Introduction
Throughout the evolution of rectal cancer resection,
an anastomotic leak (AL) has been considered the most serious
postoperative complication. Although laparoscopic anterior
resection of rectal cancer (LARRC) and intracorporeal anastomosis
possess several technical drawbacks, including the lack of direct
tactile sense, inadequate traction and an ineffective cutting angle
of the end linear surgical stapler (1), the rate of AL in LARRC is similar to
that of open surgery; reported to be between 6 and 17% (2–9).
Furthermore, previous studies have demonstrated that LARRC is safe
and feasible (10–14).
AL often results in serious outcomes, including
sepsis and emergency surgery, with ensuing prolonged hospital
stays, increased costs, and increased morbidity and mortality
(15,16). Previously, ileostomy was the only
widely used method of avoiding AL-associated complications
(17,18) despite the possibility of
stoma-associated complications and a secondary surgery to close the
stoma, which increase patient distress, overall costs and results
in additional scarring (19,20).
To protect the anastomosis and avoid stoma reversal
surgery two novel surgical methods, the Valtrac™-secured
intracolonic bypass (VIB) and the spontaneously closing cannula
ileostomy (SCCI), were developed in the Department of Colorectal
Surgery at The First Affiliated Hospital, Zhejiang University
(Hangzhou, Zhejiang, China). The use of VIB has been reported in
open surgery (21,22) and laparoscopic surgery (23), whereas the use of SCCI has been
reported in open procedures and hand-assisted laparoscopic surgery
(24–26). Suturing of the ileum to the peritoneum
could not be finished under laparoscopic guidance, therefore, SCCI
was not performed during laparoscopic surgery until the location of
the SCCI was moved in November 2013. Subsequently, the SCCI
technique was applied to LARRC. The present study assessed the
efficacy and safety of SCCI in LARRC in comparison with
ileostomy.
Patients and methods
Patients
The medical records of 41 consecutive patients with
rectal cancer who had undergone selective LARRC with SCCI or
ileostomy procedures to protect the anastomosis in the Department
of Colorectal Surgery, First Affiliated Hospital, Zhejiang
University, between November 2013 and August 2014, were
retrospectively reviewed. All patients were followed up for ≥6
months following laparoscopic surgery. Patients in the ileostomy
group were followed up for at least 3 months after reversal
surgery.
Approval for the study was obtained from the
Institutional Review Board of the First Affiliated Hospital,
College of Medicine, Zhejiang University. Although a number of
patients did not receive protective procedures in later surgery,
all patients were informed about the LARRC, ileostomy or SCCI
procedures preoperatively. Written consent was obtained from the
patients between 1 and 3 days prior to the surgery. Inclusion
criteria for the laparoscopic surgery were: i) A localized tumor
>4 and <12 cm from the anal verge; ii) compliance with
laparoscopy procedures; and iii) sufficient heart and lung function
to withstand laparoscopic surgery. Exclusion criteria for the
minimally invasive approach were: i) Cancer infiltrating contiguous
organs [T4 of the Tumor-Node-Metastasis (TNM) colorectal cancer
staging system] (27); ii)
counter-indications to the pneumoperitoneum; and iii) a long-axis
tumor size >6 cm. Preoperative study was based on locoregional
staging using transanal ultrasonography or magnetic resonance
imaging scans, and contrast-enhanced computed tomography scans of
the thorax, abdomen and pelvis.
Patients with locally advanced rectal carcinomas
(T3N0 or N+ of the TNM colorectal cancer staging system) were
routinely suggested to receive neoadjuvant chemoradiation of 25
fractions of 45 Gy over 5 weeks, with 825 mg/m2 oral
capecitabine twice daily (28).
However, these suggestions were rejected by a number of the
patients or their relatives. All patients treated with preoperative
chemoradiation underwent surgery within 6 to 8 weeks of completing
neoadjuvant therapy. Appropriate demographic information comprised
age, sex, American Society of Anesthesiologists (ASA) score, body
mass index (BMI), comorbidities, smoking status, level of tumor
(the distance from the inferior margin of tumor to the anus) and
neoadjuvant therapies. Measured outcomes included: Level of
anastomosis, surgical duration, intraoperative blood loss, number
of linear stapler firings, postoperative complications, length of
postoperative stay, cost for LARRC, total cost (which included the
cost of reversal surgery in the ileostomy group), Duke's stage,
number of harvested lymph nodes and return of bowel function.
Additionally, the protective period, the time to cannula removal
and the time to cannula stoma closure were assessed in the SCCI
group. Wounds were monitored daily until patients were discharged,
and a follow-up of between 1 and 2 weeks was performed to observe
any signs of infection.
Surgery
Surgical technique for laparoscopic anterior
resection
The patients were placed in a modified lithotomy,
right side down, in the Trendelenburg position. The open technique
was used to introduce an initial 10-mm port, inferior to the
umbilicus, prior to formation of a pneumoperitoneum using carbon
dioxide. The gas line was connected and the laparoscope introduced.
Subsequently, two 5-mm ports were inserted in the upper right and
left abdominal quadrants, and one 12-mm port was inserted in the
lower right abdominal quadrant under laparoscopic guidance. For the
very low anastomosis patients, an additional 5-mm port was inserted
between 3 and 4 cm superior to the upper margin of the pubic
symphysis.
The procedure permitted the laparoscopic no-touch
isolation technique, the so-called ‘medial to lateral’ approach and
total mesorectal excision (TME) principles. Following mobilization
of the left colon, if necessary, mobilization of the splenic
flexure was performed; intracorporeal ligation of the inferior
mesenteric vessels was performed, followed by mobilization of the
rectum and the mesorectum. The peritoneum was cut from the lateral
side to complete full mobilization prior to intracorporeal
transection of the distal bowel, using a 45- or 60-mm Endo GIA
stapler (Ethicon, Inc.; Johnson & Johnson, Cincinnati, OH,
USA). The bowel was extracted through a small incision made beneath
a wound protector in the left lower quadrant port and subsequently
removed from associated proximal bowel. Purse string sutures were
applied at the proximal stumps using straight needles (3–0 Maxon™,
United States Surgical, Norwalk, CT, USA) and monofilament
absorbable thread. A pocket was created locally to the proximal end
of the sigmoid colon, and the anvil of an end-to-end anastomotic
stapler (Ethicon Inc, Johnson & Johnson, Miami, FL, USA) was
inserted and fixed. The stapler was inserted through the anus into
the pelvic cavity. Following stapling, the anastomotic site and
stapler were evaluated, and any anastomotic tears were sutured.
Subsequently, SCCI or ileostomy was performed.
SCCI technique
Previously, two SCCI techniques have been reported
by the Department of Colorectal Surgery, First Affiliated Hospital,
Zhejiang University (24,26). An appropriate diversion of the distal
small bowel was performed in the two methods to provide temporary
diversion; the distinction was whether a stapler was used to block
the distal ileum or not. In the present study, the SCCI protocol
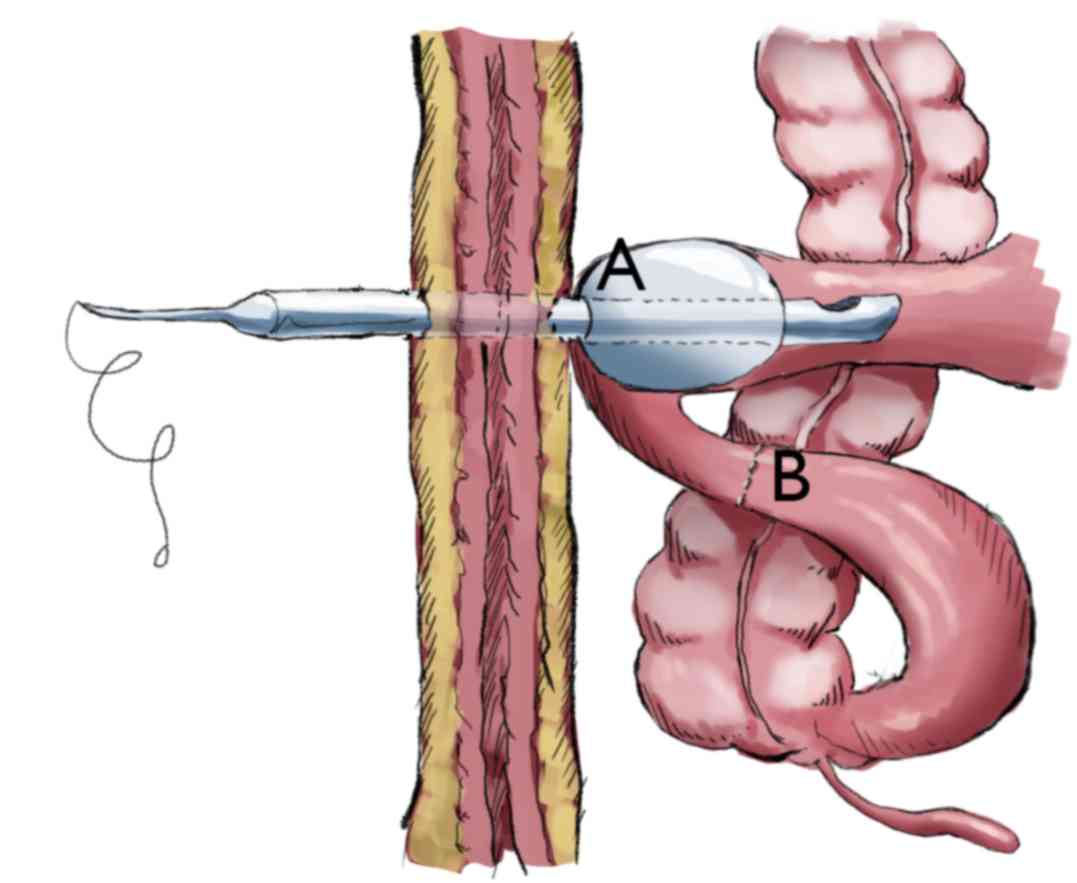
followed the method of Xu et al (26) (Fig.
1).
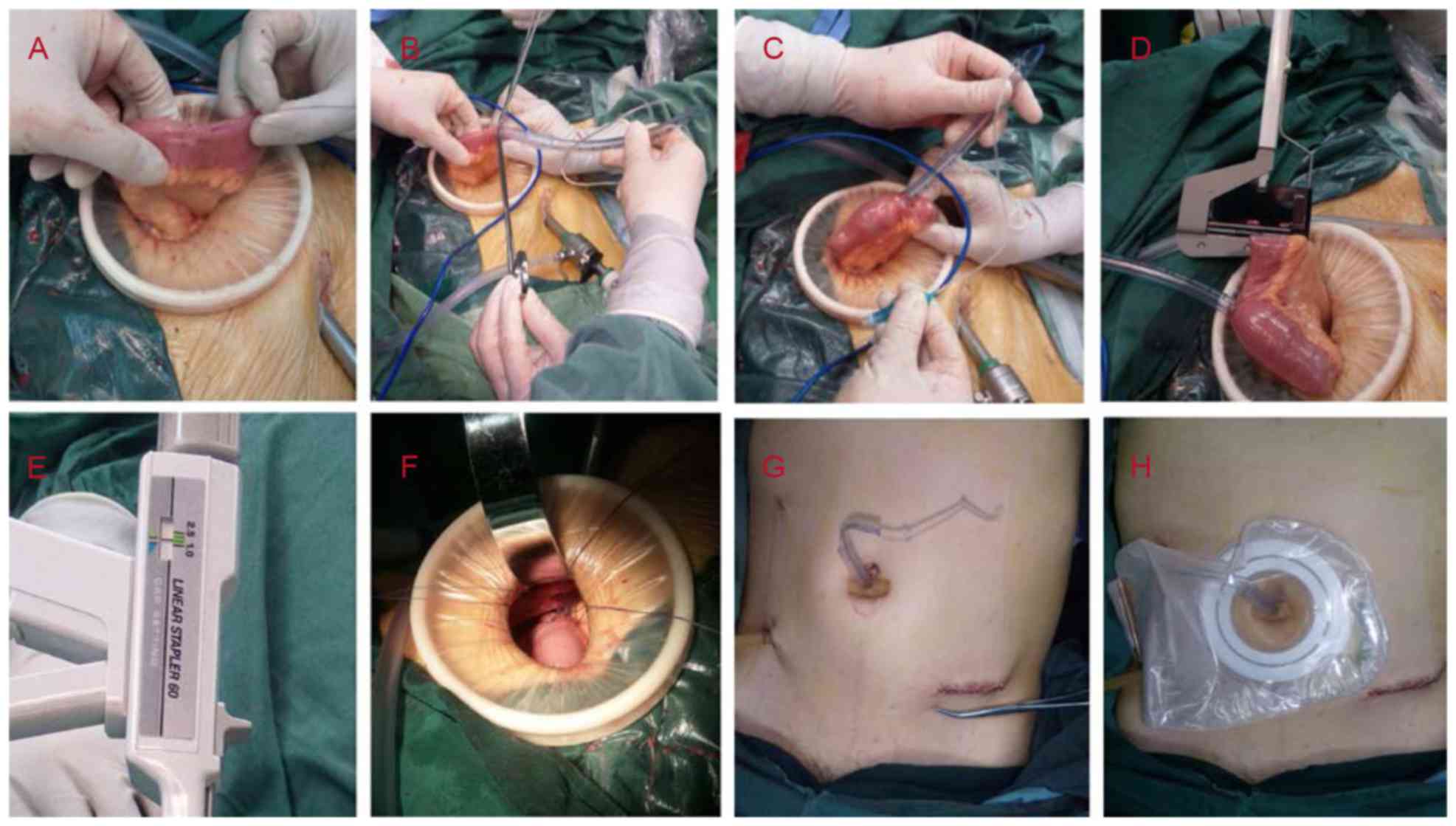
First, a purse string (3–0 Vicryl™; Johnson &
Johnson, New Brunswick, NJ, USA) was made in the distal ileum 20–28
cm from the ileocecal valve (Fig.
2A). The diameters of the purse-string rings were ~10 mm.
Thereafter, a small incision was created within the purse string; a
7.5-mm diameter hard trachea cannula (QC-01; Hangzhou Jinlin
Medical Appliances Co., Ltd., Hangzhou, China) was then inserted,
directed toward the proximal lumen (Fig.
2B). Normal saline (7–10 ml) was then injected into the air bag
of the cannula until the ileum wall began to turn pale (Fig. 2C). The quantity of liquid was
dependent on the inner diameter of the ileum cavity. The filled air
bag prevented the cannula from slipping off of the ileum and also
prevented downward intestinal content travel to a certain
degree.
Second, the small bowel was blocked at B position
(presented in Fig. 1) 5–8 cm from the
purse string and 15–20 cm from the ileocecal valve, using a TL60
stapler (Ethicon Inc.; Johnson & Johnson; Fig. 2D). One row of nails, instead of the
original two rows, was applied, with an appropriate closing
thickness of between 1.5 and 2.0 mm (Fig.
2E).
Third, the cannula was pulled out of the abdominal
cavity through the initial 10-mm port inferior to the umbilicus.
The seromuscular layer of the ileum was sutured to the same
location in the peritoneum, close to the tube; and the interrupted
cyclic fixation was completed using 3 or 4 absorbable sutures (3–0
Vicryl; Johnson & Johnson; Fig.
2F). The tube was then pulled tight and the sutures at the
fixation site were tightly knotted. The suturing process is
important to avoid abdominal infection following tube extraction
and it aintains the adhesion of bowel and peritoneum close to the
tube. Finally, a short flexible rubber tube was set around the tube
outside the abdominal skin to fix the tracheal tube (Fig. 2G), and then an ostomy bag was placed
to collect intestinal contents (Fig.
2H).
Ileostomy technique
The technical aspects of ileostomy have been well
studied (24). The stomas were placed
on the lower right side of the abdomen and were closed between 3
and 6 months later.
Assessment and definition
Bowel function
Return of bowel function was defined as the first
passage of flatus or bowel movement with tolerance of an oral diet.
Ileus was defined as a delayed return of bowel function. Observed
symptoms of ileus included abdominal distention, intolerance of an
oral diet, nausea or radiographic evidence of dilated bowel with no
evident obstruction. Bowel obstruction was defined as exhibiting
similar symptoms to ileus with the distinction that these symptoms
were accompanied by radiographic evidence of the dilated bowel with
a clear obstruction observed. In the SCCI group, once bowel
function recovered, the patients were provided a liquid diet or a
no-residue semiliquid diet until the cannula was removed. The
special diet supplement was administered in order to prevent
obstruction of the minor-caliber cannula stoma.
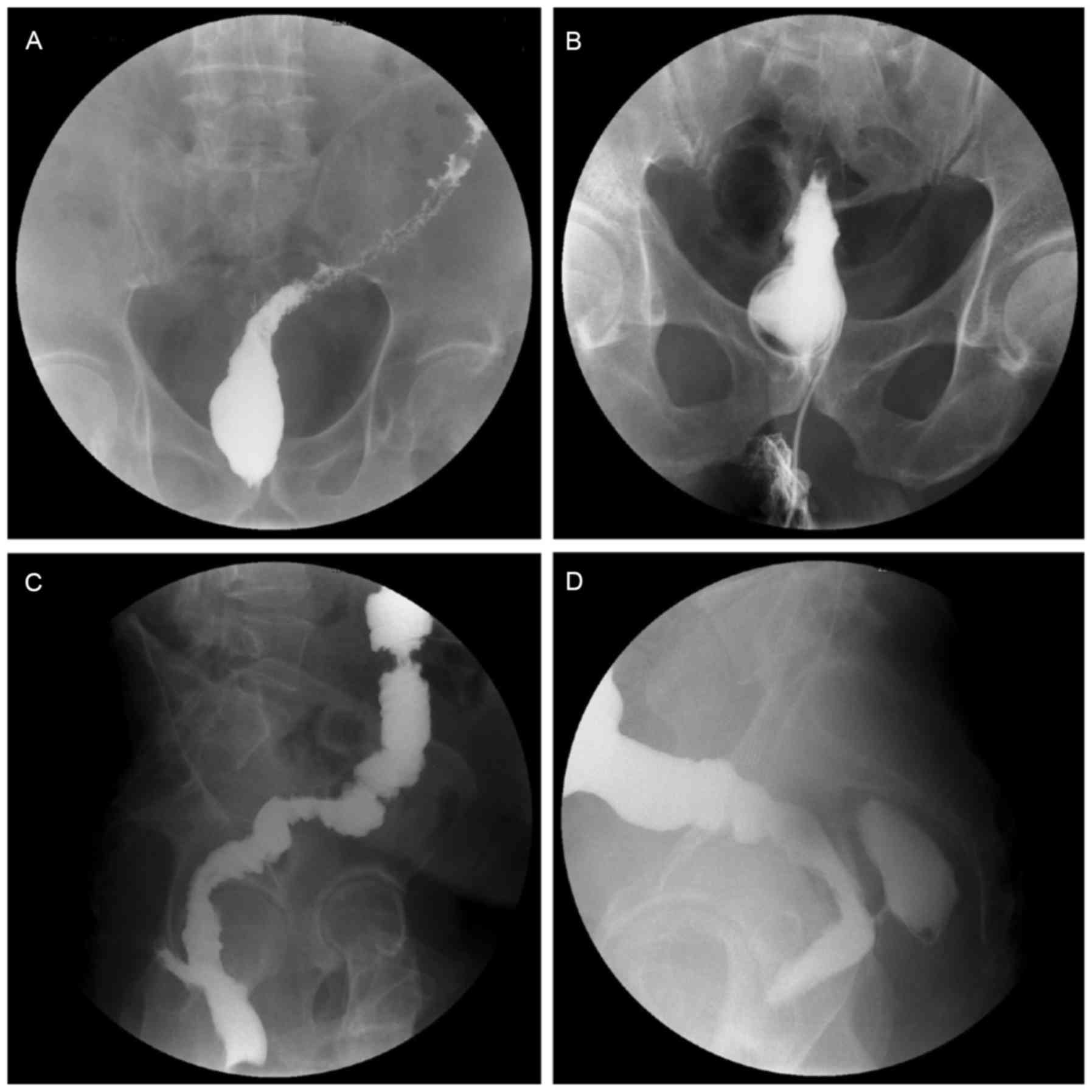
AL, dehiscence and stenosis
Routine examination of the anastomosis was performed
at ~7 and 14 days postoperatively by digital rectal examination. If
necessary, a Urografin enema colonography was performed to assess
the anastomosis. Digital rectal examination and colonography were
conducted on all patients in the ileostomy group prior to the
reversal surgery in order to avoid AL, dehiscence and stenosis
(Fig. 3).
AL was defined as a defect in intestinal wall
integrity at the colorectal or coloanal anastomosis site, leading
to communication between the intra- and extra-luminal compartments,
and may be diagnosed through digital rectal examination or
colonography. Anastomotic dehiscence was defined as incomplete
anastomosis, usually detected through digital rectal examination
and lacking clinical manifestations. Anastomotic stenosis was
detected upon digital rectal examination or colonography, and an
additional procedure to relieve the stenosis was inevitably
required prior to undertaking the reversal surgery.
Time to cannula removal and cannula stoma
closure
Time to cannula removal was defined as the duration
from laparoscopic surgery to cannula removal. The criteria for
removing cannula were: i) A period of at least 14 days
postoperatively; ii) recovered anal defecation; and iii) absence of
evident dehiscence on digital examination, and colonography if
required. When AL occurred, if an emergency surgery was not
required, the cannula was retained and the patient received total
parenteral nutrition or enteral nutrition until the AL was closed.
Time to cannula stoma closure was defined as the duration from
cannula removal to the closure of cannula stoma. This time impacted
patient quality of life.
Protective period and stoma period
The stoma period in the SCCI group was defined as
the period from the surgery to the closure of cannula stoma, which
included the time to cannula removal and the time to cannula stoma
closure, as aforementioned. The stoma period in the ileostomy group
refers to the duration between the laparoscopic surgery and the
reversal surgery. This time impacted the patient quality of
life.
Statistical analysis
Due to the small sample size of the two treatment
groups, continuous and ordinal data were presented as median with
interquartile range (IQR; range between the 25 and 75th
percentiles). Comparisons of non-parametric data between groups
were performed using the Mann-Whitney U test. The categorical data
were presented as numbers and percentages, and Fisher's exact test
was performed for comparisons between groups. The categorical data
of Dukes' stage was evaluated using linear-by-linear association. A
two-tailed P-value of <0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS (version 16.0; SPSS, Inc., Chicago, IL,
USA).
Results
Between November 2013 and August 2014 the SCCI and
ileostomy techniques were performed in 19 and 22 cases of LARRC,
respectively. The demographic data and preoperative symptoms of the
patients were similar in the two groups, including age, sex, ASA
score, BMI, smoking status, preexisting diseases, level of tumor
(the distance from the inferior margin of tumor to the anus) and
neoadjuvant radio-chemotherapy (Table
I).
 | Table I.General patient characteristics. |
Table I.
General patient characteristics.
| Characteristic | All patients
(n=41) | SCCI patients
(n=19) | Ileostomy patients
(n=22) | P-value |
|---|
| Agea, years | 64 (61–67.5) | 64 (59–68) | 64.5 (62–67.8) | 0.409 |
| Sexb |
|
|
| >0.999 |
|
Female | 17 (41.5) | 8 (42.1) | 9 (40.9) |
|
|
Male | 24 (58.5) | 11 (57.9) | 13 (59.1) |
|
| ASA
scorea | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.286 |
| BMIa, kg/m2 | 23.6
(22.9–24.1) | 23.5
(22.5–24.1) | 23.6
(23.1–24.5) | 0.505 |
|
Diabetesb | 8 (19.5) | 4 (21.1) | 4 (18.2) | >0.999 |
|
Hypertensionb | 11 (26.8) | 5 (26.3) | 6 (27.3) | >0.999 |
| Smokerb | 10 (24.4) | 5 (26.3) | 5 (22.7) | >0.999 |
| Level of
tumora, cm | 7 (6–8) | 7 (6–8) | 7 (5–8) | 0.698 |
| Neoadjuvant
radio-chemotherapyb | 6 (14.6) | 3 (15.8) | 3 (13.6) | >0.999 |
The intraoperative and postoperative patient data
are presented in Table II. No
significant differences in the level of anastomosis, surgical time,
intraoperative blood loss, number of linear stapler firings,
adverse events, cost of LARRC, distribution of Duke's stage, number
of harvested lymph nodes or time of bowel function recovery were
identified (P>0.05). Due to the inevitability of reversal
surgery in the ileostomy group, the overall postoperative hospital
stay was significantly increased compared with that in the SCCI
group (median, 13 vs. 7 days; P<0.001); the total cost in the
ileostomy group was also significantly increased compared with that
in the SCCI group (median, $11,350 vs. $6,500; P<0.001).
 | Table II.Surgical patient data. |
Table II.
Surgical patient data.
| Characteristic | All patients
(n=41) | SCCI patients
(n=19) | Ileostomy patents
(n=22) | P-value |
|---|
| Level of
anastomosisa, cm | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.559 |
| Surgical
timea, min | 200
(177.5–214.5) | 199 (175–214) | 201 (177–223) | 0.763 |
| Intraoperative
blood lossa | 100 (50–100) | 50 (50–100) | 100 (50–100) | 0.277 |
| Number of linear
stapler firingsa | 3 (3–3) | 3 (2–3) | 3 (3–4) | 0.077 |
| Adverse events |
|
|
|
|
|
Staple-line
bleedingb | 3 (7.3) | 1 (5.3) | 2 (9.1) | >0.999 |
|
Anastomotic leakb | 1 (2.4) | 1 (5.3) | 0 (0.0) | 0.463 |
|
Anastomotic
dehiscenceb | 3 (7.3) | 1 (5.3) | 2 (9.1) | >0.999 |
| Wound
infectionb,c | 5 (12.2) | 2 (10.5) | 3 (13.6) | >0.999 |
| Stapled
anastomotic stenosisb | 2 (4.9) | 0 (0.0) | 2 (9.1) | 0.490 |
| Length of
post-operative stay, daysa,b,d | 12 (7–13.5) | 7 (7–9) | 13 (13–15.25) | <0.001 |
| Cost in
$1,000s |
|
|
|
|
| Cost
for LARRCa | 6.6 (6.4–6.75) | 6.5 (6.4–6.7) | 6.6
(6.4–6.825) | 0.502 |
| Total
costa,c,d | 10.9
(6.55–11.4) | 6.5 (6.4–6.7) | 11.35
(11.1–11.6) | <0.001 |
| Duke's
stagese |
|
|
|
|
| A
(T1-2N0M0) | 4 (9.8) | 2 (10.5) | 2 (9.1) | 0.814 |
| B
(T3-4N0M0) | 30 (73.2) | 14 (73.7) | 16 (72.7) |
|
| C
(TXN1-2M0) | 7 (17.1) | 3 (15.8) | 4 (18.2) |
|
| Number of harvested
lymph nodesa | 13 (12–14.5) | 14 (12,15) | 13 (12–13.25) | 0.100 |
| Time of bowel
function recovery, days |
|
|
|
|
| First
flatus postoperativelya | 3 (3–3) | 3 (3–3) | 3 (3–4) | 0.162 |
| Oral
intakea | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.541 |
AL occurred in 1 case in the SCCI group, at day 6
postoperatively, in a patient exhibiting a fever (38.5–38.8°C for 2
days and 37.8–38.2°C for 3 days) and drainage of muddy liquid
(10–20 ml, duration of 6 days). There was no serious sepsis or
emergency surgery required in this patient, which was a benefit of
the protection afforded by SCCI. For patients in the protective
period, feces had not passed through the anastomosis. A total of 2
patients with stapled anastomotic stenosis were identified in the
ileostomy group where an additional procedure to relieve the
stenosis was subsequently performed.
With the key aim of avoiding emergency surgery as a
result of AL, in the SCCI group the median protective period was 22
days (IQR, 19–22 days). The median time to cannula removal and
cannula stoma closure were 23 days (IQR, 20–24 days) and 12 days
(IQR, 11–13 days), respectively. The stoma period in the SCCI group
was significantly decreased compared with that in the ileostomy
group (median, 34 vs. 95 days; P<0.001), which led to improved
patient quality of life (Table
III).
 | Table III.Protective period, time to cannula
removal, time to cannula stoma closure and stoma period. |
Table III.
Protective period, time to cannula
removal, time to cannula stoma closure and stoma period.
| Parameter,
days | All patients
(n=41) | SCCI patients
(n=19) | Ileostomy patients
(n=22) | P-value |
|---|
| Protective
period |
| 22 (19–22) |
|
|
| Time to cannula
removal |
| 23 (20–24) |
|
|
| Time to cannula
stoma closure |
| 12 (11–13) |
|
|
| Stoma
perioda | 89 (34–95) | 34 (31–37) | 95 (91–99) | <0.001 |
Discussion
AL remains the most serious early postoperative
complication following rectal cancer resection using open and
laparoscopic techniques (2–5,7–9,29–32), frequently exhibiting early adverse
effects on bowel function and quality of life (33). Furthermore, numerous studies suggested
that the increased incidence of negative prognostic factors,
including local recurrence, functional disorders and mortality,
were associated with AL (2,15,16,34–38).
Despite much effort, even the most experienced surgeon is unable to
avoid AL in all cases. The major risk factors for AL include being
male, older age, smoking, obesity, TME, blood loss, neoadjuvant
radiotherapy, corticosteroid treatment, diabetes, hypertension,
cardiovascular disease, weight loss, hypoproteinemia, anemia,
metabolic disorders, the distance of the anastomosis from the anal
verge and the experience of the surgeon (2–5,16,29,39–45).
Additionally, laparoscopic rectal transection in the lower rectum
utilizes at least two linear staplers (in the present study, the
median number of linear stapler firings in the two groups was 3)
with a cutter, which may lead to an excessively long stapling line
with an inadequate cutting angle, which may increase the incidence
of AL (1,10). Thereafter, protection of the
anastomosis in LARRC was inevitable in certain high-risk
patients.
To protect the anastomosis and avoid additional
reversal surgery, the Department of Colorectal Surgery, First
Affiliated Hospital, Zhejiang University, developed two surgical
methods, VIB and SCCI. However, the VIB technique was not suitable
for patients in whom the anastomosis was too far from the anal
verge (>7 cm) or in whom the colon wall was too thick.
Furthermore, the protected period in the VIB group could not be
controlled, and so could not be prolonged artificially when the AL
persisted. Nevertheless, SCCI is able to solve these problems and
has been used in open procedures and hand-assisted laparoscopic
surgery (24–26). The interrupted cyclic fixation step
from the ileum to the peritoneum was completed through the
abdominal incision, as settling of the stoma position in the right
lower abdomen prevented completion of this step under the
laparoscopic incision. Furthermore, this step was key to preventing
abdominal infection following tube extraction and maintaining
adhesion of the bowel and peritoneum to the tube. These issues
prevented use of the SCCI method in LARRC. In the present study,
the stoma position was transferred from the right lower abdomen to
the puncture hole closely inferior to the umbilicus. Subsequently,
the interrupted cyclic fixation step was able to be finished
through the laparoscopic incision, as the distance from the
incision to the cannula ileostomy was sufficiently decreased.
Additionally, there was an adjustment to the surgical detail; a
previous study indicated that a double row of concentric purse
string sutures was required (24),
whereas the patients in the present study accepted a single row of
purse string sutures in the distal ileum. However, no
suture-associated adverse events were detected in any cases.
Therefore, the results of the present study suggest that a single
row of purse string sutures is sufficient, which will decrease the
duration of surgery by several min.
No statistical significance was evident in the
majority of the parameters between the SCCI and ileostomy groups in
LARRC; however, the total cost, length of postoperative stay and
stoma period were significantly improved in the SCCI group
(P<0.001), with reversal surgery in the ileostomy group as an
underlying influencing factor. These results suggested that the
SCCI procedure in LARRC obviates the requirement for reversal
surgery, and exhibits safety comparable to the ileostomy group in
the primary surgery. The results of the present study supported
previous data from a large sample study in open and hand-assisted
laparoscopic low anterior resection (24), in which it was revealed that the
overall postoperative hospital stay (P<0.01) and cost
(P<0.01) were increased in the Ileostomy group compared with the
SCCI group.
As aforementioned, 1 patient in the SCCI group
experienced AL at day 6 postoperatively, with the manifestation of
a fever (38.5–38.8°C for 2 days and 37.8–38.2°C for 3 days) and
drainage of a small amount of muddy liquid (10–20 ml for 6 days).
Due to the protection offered by SCCI, feces had not passed through
the anastomosis, which may have contributed to the absence of
serious peritonitis. Therefore, this patient was cured without
requiring emergency surgery. Whether defunctioning stoma can
prevent AL remains unknown, however, it decreases the incidence of
sepsis and emergency surgery due to fecal diversion (17,18,46,47).
Furthermore, AL typically presents 2–17 days postoperatively
(48), therefore the key stage during
which fecal diversion must be maintained is 3–4 weeks
postoperatively. In the present study, the median protected period
was 22 days, during which the anastomosis was effectively
protected. Additionally, the air bag filled with normal saline
prevented downward intestinal content travel; although the ileum
cavity was slowly expanded to relieve the block, more normal saline
could be injected into the air bag to maintain the blocking of
feces. To prolong the protected period, use of two separate
single-row nails to block the ileum is currently under
investigation. The two novel aforementioned methods may prolong the
protected period significantly.
A total of 2 patients had stapled anastomotic
stenosis in the ileostomy group; thus, an additional procedure to
relieve the stenosis was required. These 2 patients were among the
3 patients who underwent neoadjuvant radio-chemotherapy
preoperatively. However, no stenosis occurred in the SCCI group,
although 3 patients received neoadjuvant radio-chemotherapy
preoperatively. According to the National Comprehensive Cancer
Network guidelines (49), patients
with locally advanced cancer are suggested to undergo neoadjuvant
radio-chemotherapy preoperatively. However, these patients exhibit
an increased rate of anastomotic stenosis, which is associated with
the period for the absence of feces passing through the anastomosis
(50–54). For the majority of patients in the
SCCI group in the present study, anal defecation recovered in less
than a month, which may prevent stenosis and the requirement for
additional surgery. Additionally, SCCI did not affect postoperative
chemotherapy; chemotherapy was able to be administered prior to
cannula stoma closure or even prior to cannula removal.
In conclusion, despite the small size of the
treatment groups and the non-randomized nature of the present
study, results demonstrated that in LARRC, the SCCI procedure
appeared to be a safe, feasible diverting technique to protect the
anastomosis. Compared with ileostomy, the SCCI procedure obviated
the requirement for stoma reversal surgery, which resulted in a
decreased postoperative hospital stay, hospitalization costs and
stoma period.
Acknowledgements
The present study was supported by the Public
Welfare Technology Research Project and the Science and Technology
Department of Zhejiang, China (grant no. 2015C33208).
Glossary
Abbreviations
Abbreviations:
|
AL
|
anastomotic leak
|
|
ASA
|
American Society of
Anesthesiologists
|
|
BMI
|
body mass index
|
|
LARRC
|
laparoscopic anterior resection of
rectal cancer
|
|
SCCI
|
spontaneously closing cannula
ileostomy
|
|
TME
|
total mesorectal excision
|
|
VIB
|
Valtrac™-secured intracolonic
bypass
|
References
|
1
|
Targarona EM, Balague C, Pernas JC,
Martinez C, Berindoague R, Gich I and Trias M: Can we predict
immediate outcome after laparoscopic rectal surgery? Multivariate
analysis of clinical, anatomic, and pathologic features after
3-dimensional reconstruction of the pelvic anatomy. Ann Surg.
247:642–649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Branagan G and Finnis D: Wessex Colorectal
Cancer Audit Working Group: Prognosis after anastomotic leakage in
colorectal surgery. Dis Colon Rectum. 48:1021–1026. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matthiessen P, Hallböök O, Andersson M,
Rutegård J and Sjödahl R: Risk factors for anastomotic leakage
after anterior resection of the rectum. Colorectal Dis. 6:462–469.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JS, Choi GS, Kim SH, Kim HR, Kim NK,
Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, et al: Multicenter
analysis of risk factors for anastomotic leakage after laparoscopic
rectal cancer excision: The Korean laparoscopic colorectal surgery
study group. Ann Surg. 257:665–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh CY, Changchien CR, Wang JY, Chen JS,
Chen HH, Chiang JM and Tang R: Pelvic drainage and other risk
factors for leakage after elective anterior resection in rectal
cancer patients: A prospective study of 978 patients. Ann Surg.
241:9–13. 2005.PubMed/NCBI
|
|
6
|
Anthuber M, Fuerst A, Elser F, Berger R
and Jauch KW: Outcome of laparoscopic surgery for rectal cancer in
101 patients. Dis Colon Rectum. 46:1047–1053. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feliciotti F, Guerrieri M, Paganini AM, De
Sanctis A, Campagnacci R, Perretta S, D'Ambrosio G and Lezoche E:
Long-term results of laparoscopic versus open resections for rectal
cancer for 124 unselected patients. Surg Endosc. 17:1530–1535.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JS, Choi GS, Jun SH, Hasegawa S and
Sakai Y: Laparoscopic versus open intersphincteric resection and
coloanal anastomosis for low rectal cancer: Intermediate-term
oncologic outcomes. Ann Surg. 254:941–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng
Z, Li L, Shu Y and Wang TC: Laparoscopic versus open total
mesorectal excision with anal sphincter preservation for low rectal
cancer. Surg Endosc. 18:1211–1215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akiyoshi T, Ueno M, Fukunaga Y, Nagayama
S, Fujimoto Y, Konishi T, Kuroyanagi H and Yamaguchi T: Incidence
of and risk factors for anastomotic leakage after laparoscopic
anterior resection with intracorporeal rectal transection and
double-stapling technique anastomosis for rectal cancer. Am J Surg.
202:259–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YW, Huang LY, Song CL, Zhuo CH, Shi
DB, Cai GX, Xu Y, Cai SJ and Li XX: Laparoscopic vs open
abdominoperineal resection in the multimodality management of low
rectal cancers. World J Gastroenterol. 21:10174–10183. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Kang L, Huang M, Luo Y, Wang L, Lan
P, Cui J and Wang J: Open surgery against laparoscopic surgery for
mid-rectal or low-rectal cancer of male patients: better
postoperative genital function of laparoscopic surgery. Surg
Laparosc Endosc Percutan Tech. 25:444–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S, Jiang F, Tu J and Zheng X: Long-term
oncologic outcomes of laparoscopic versus open surgery for middle
and lower rectal cancer. PLoS One. 10:e01358842015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vargas GM, Sieloff EP, Parmar AD, Tamirisa
NP, Mehta HB and Riall TS: Laparoscopy decreases complications for
obese patients undergoing elective rectal surgery. Surg Endosc.
30:1826–1832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bell SW, Walker KG, Rickard MJ, Sinclair
G, Dent OF, Chapuis PH and Bokey EL: Anastomotic leakage after
curative anterior resection results in a higher prevalence of local
recurrence. Br J Surg. 90:1261–1266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung SH, Yu CS, Choi PW, Kim DD, Park IJ,
Kim HC and Kim JC: Risk factors and oncologic impact of anastomotic
leakage after rectal cancer surgery. Dis Colon Rectum. 51:902–908.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matthiessen P, Hallbook O, Rutegard J,
Simert G and Sjödahl R: Defunctioning stoma reduces symptomatic
anastomotic leakage after low anterior resection of the rectum for
cancer: A randomized multicenter trial. Ann Surg. 246:207–214.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huser N, Michalski CW, Erkan M, Schuster
T, Rosenberg R, Kleeff J and Friess H: Systematic review and
meta-analysis of the role of defunctioning stoma in low rectal
cancer surgery. Ann Surg. 248:52–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiomi A, Ito M, Saito N, Ohue M, Hirai T,
Kubo Y and Moriya Y: Diverting stoma in rectal cancer surgery. A
retrospective study of 329 patients from Japanese cancer centers.
Int J Colorectal Dis. 26:79–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo SB, Jeong SY, Lim SB, Park JW, Choi HS
and Oh JH: Left-sided ileostomy at specimen extraction site in
laparoscopic-assisted low anterior resection for rectal cancer. J
Laparoendosc Adv Surg Tech A. 23:22–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang D, Zhang Y, Dang C, Zhu K, Li K,
Chen D and Chen W: Prevention of anastomotic leakage after low
anterior resection in rectal cancers. Hepatogastroenterology.
57:477–481. 2010.PubMed/NCBI
|
|
22
|
Ye F, Wang D, Xu X, Liu F and Lin J: Use
of intracolonic bypass secured by a biodegradable anastomotic ring
to protect the low rectal anastomosis. Dis Colon Rectum.
51:109–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye F, Chen D, Wang D, Lin J and Zheng S:
Use of Valtrac™-secured intracolonic bypass in laparoscopic rectal
cancer resection. Medicine (Baltimore). 93:e2242014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua H, Xu J, Chen W, Zhou X, Wang J, Sheng
Q and Lin J: Defunctioning cannula ileostomy after lower anterior
resection of rectal cancer. Dis Colon Rectum. 57:1267–1274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Ke B, Lin J, Xu J and Chen W:
Application of a spontaneously closed protective stoma in an ileal
pouch-anal anastomosis: A preliminary study. Int J Clin Exp Med.
8:1281–1285. 2015.PubMed/NCBI
|
|
26
|
Xu JZ, Wang J, Hua H, Lin C, Liu F, Li Y
and Chen W: Spontaneously closed protective stoma: A preliminary
report of exploratory study. Chin J Practical Surg. 32:1040–1042.
2012.
|
|
27
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reyngold M, Niland J, ter Veer A, Milne D,
Bekaii-Saab T, Cohen SJ, Lai L, Schrag D, Skibber JM, Small W Jr,
et al: Neoadjuvant radiotherapy use in locally advanced rectal
cancer at NCCN member institutions. J Natl Compr Canc Netw.
12:235–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peeters KC, Tollenaar RA, Marijnen CA,
Kranenbarg Klein E, Steup WH, Wiggers T, Rutten HJ and van de Velde
CJ: Dutch Colorectal Cancer Group: Risk factors for anastomotic
failure after total mesorectal excision of rectal cancer. Br J
Surg. 92:211–216. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rudinskaite G and Pavalkis D: Coloanal
anastomosis in rectal cancer surgery. Medicina (Kaunas).
38:624–630. 2002.PubMed/NCBI
|
|
31
|
Karanjia ND, Corder AP, Bearn P and Heald
RJ: Leakage from stapled low anastomosis after total mesorectal
excision for carcinoma of the rectum. Br J Surg. 81:1224–1226.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dehni N, Schlegel RD, Cunningham C,
Guiguet M, Tiret E and Parc R: Influence of a defunctioning stoma
on leakage rates after low colorectal anastomosis and colonic J
pouch-anal anastomosis. Br J Surg. 85:1114–1117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashburn JH, Stocchi L, Kiran RP, Dietz DW
and Remzi FH: Consequences of anastomotic leak after restorative
proctectomy for cancer: Effect on long-term function and quality of
life. Dis Colon Rectum. 56:275–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Law WL, Choi HK, Lee YM, Ho JW and Seto
CL: Anastomotic leakage is associated with poor long-term outcome
in patients after curative colorectal resection for malignancy. J
Gastrointest Surg. 11:8–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laxamana A, Solomon MJ, Cohen Z, Feinberg
SM, Stern HS and McLeod RS: Long-term results of anterior resection
using the double-stapling technique. Dis Colon Rectum.
38:1246–1250. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mirnezami A, Mirnezami R, Chandrakumaran
K, Sasapu K, Sagar P and Finan P: Increased local recurrence and
reduced survival from colorectal cancer following anastomotic leak:
Systematic review and meta-analysis. Ann Surg. 253:890–899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rullier E, Laurent C, Garrelon JL, Michel
P, Saric J and Parneix M: Risk factors for anastomotic leakage
after resection of rectal cancer. Br J Surg. 85:355–358. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scott N, Jackson P, al-Jaberi T, Dixon MF,
Quirke P and Finan PJ: Total mesorectal excision and local
recurrence: A study of tumour spread in the mesorectum distal to
rectal cancer. Br J Surg. 82:1031–1033. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buchs NC, Gervaz P, Secic M, Bucher P,
Mugnier-Konrad B and Morel P: Incidence, consequences and risk
factors for anastomotic dehiscence after colorectal surgery: A
prospective monocentric study. Int J Colorectal Dis. 23:265–270.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buie WD, MacLean AR, Attard JA, Brasher PM
and Chan AK: Neoadjuvant chemoradiation increases the risk of
pelvic sepsis after radical excision of rectal cancer. Dis Colon
Rectum. 48:1868–1874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi HK, Law WL and Ho JW: Leakage after
resection and intraperitoneal anastomosis for colorectal
malignancy: Analysis of risk factors. Dis Colon Rectum.
49:1719–1725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leichtle SW, Mouawad NJ, Welch KB, Lampman
RM and Cleary RK: Risk factors for anastomotic leakage after
colectomy. Dis Colon Rectum. 55:569–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lipska MA, Bissett IP, Parry BR and Merrie
AE: Anastomotic leakage after lower gastrointestinal anastomosis:
Men are at a higher risk. ANZ J Surg. 76:579–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martel G, Al-Suhaibani Y, Moloo H, Haggar
F, Friedlich M, Mamazza J, Poulin EC, Stern H and Boushey RP:
Neoadjuvant therapy and anastomotic leak after tumor-specific
mesorectal excision for rectal cancer. Dis Colon Rectum.
51:1195–1201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zaharie F, Mocan L, Tomuş C, Mocan T,
Zaharie R, Bartoş D, Bartoş A, Vlad L and Iancu C: Risk factors for
anastomotic leakage following colorectal resection for cancer.
Chirurgia (Bucur). 107:27–32. 2012.PubMed/NCBI
|
|
46
|
Smith JD, Paty PB, Guillem JG, Temple LK,
Weiser MR and Nash GM: Anastomotic leak is not associated with
oncologic outcome in patients undergoing low anterior resection for
rectal cancer. Ann Surg. 256:1034–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin JK, Yueh TC, Chang SC, Lin CC, Lan YT,
Wang HS, Yang SH, Jiang JK, Chen WS and Lin TC: The influence of
fecal diversion and anastomotic leakage on survival after resection
of rectal cancer. J Gastrointest Surg. 15:2251–2261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tai JD, Liu YS and Wang GY: Risk factors
and the management of anastomotic leakage after anus-preserving
operation for rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
10:153–156. 2007.(In Chinese). PubMed/NCBI
|
|
49
|
Network, . NCC: NCCN Clinical Practice
Guidelines in Oncology: Rectal cancer. Version I. Washington,
National Comprehensive Cancer Network. 2015.
|
|
50
|
Widder J, Herbst F, Dobrowsky W, Schmid R,
Pokrajac B, Jech B, Chiari C, Stift A, Maier A, Karner-Hanusch J,
et al: Preoperative short-term radiation therapy (25 Gy, 2.5 Gy
twice daily) for primary resectable rectal cancer (phase II). Br J
Cancer. 92:1209–1214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Luna-Pérez P, Rodríguez-Ramírez S,
Rodriguez-Coria DF, Fernández A, Labastida S, Silva A and López MJ:
Preoperative chemoradiation therapy and anal sphincter preservation
with locally advanced rectal adenocarcinoma. World J Surg.
25:1006–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luna-Pérez P, Rodríguez-Ramírez S,
Hernández-Pacheco F, De La Barrera Gutiérrez M, Fernández R and
Labastida S: Anal sphincter preservation in locally advanced low
rectal adenocarcinoma after preoperative chemoradiation therapy and
coloanal anastomosis. J Surg Oncol. 82:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chan A, Wong A, Langevin J and Khoo R:
Preoperative concurrent 5-fluorouracil infusion, mitomycin C and
pelvic radiation therapy in tethered and fixed rectal carcinoma.
Int J Radiat Oncol Biol Phys. 25:791–799. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Brigand C, Rohr S and Meyer C: Colorectal
stapled anastomosis: Results after anterior resection of the rectum
for cancer. Ann Chir. 129:427–432. 2004. View Article : Google Scholar : PubMed/NCBI
|