Introduction
A number of biochemical, physiological and
behavioral processes have demonstrated that an internal
time-keeping mechanism, referred to as the biological clock,
regulates circadian rhythms. The master circadian clock coordinates
peripheral clocks elsewhere in the body and is located in the
suprachiasmatic nuclei (SCN) within the anterior hypothalamus
(1). The core oscillator driving this
clock is intergrated by an auto-regulatory transcription-(post)
translation-based feedback loop, which is comprised of genes
related to the circadian rhythm (1,2).
Epidemiological studies have suggested that
disruption of the circadian clock may increase cancer risk in
humans (3–5). In particular, it has been observed that
shift workers have an increased risk of developing malignancies,
including breast, endometrial, prostate and colorectal cancer, due
to their disrupted circadian cycles (5–9). Fu et
al (10) previously demonstrated
that a period circadian protein homolog 2 (Per2) mutation
induced upregulation of c-Myc and downregulation of p53
transcription in mice; furthermore, the incidence of spontaneous
and radiation-induced lymphoma increased, as did
lymphoma-associated mortality. Other in vivo studies have
identified an association between alterations of the circadian
rhythm and tumorigenesis (2,9,11). In a
number of types of human solid cancer, including breast,
endometrial and colorectal cancer, the dysregulated expression of
circadian genes has been investigated by immunohistochemistry
and/or reverse transcription quantitative polymerase chain reaction
(RT-qPCR) (9,12–14).
The aim of the present study was to investigate the
clinical significance of the mRNA expression of clock genes in
human colorectal carcinoma and adenoma tissues, using in
situ hybridization.
Patients and methods
Patients and tumor samples
A total of 51 patients (32 males and 19 females)
with colorectal carcinoma, and 10 patients with colorectal adenoma
were examined. All patients underwent endoscopic or surgical
resection to completely remove tumors in the Department of Organ
Regulatory Surgery, Fukushima Medical University Hospital
(Fukushima, Japan) between April 1999 and July 2005. In several
tissue specimens, the surrounding normal mucosa was also examined.
None of the patients had received prior chemotherapy or irradiation
or had experienced any other form of cancer. The
clinicopathological characteristics of the 51 patients with
colorectal cancer investigated in this study are summarized in
Table I.
 | Table I.Associations between clock gene
expression and clinicopathological variables. |
Table I.
Associations between clock gene
expression and clinicopathological variables.
|
|
| Per1 |
| Per2 |
| Cry1 |
| Clock |
| Bmal1 |
| CKIε |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
| Total | Negative | Positive |
| Negative | Positive |
| Negative | Positive |
| Negative | Positive |
| Negative | Positive |
| Negative | Positive |
|
|---|
| Variables | 51 | 27 | 24 | P-value | 25 | 26 | P-value | 27 | 24 | P-value | 30 | 21 | P-value | 31 | 20 | P-value | 37 | 14 | P-value |
|---|
| Age |
|
|
| 0.242 |
|
| 0.048 |
|
| 0.820 |
|
| 0.931 |
|
| 0.423 |
|
| 0.727 |
|
Mean | 65.1 | 63.7 | 67.6 |
| 68.9 | 62.2 |
| 65.1 | 65.9 |
| 65.6 | 65.4 |
| 65.3 | 65.9 |
| 65.8 | 64.6 |
|
|
Range | 33–84 | 33–81 | 43–84 |
| 49–84 | 33–81 |
| 33–84 | 41–84 |
| 41–81 | 33–84 |
| 41–84 | 33–84 |
| 33–84 | 46–80 |
|
| Gender |
|
|
| 1 |
|
| 1 |
|
| 0.772 |
|
| 0.563 |
|
| 0.774 |
|
| 0.333 |
|
Male | 32 | 17 | 15 |
| 16 | 16 |
| 16 | 16 |
| 20 | 12 |
| 20 | 12 |
| 25 | 7 |
|
|
Female | 19 | 10 | 9 |
| 9 | 10 |
| 11 | 8 |
| 10 | 9 |
| 11 | 8 |
| 12 | 7 |
|
| Tumor size
(mm) |
|
|
| 0.012a |
|
| 0.011a |
|
| 0.164 |
|
| 0.009a |
|
| 0.082a |
|
| 0.342 |
|
<50 | 29 | 20 | 9 |
| 19 | 10 |
| 18 | 11 |
| 22 | 7 |
| 21 | 8 |
| 23 | 6 |
|
|
≥50 | 22 | 7 | 15 |
| 6 | 16 |
| 9 | 13 |
| 8 | 14 |
| 10 | 12 |
| 14 | 8 |
|
| Tumor location |
|
|
| 0.782 |
|
| 1 |
|
| 0.577 |
|
| 0.578 |
|
| 0.567 |
|
| 0.363 |
|
Colon | 24 | 12 | 12 |
| 12 | 12 |
| 14 | 10 |
| 13 | 11 |
| 16 | 8 |
| 19 | 5 |
|
|
Rectum | 27 | 15 | 12 |
| 13 | 14 |
| 13 | 14 |
| 17 | 10 |
| 15 | 12 |
| 18 | 9 |
|
| Histological
differentiation |
|
|
| 0.636 |
|
| 0.972 |
|
| 0.991 |
|
| 0.754 |
|
| 0.422 |
|
| 0.212 |
|
Well | 34 | 17 | 17 |
| 17 | 17 |
| 18 | 16 |
| 20 | 14 |
| 22 | 12 |
| 27 | 7 |
|
|
Moderately | 13 | 7 | 6 |
| 6 | 7 |
| 7 | 6 |
| 7 | 6 |
| 6 | 7 |
| 7 | 6 |
|
|
Poorly | 0 | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
|
Mucinous | 4 | 3 | 1 |
| 2 | 2 |
| 2 | 2 |
| 3 | 1 |
| 3 | 1 |
| 3 | 1 |
|
| Depth of
invasion |
|
|
| 0.482 |
|
| 0.103 |
|
| 0.978 |
|
| 0.303 |
|
| 0.580 |
|
| 0.240 |
|
pT1 | 5 | 3 | 2 |
| 4 | 1 |
| 3 | 2 |
| 4 | 1 |
| 2 | 3 |
| 2 | 3 |
|
|
pT2 | 7 | 4 | 3 |
| 5 | 2 |
| 4 | 3 |
| 5 | 2 |
| 4 | 3 |
| 6 | 1 |
|
|
pT3 | 31 | 14 | 17 |
| 11 | 20 |
| 16 | 15 |
| 15 | 16 |
| 21 | 10 |
| 22 | 9 |
|
|
pT4 | 8 | 6 | 2 |
| 5 | 3 |
| 4 | 4 |
| 6 | 2 |
| 4 | 4 |
| 7 | 1 |
|
| Depth of
invasion |
|
|
| 0.749 |
|
| 0.052 |
|
| 0.749 |
|
| 0.315 |
|
| 0.502 |
|
| 0.715 |
|
pT1-2 | 12 | 7 | 5 |
| 9 | 3 |
| 7 | 5 |
| 9 | 3 |
| 6 | 6 |
| 8 | 4 |
|
|
pT3-4 | 39 | 20 | 19 |
| 16 | 23 |
| 20 | 19 |
| 21 | 18 |
| 25 | 14 |
| 29 | 10 |
|
| Lymph node
metastasis |
|
|
| 1 |
|
| 1 |
|
| 0.264 |
|
| 1 |
|
| 1 |
|
| 0.363 |
|
Absent | 27 | 14 | 13 |
| 13 | 14 |
| 12 | 15 |
| 16 | 11 |
| 16 | 11 |
| 18 | 9 |
|
|
Present | 24 | 13 | 11 |
| 12 | 12 |
| 15 | 9 |
| 14 | 10 |
| 15 | 9 |
| 19 | 5 |
|
| Stage |
|
|
| 0.723 |
|
| 0.240 |
|
| 0.506 |
|
| 0.180 |
|
| 0.987 |
|
| 0.363 |
| I | 9 | 6 | 3 |
| 6 | 3 |
| 5 | 4 |
| 8 | 1 |
| 5 | 4 |
| 6 | 3 |
|
| II | 16 | 7 | 9 |
| 6 | 10 |
| 6 | 10 |
| 7 | 9 |
| 10 | 6 |
| 10 | 6 |
|
|
III | 21 | 11 | 10 |
| 12 | 9 |
| 13 | 8 |
| 12 | 9 |
| 13 | 8 |
| 18 | 3 |
|
| IV | 5 | 3 | 2 |
| 1 | 4 |
| 3 | 2 |
| 3 | 2 |
| 3 | 2 |
| 3 | 2 |
|
| Stage |
|
|
| 0.473 |
|
| 0.291 |
|
| 1 |
|
| 0.064 |
|
| 0.724 |
|
| 0.692 |
| I | 9 | 6 | 3 |
| 6 | 3 |
| 5 | 4 |
| 8 | 1 |
| 5 | 4 |
| 6 | 3 |
|
|
II–IV | 42 | 21 | 21 |
| 19 | 23 |
| 22 | 20 |
| 22 | 20 |
| 26 | 16 |
| 31 | 11 |
|
All tissue samples were embedded in optimal cutting
temperature (OCT) compound (Sakura Finetek USA, Inc., Torrance, CA,
USA) and immediately stored at −8°C. Tumors were
histopathologically classified as well-differentiated, moderately
differentiated, poorly differentiated or mucinous adenocarcinomas
(15), and tumor size was defined as
the largest diameter of the tumor. Histopathological diagnoses were
performed at the Department of Pathology, Fukushima Medical
University Hospital following standard procedures. Informed consent
was obtained from each patient and the Fukushima Medical University
Committee approved the protocol of the present study.
In situ hybridization
Digoxigenin (DIG)-UTP labeled cRNA probes were used
to evaluate the mRNA expression of clock genes. The DIG-labeled
cRNA probes were synthesized using a DIG RNA Labeling kit (Roche
Diagnostics, Basel, Switzerland), and were labeled with SP6 or T7
RNA polymerase in the presence of DIG-UTP. Sections 5-µm thick were
formed from the embedded tissue specimens, sufficiently dried with
cold air and fixed in 4% paraformaldehyde diluted with
phosphate-buffered saline for 30 min. Sections were hybridized
overnight at 42°C in hybridization buffer containing 1 µg/ml of
DIG-labeled probe. The DIG-labeled probes were diluted to 1 µg/ml
with hybridization buffer (Nippon Gene Co., Ltd., Tokyo, Japan) and
dropped to the sections, which were incubated at 42°C for 16–20 h
to hybridize with each probe. Following hybridization, the sections
were washed in 2 × standard citrate buffered saline and 0.2 ×
saline sodium citrate buffer at 5°C for 20 min and treated with 1%
blocking solution at room temperature for 30 min, using the DIG
Nucleic Acid Detection Kit (Roche Diagnostics). The sections were
subsequently incubated at room temperature for 30 min with alkaline
phosphatase labeled anti-digoxigenin antibody (Roche) diluted with
a blocking solution (1:5,000). Color reaction was conducted using
NTB/BCIP at 4°C for 12 h. As a negative control, the serial section
was hybridized with a sense probe. Sections were simultaneously
evaluated by two investigators. The tumor cells were classified
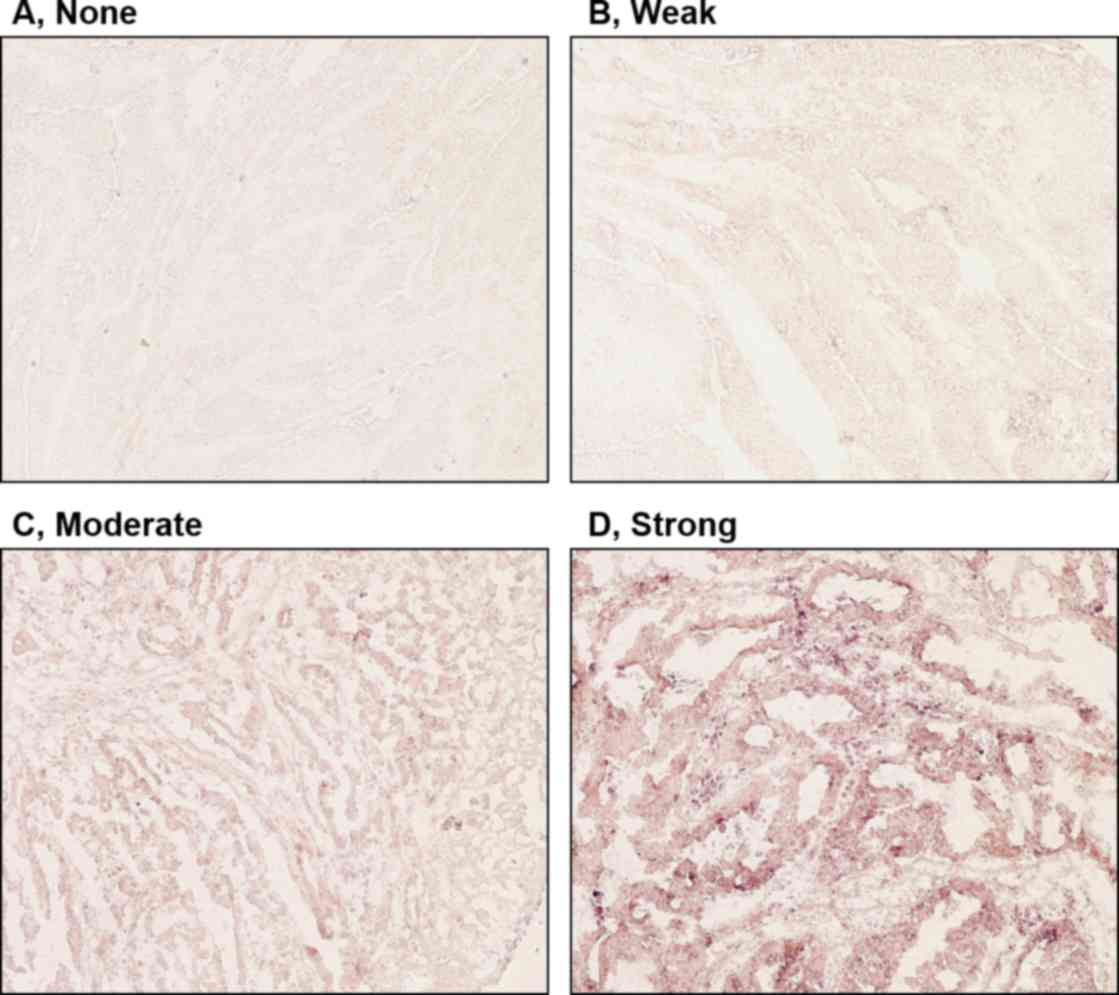
into 4 groups based on intensity of staining (none, weak, moderate
or strong) indicating levels of gene expression within the
cells.
Statistical analysis
Differences between groups were evaluated by the
χ2 test, Fisher's exact test, Student's t test or
the Mann-Whitney U test. Cumulative survival was estimated by the
Kaplan-Meier method and differences were analyzed by the log-rank
test. All statistical analyses were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
Potential involvement of clock genes
in colorectal tumor progression
mRNA expression of the clock genes, including
Per1 and 2, cryptochrome 1 (Cry1), circadian
locomoter output cycles protein kaput (Clock), brain and
muscle ARNT-like protein 1 (Bmal1) and casein kinase 1ε
(CK1ε), was examined in colorectal cancer tissues by in
situ hybridization. As presented in Fig. 1, sense probes as negative controls
exhibited no staining (Fig. 1 panels
3–4), whereas various levels of staining were observed in tumor
cells detected by anti-sense probes (Fig.
1, panels 1–2). Normal epithelial areas were evaluated in a
number of specimens where the surrounding normal mucosa was
available, however, no clear staining was detected by any of the
probes (Fig. 1. panels 5–6). The
levels of expression of clock genes in tumor cells were classified
into four groups (none, weak, moderate or strong), based on the
intensity of staining (Fig. 2).
Tumors with no or weak staining were further defined as a negative
group, while tumors with moderate and strong staining were a
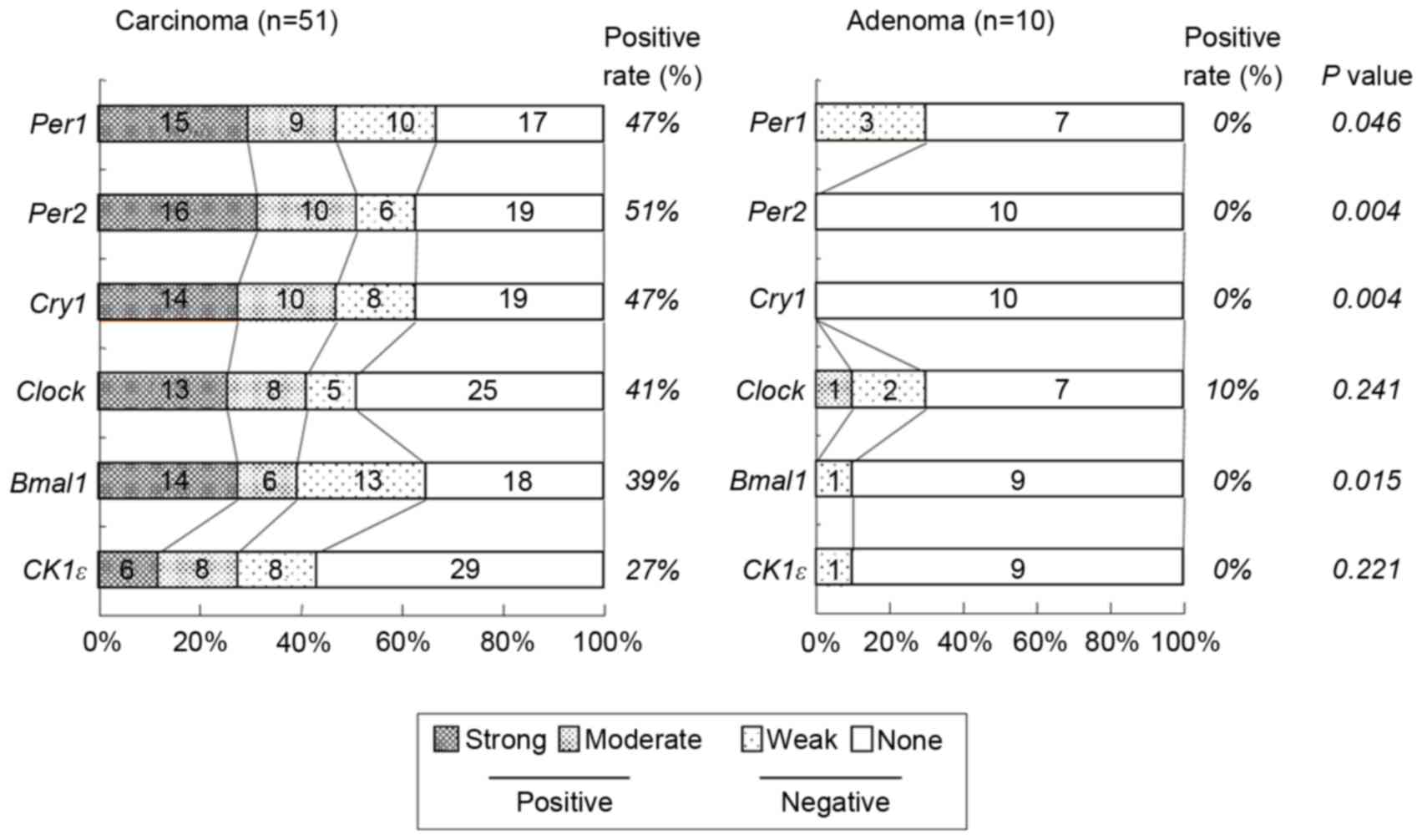
positive group. Of the 51 colorectal carcinomas evaluated, positive
staining for Per1, Per2, Cry1, Clock,
Bmal1 and CK1ε was observed in 24 (47%), 26 (51%), 24
(47%), 21 (41%), 20 (39%) and 14 (27%) tumors, respectively
(Table I and Fig. 3). However, no significant associations
were observed between levels of clock gene expression and
histopathological type, depth of invasion, lymph node metastasis or
disease stage (Table I). Although
positive Per2 and positive Clock groups tended to be
associated with a deeper depth of invasion and advanced stage,
respectively, these associations were not significance (P=0.052 and
P=0.064, respectively). By contrast, positive-Per1,
Per2 and Clock groups were each associated with
larger tumor size (>50 mm; P=0.012, P=0.011 and P=0.009,
respectively; Table I). Similar
results were obtained when tumor size was treated as a continuous
variable (Fig. 4), therefore, the
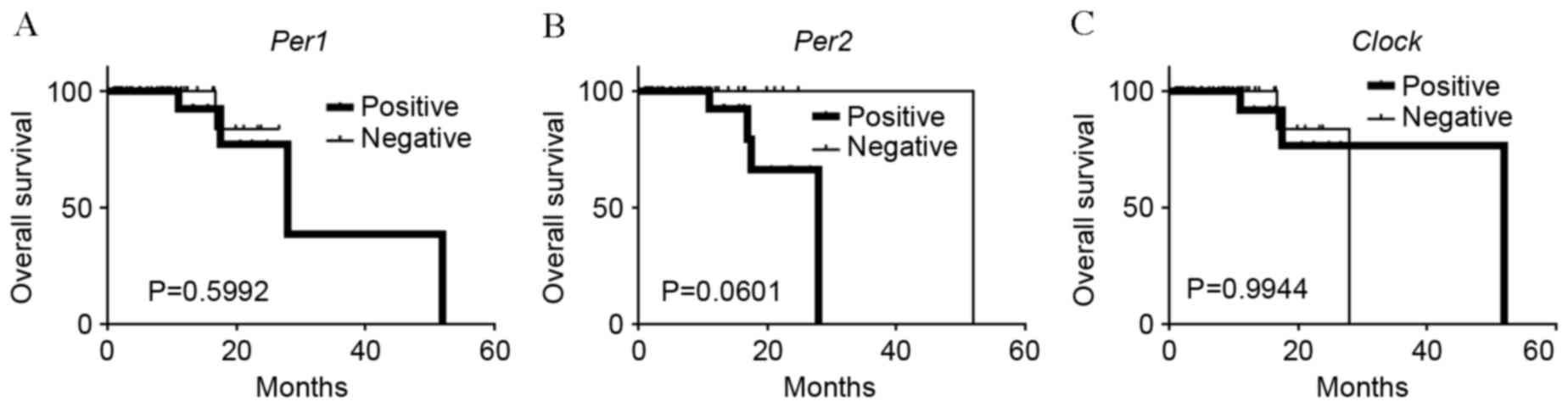
potential prognostic significance of Per1, Per2 and
Clock was investigated. No association was observed between
Per1 or Clock positive expression and overall
survival rates (P=0.0599 and P=0.994, respectively; Fig. 5A and B). On the other hand, patients
with carcinomas exhibiting positive-Per2 expression tended
to have lower rates of survival than patients with
negative-Per2 carcinomas, although this association was not
significant (P=0.060; Fig. 5C).
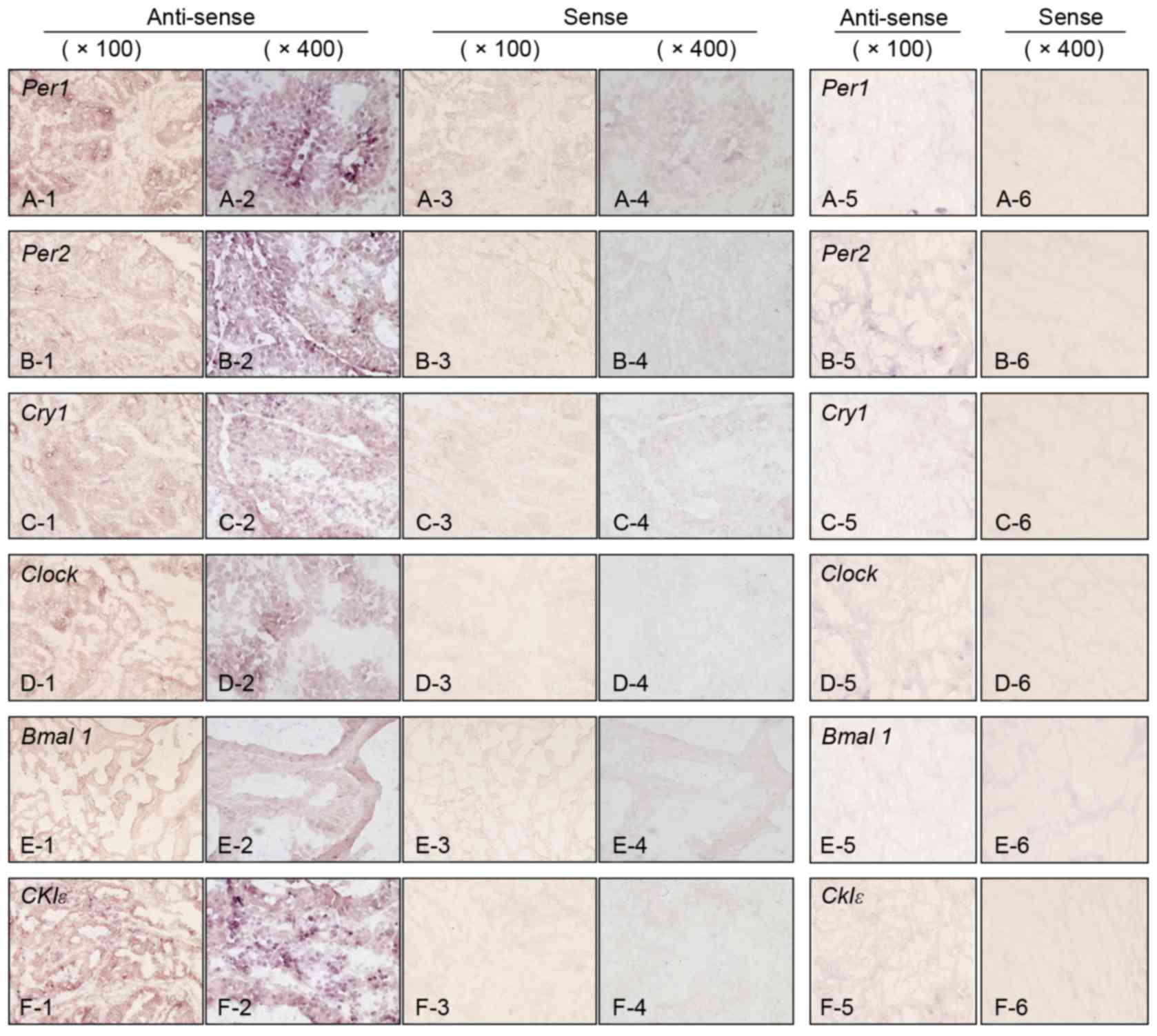 | Figure 1.Expression of clock genes detected by
in situ hybridization in colorectal carcinoma and
surrounding normal mucosa. (A) Per1, (B) Per2, (C)
Cry1, (D) Clock, (E) Bmal1 and (F)
CK1ε. Panels 1 and 2 represent carcinoma tissue with
anti-sense probe (magnification, ×100 and ×400, respectively).
Panels 3 and 4 represent carcinoma tissues with sense probe
(magnification, ×100 and ×400, respectively). Panels 5 and 6
represent normal mucosa tissues with anti-sense and sense probes,
respectively (magnification, ×100 for both). Per, Period circadian
protein homolog; Cry1, Cryptochrome 1; Clock, Circadian locomoter
output cycles protein kaput; Bmal1, Brain and Muscle ARNT-like
Protein 1; CK1ε, Casein Kinase 1ε. |
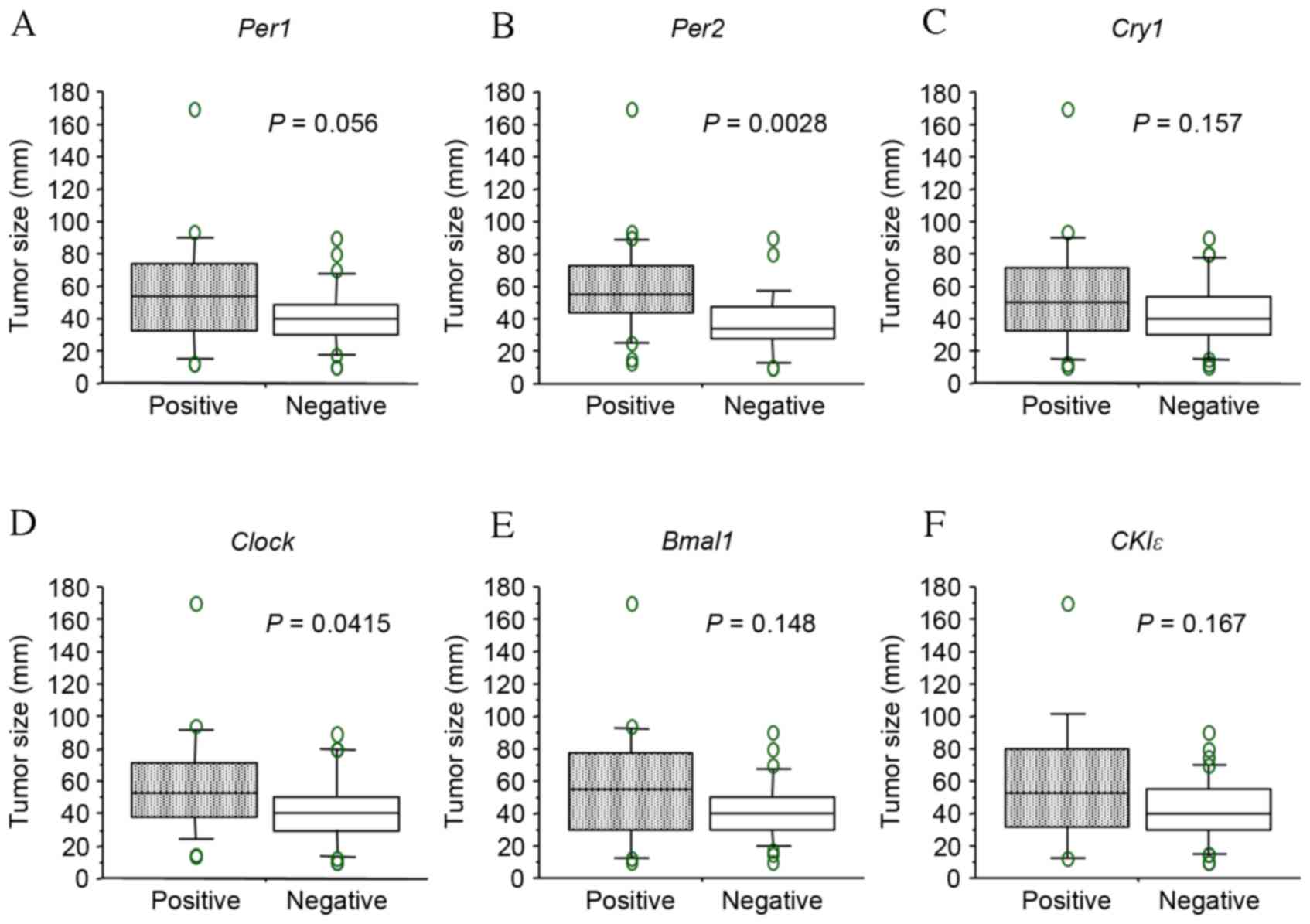 | Figure 4.Comparison of tumor size between
positive and negative staining of each gene. (A) Per1, (B)
Per2, (C) Cry1, (D) Clock, (E) Bmal1
and (F) CKIε. Boxes correspond to the inter-quartile ranges,
with the lower boundary of the box representing the 25th percentile
and the upper boundary representing the 75th percentile.
Differences between groups were analyzed by Student's
t-test. Per, period circadian protein homolog; Cry1,
cryptochrome 1; Clock, circadian locomoter output cycles protein
kaput; Bmal1, brain and muscle ARNT-like protein 1; CK1ε, casein
kinase 1ε. |
The expression of clock genes in
colorectal adenoma
To investigate the expression of clock genes in
precancerous lesions compared with cancer tissues, 10 colorectal
adenomas were examined by in situ hybridization. In contrast
to cancer tissues, the expression of each clock gene was
undetectable in the majority of adenoma tissue (Fig. 3). No adenomas (0%) exhibited positive
staining for Per1, Per2, Cry1, Bmal1 or
CK1ε and only 10% of adenomas exhibited positive expression
of Clock. Hence, the proportion of tissues indicating
positive Per1, Per2, Cry1 and Cry2
expression in colorectal carcinoma was significantly higher than in
colorectal adenoma (P=0.046, P=0.004, P=0.004 and P=0.015,
respectively; Fig. 3).
Discussion
Epidemiological studies have suggested that
disruption of the circadian rhythm is associated with increased
cancer incidence and poorer disease outcome (3–5,8). Previous studies have indicated that in
Per2 mutant mice, Bmal1 expression decreased causing an
increase in c-Myc transcription, thus disrupting the circadian
rhythm and increasing cancer risk (10). In colorectal cancer, animal studies
using chemically induced models as well as
APCMin/+ mice, have suggested a link between
alterations of circadian genes and colorectal tumor development and
progression (16–18). Other studies have used RT-qPCR to
demonstrate that clock gene expression is dysregulated in human
colorectal cancer (9,19,20).
In the present study, unlike previous studies, the
in situ hybridization technique was utilized to detect clock
gene mRNA expression in colorectal tumor tissues, including
precancerous and cancerous lesions. The proportion of colorectal
carcinomas with positive Per1, Per2, Cry1 and
Cry2 expression was observed to be significantly higher than
the proportion of adenomas, suggesting that dysregulated clock gene
expression may be involved in colorectal tumorigenesis. Colorectal
carcinoma tumors exhibiting positive staining of Per1,
Per2 and Clock were significantly larger than those
exhibiting negative staining. Correspondingly, tumors with positive
staining of Per2 and Clock tended to be associated
with deeper depth of invasion and a more advanced stage of cancer.
Furthermore, an association was observed between
positive-Per2 expression and poorer overall survival
outcome, though this was not technically significant. However, due
to the relatively small sample size and short follow-up time of the
present study, the prognostic impact of positive-Per2
expression remains to be fully determined.
Clock genes are involved in cell cycle regulation
(21). A positive factor, the
Clock-Bmal1 dimer, is required for transcription initiation of the
Per and Cry genes oscillating mechanism in the
feedback mechanisms of the clock genes (21). By contrast, Per and Cry proteins are
supposed to act as negative factors and promote oscillation
(22). The Clock-Bmal1 dimer promotes
transcription through the E-box of Wee1, suppressing the cell cycle
at M-phase (22). The results of
previous studies have demonstrated a connection between the
alterations of clock genes, and cell cycle progression and
proliferation through c-Myc/p21 signaling and the Wnt/β-catenin
pathway, which are implicated in the molecular pathogenesis of
colorectal cancer (11,17,18). Taken
together with the results of the present study, this indicates that
the imbalance of clock gene expression levels, which results in the
dysregulation of cell cycle, may stimulate the adenoma-carcinoma
transition and tumor progression during colorectal carcinogenesis.
However, the current study did not address whether clock gene
expression directly contributes to cell cycle dysregulation and
tumorigenesis. The biological significance of in situ clock
gene expression remains to be elucidated. Clock gene expression
in situ may at least in part represent the dysregulated
rhythms in carcinomas, therefore, future studies are required to
address the molecular mechanisms by which the imbalance of clock
genes expression contributes to dysregulated circadian rhythms and
consequently, to tumorigenesis.
In conclusion, mRNA expression of key clock genes,
including Per1, Per2, Cry1 and Cry2 was frequently found in
carcinomas, but not in adenomas, using in situ
hybridization. Also, the expression of some clock genes were
associated with tumor size, and tended to be associated with depth
of invasion and survival outcome. Therefore, the present study
suggests that dysregulated clock gene expression may serve an
important role in human colorectal tumorigenesis.
Glossary
Abbreviations
Abbreviations:
|
SCN
|
suprachiasmatic nuclei
|
|
Per
|
period circadian protein homolog
|
|
Bmal1
|
brain and Muscle ARNT-Like Protein
1
|
|
Clock
|
circadian locomoter output cycles
protein kaput
|
|
Cry
|
cryptochrome
|
|
CK1ε
|
casein Kinase 1ε
|
References
|
1
|
Fu L and Lee CC: The circadian clock:
Pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hunt T and Sassone-Corsi P: Riding tandem:
Circadian clocks and the cell cycle. Cell. 129:461–464. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devilee P, Schuuring E, van de Vijver MJ
and Cornelisse CJ: Recent developments in the molecular genetic
understanding of breast cancer. Crit Rev Oncog. 5:247–270. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ronco A, De Stefani E, Mendilaharsu M and
Deneo-Pellegrini H: Meat, fat and risk of breast cancer: A
case-control study from Uruguay. Int J Cancer. 65:328–331. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stevens RG, Brainard GC, Blask DE, Lockley
SW and Motta ME: Breast cancer and circadian disruption from
electric lighting in the modern world. CA Cancer J Clin.
64:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambrosone CB, Freudenheim JL, Graham S,
Marshall JR, Vena JE, Brasure JR, Michalek AM, Laughlin R, Nemoto
T, Gillenwater KA and Shields PG: Cigarette smoking,
N-acetyltransferase 2 genetic polymorphisms, and breast cancer
risk. JAMA. 276:1494–1501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magnusson C, Baron J, Persson I, Wolk A,
Bergström R, Trichopoulos D and Adami HO: Body size in different
periods of life and breast cancer risk in post-menopausal women.
Int J Cancer. 76:29–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schernhammer ES, Laden F, Speizer FE,
Willett WC, Hunter DJ, Kawachi I, Fuchs CS and Colditz GA:
Night-shift work and risk of colorectal cancer in the nurses'
health study. J Natl Cancer Inst. 95:825–828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karantanos T, Theodoropoulos G, Pektasides
D and Gazouli M: Clock genes: Their role in colorectal cancer.
World J Gastroenterol. 20:1986–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu L, Pelicano H, Liu J, Huang P and Lee
C: The circadian gene Period2 plays an important role in tumor
suppression and DNA damage response in vivo. Cell. 111:41–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huisman SA, Oklejewicz M, Ahmadi AR,
Tamanini F, Ijzermans JN, van der Horst GT and de Bruin RW:
Colorectal liver metastases with a disrupted circadian rhythm phase
shift the peripheral clock in liver and kidney. Int J Cancer.
136:1024–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh KT, Yang MY, Liu TC, Chen JC, Chan WL,
Lin SF and Chang JG: Abnormal expression of period 1 (PER1) in
endometrial carcinoma. J Patol. 206:111–120. 2005.
|
|
13
|
Shih HC, Choo KB, Chang TJ, Yang MY, Shih
MC, Yeh KT, Liu TC, Lin SF and Chang JG: Disturbance of circadian
gene expression in endometrial cancer: Detection by real-time
quantitative RT-PCR. Oncol Rep. 14:1533–1538. 2005.PubMed/NCBI
|
|
14
|
Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ
and Chang JG: Deregulated expression of the PER1, PER2 and PER3
genes in breast cancers. Carcinogenesis. 26:1241–1246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH: World Health Organization
international histological classification of tumors: Histological
typing of intestinal tumors. 2nd. New York: Springer-Verlag;
1989
|
|
16
|
Wood PA, Yang X, Taber A, Oh EY, Ansell C,
Ayers SE, Al-Assaad Z, Carnevale K, Berger FG, Peña MM and
Hrushesky WJ: Period 2 mutation accelerates ApcMin/+ tumorigenesis.
Mol Cancer Res. 6:1786–1793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Wood PA, Ansell CM, Ohmori M, Oh
EY, Xiong Y, Berger FG, Peña MM and Hrushesky WJ: Beta-catenin
induces beta-TrCP-mediated PER2 degradation altering circadian
clock gene expression in intestinal mucosa of ApcMin/+ mice. J
Biochem. 145:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soták M, Polidarová L, Ergang P, Sumová A
and Pácha J: An association between clock genes and
clock-controlled cell cycle genes in murine colorectal tumors. Int
J Cancer. 132:1032–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oshima T, Takenoshita S, Akaike M,
Kunisaki C, Fujii S, Nozaki A, Numata K, Shiozawa M, Rino Y, Tanaka
K, et al: Expression of circadian genes correlates with liver
metastasis and outcomes in colorectal cancer. Oncol Rep.
25:1439–1446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karantanos T, Theodoropoulos G, Gazouli M,
Vaiopoulou A, Karantanou C, Lymberi M and Pektasides D: Expression
of clock genes in patients with colorectal cancer. Int J Biol
Markers. 28:280–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuo T, Yamaguchi S, Mitsui S, Emi A,
Shimoda F and Okamura H: Control mechanism of the circadian clock
for timing of cell division in vivo. Science. 302:255–259. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yagita K, Tamanini F, Yasuda M,
Hoeijmakers JH, van der Horst GT and Okamura H: Nucleocytoplasmic
shuttling and mCRY-dependent inhibition of ubiquitylation of the
mPER2 clock protein. EMBO J. 21:1301–1314. 2002. View Article : Google Scholar : PubMed/NCBI
|