tan X, Ding
Y, Luo Y, Cai H, Liu Y, Gao X, et al: Periostin expression in
intra-tumoral stromal cells is prognostic and predictive for
colorectal carcinoma via creating a cancer-supportive niche.
Oncotarget. 7:798–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma-derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee YJ, Kim IS, Park SA, Kim Y, Lee JE,
Noh DY, Kim KT, Ryu SH and Suh PG: Periostin-binding DNA aptamer
inhibits breast cancer growth and metastasis. Mol Ther.
21:1004–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Choi KU, Yun JS, Lee IH, Heo SC, Shin SH,
Jeon ES, Choi YJ, Suh DS, Yoon MS and Kim JH: Lysophosphatidic
acid-induced expression of periostin in stromal cells: Prognoistic
relevance of periostin expression in epithelial ovarian cancer. Int
J Cancer. 128:332–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu M, Fejzo MS, Anderson L, Dering J,
Ginther C, Ramos L, Gasson JC, Karlan BY and Slamon DJ: Periostin
promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol.
119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan W and Shao R: Transduction of a
mesenchyme-specific gene periostin into 293T cells induces cell
invasive activity through epithelial-mesenchymal transformation. J
Biol Chem. 281:19700–19708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ouyang G, Liu M, Ruan K, Song G, Mao Y and
Bao S: Upregulated expression of periostin by hypoxia in
non-small-cell lung cancer cells promotes cell survival via the
Akt/PKB pathway. Cancer Lett. 281:213–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hong LZ, Wei XW, Chen JF and Shi Y:
Overexpression of periostin predicts poor prognosis in non-small
cell lung cancer. Oncol Lett. 6:1595–1603. 2013.PubMed/NCBI
|
|
51
|
Tischler V, Fritzsche FR, Wild PJ, Stephan
C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J,
Schraml P, et al: Periostin is up-regulated in high grade and high
stage prostate cancer. BMC Cancer. 10:2732010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tai IT, Dai M and Chen LB: Periostin
induction in tumor cell line explants and inhibition of in vitro
cell growth by anti-periostin antibodies. Carcinogenesis.
26:908–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang L, Murota H, Serada S, Fujimoto M,
Kudo A, Naka T and Katayama I: Histamine contributes to tissue
remodeling via periostin expression. J Invest Dermatol.
134:2105–2113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li P, Oparil S, Feng W and Chen YF:
Hypoxia-responsive growth factors upregulate periostin and
osteopontin expression via distinct signaling pathways in rat
pulmonary arterial smooth muscle cells. J Appl Physiol (1985).
97:1549–1558. 2004. View Article : Google Scholar
|
|
55
|
Erkan M, Kleeff J, Gorbachevski A, Reiser
C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA and Friess
H: Periostin creates a tumor-supportive microenvironment in the
pancreas by sustaining fibrogenic stellate cell activity.
Gastroenterology. 132:1447–1464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oshima A, Tanabe H, Yan T, Lowe GN,
Glackin CA and Kudo A: A novel mechanism for the regulation of
osteoblast differentiation: Transcription of periostin, a member of
the fasciclin I family, is regulated by the bHLH transcription
factor, twist. J Cell Biochem. 86:792–804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takayama G, Arima K, Kanaji T, Toda S,
Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T and Izuhara
K: Periostin: A novel component of subepithelial fibrosis of
bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy
Clin Immunol. 118:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, et al: Periostin, a cell adhesion molecule,
facilitates invasion in the tumor microenvironment and annotates a
novel tumor-invasive signature in esophageal cancer. Cancer Res.
70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kikuchi Y, Kunita A, Iwata C, Komura D,
Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita
Y, et al: The niche component periostin is produced by
cancer-associated fibroblasts, supporting growth of gastric cancer
through ERK activation. Am J Pathol. 184:859–870. 2014. View Article : Google Scholar : PubMed/NCBI
|