Introduction
Bladder cancer is one of the most common malignant
tumors of the urinary system and is a major cause of mortality
worldwide. An estimated 429,800 cases of bladder cancer are
diagnosed every year and 165,100 mortalities occurred in 2012
worldwide as a result of the disease (1). In the United States of America, a total
of 79,030 new bladder cancer cases and 16,870 bladder
cancer-associated mortalities were projected to occur in 2017
(2,3).
Therefore, the identification of genes associated with bladder
cancer and novel therapeutic targets is necessary.
LAG1 longevity assurance homolog 2 (LASS2), also
known as tumor metastasis suppressor gene 1, was identified as a
tumor suppressor gene (3). LASS2 has
been correlated with the degree of invasion and recurrence of
carcinomas of the prostate (4–6), liver
(7), breast (8,9) and
bladder (10). However, to the best
of our knowledge, the precise role LASS2 serves in regulating
bladder cancer cell tumorigenicity and growth in vivo has
not yet been investigated in animal or clinical studies.
In previous studies, an implantation metastasis
model of the highly invasive human bladder cancer EJ-M3 cell line
was established in nude mice (11,12).
Another study identified that LASS2 expression was the highest in
the EJ-M3 cell line compared with other human bladder carcinoma
cell lines (BIU-87, T24 and EJ), and was significantly correlated
with the proliferation, metastasis and invasion of human bladder
cancer (13). In the present study,
EJ-M3 cells were selected for a lentivirus-mediated LASS2
interference strategy and LASS2 overexpression, in order to
investigate the effects of LASS2 expression on bladder cancer cell
tumorigenicity and growth in vitro and in nude mice.
Materials and methods
Cell line and cell culture
The highly invasive human bladder cancer EJ-M3 cell
line was established and preserved at the Department of Urology of
the Second Affiliated Hospital of Kunming Medical University
(Yunnan Institute of Urology, Kunming, China) (11). Cells were maintained in RPMI-1640
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2. All reagents were purchased from EMD Millipore
(Billerica, MA, USA).
Construction of the lentiviral vector
and cell transfection
EJ-M3 cells were transfected with a LASS2 shRNA
plasmid (cat no. HSH008629-CU6) or a LASS2 overexpression plasmid
(cat no. EX-T3019-M98-5) (both GeneCopoeia, Inc., Rockville, MD,
USA). Briefly, the plasmids were amplified in DH5α Escherichia
coli cells (Takara Biotechnology Co., Ltd., Dalian, China),
purified using an E.Z.N.A.® Endo-Free Plasmid Mini Kit I
(Omega Bio-Tek, Inc., Norcross, GA, USA), and transfected into 80%
confluent EJ-M3 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
lentiviral vector expressing green fluorescent protein (GFP) alone
was used as the control. At 72 h following transfection, LASS2
overexpression and LASS2 shRNA positive cells were selected using 1
µg/ml neomycin or puromycin, respectively, and the transfection
efficiency was examined by fluorescence microscopy (Olympus BX53;
Olympus Corporation, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse
transcribed into cDNA using Quant Reverse Transcriptase (Tiangen
Biotech Co., Ltd., Beijing, China) following the manufacturer's
protocol. β-Actin was used as the internal control. RT-qPCR was
performed in a 20 µl reaction containing 1 µl cDNA template, 1 µl
primer and 10 µl FastStart Universal SYBR Green Master (ROX) (Roche
Ltd., Mannheim, Germany), using an Applied Biosystems 7900HT
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific
Ltd., Waltham, MA, USA). The primers sequences were as follows:
β-actin forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; and reverse,
5′-GAAGATGGTGATGGGATTTC-3′; LASS2 forward,
5′-TCTCCTGGTTTGCCAATTACG-3′; and reverse,
5′-CCGGGCAGGGACCCTCATCA-3′. All the primers were synthesized by
GeneCopoeia, Inc. The amplification program consisted of
denaturation at 95°C for 1 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing at 60°C for 30 sec,
and then extension at 60°C for 30 sec. All experiments were
repeated at least three times. The relative expression of LASS2
mRNA was calculated using the 2−ΔΔCq method (14).
Western blot analysis
Cell lysates were extracted using RIPA Lysis Buffer
(Beyotime Institute of Biotechnology, Haimen, China). Total
proteins were separated via SDS-PAGE on a 10% gel, transferred onto
0.45 µm polyvinylidene difluoride membranes (EMD Millipore), and
blocked with 5% skimmed milk. Using β-actin as the internal
control, the membranes were incubated with a mouse anti-LASS2 (cat.
no. sc-390745; dilution, 1:500) or anti-β-actin monoclonal antibody
(cat. no. sc-47778; dilution, 1:500) (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) overnight at 4°C, followed by incubation
with a horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (cat. no. sc-2005; dilution, 1:2,500; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. Protein bands
were visualized through enhanced chemiluminescence by using
Immobilon Western Chemiluminescent HRP substrate (EMD Millipore
Corporation, Billerica, MA, USA) and captured using a MicroChemi
4.2 system (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
Relative protein quantitative quantification was performed using
ImageJ image analysis software (version 1.34; National Institutes
of Health, Bethesda, MD, USA).
Establishment of a human bladder
cancer xenograft model in nude mice
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Kunming Medical
University (Kunming, China) (approval no. KYLL20140071), and were
performed in compliance with all regulatory institutional
guidelines for animal welfare (the National Institutes of Health
Publications no. 80-23). Fifteen 6-week-old female nude mice
(BALB/c-nu/nu) weighing 16–20 g, were purchased from Beijing HFK
Bioscience Company (Beijing, China), and were randomly divided into
the following 3 groups (5/group): A non-transfected EJ-M3 cell
group [control (Con)]; a LASS2-overexpression EJ-M3 cell group
(LASS2-over); and a LASS2-shRNA EJ-M3 cell group (LASS2-shRNA). All
nude mice were housed in a sterile environment with a room
temperature of 20–25°C, a relative humidity of 30–70% and a 12 h
light/dark cycle. All mice were given free access to food and
water, and the bedding materials, drinking water, feeding cages and
other items in contact with the animals were all autoclaved prior
to use. Cell suspensions (200 µl, 2×106/ml) of
non-transfected, LASS2-over or LASS2-shRNA EJ-M3 cells were
subcutaneously injected into the right side of the dorsal aspect of
the nude mice using a 6-gauge needle. The tumor size and body
weight of each mouse were measured once a week. The tumor volumes
were calculated using the following formula: V=π/6 × largest
diameter × smallest diameter2 (15), and tumor growth curves were plotted.
The tumor inhibition rate (TIR) was determined using the formula:
TIR (%)=[1-(mean tumor volume of experimental group/mean tumor
volume of control group)]x100. All mice were sacrificed 6 weeks
following injection, and the tumors were resected. One part of each
tumor was fixed in 4% formalin, dehydrated with gradient alcohol,
cleared with xylene, embedded in paraffin, sectioned serially
(4-µm-thick sections), and then mounted on a slide for hematoxylin
and eosin staining and immunohistochemical analysis. Another part
of each tumor was stored at −80°C for the detection of matrix
metalloproteinase (MMP)-2/−9 activity. The slides were stained with
hematoxylin and eosin (H&E), and the properties of the
xenograft tumors were assessed under an optical microscope (Eclipse
E200; Nikon Corporation, Tokyo, Japan).
Immunohistochemical analysis
The slides were heated and subsequently put in
xylene, washed with gradient alcohol for deparaffinization, and
then rehydrated with distilled water. After that, antigen retrieval
was performed in 1 mM boiling EDTA buffer (pH 8.0; EMD Millipore)
for 15 min at 92–100°C. The slides were incubated with 3% hydrogen
peroxide for 30 min, washed, and blocked with 5% goat serum (EMD
Millipore) for 60 min at room temperature. A mouse monoclonal
anti-LASS2 antibody (cat. no. sc-390745; dilution, 1:100, Santa
Cruz Biotechnology, Inc.) was applied to each slide overnight at
4°C. Following washing with PBS (pH 7.4), the slides were incubated
for 30 min with a rabbit anti-mouse secondary antibody (cat. no.
A-11062, dilution, 1:400; Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, the slides were stained with diaminobenzidine,
counterstained with hematoxylin, dehydrated in ethanol, cleared in
xylene, mounted on coverslips and examined under an optical
microscope (Eclipse E200; Nikon Corporation, Tokyo, Japan). The
cells with yellow-brown staining in their cytoplasm were considered
LASS2-positive. The mean density of staining, calculated using
Image Pro-Plus (Media Cybernetics, Inc., Rockville, MD, USA) as
previously described (16), was used
to quantify the LASS2 staining.
MMP zymography
To investigate MMP-2 and MMP-9 activity in the
xenografts expressing different LASS2 levels, gelatin zymography
was used. SDS-PAGE on a 10% gel containing 0.1 mg/ml gelatin was
used for electrophoresis. The protein samples (20 µg of each) were
loaded into each lane, and electrophoresis was performed at 100 V
for 1.5 h at 4°C. Subsequently, the gel was washed twice with 100
ml solution containing 2.5% Triton X-100 on a rotary shaker for 40
min at room temperature, then incubated in 100 ml reaction buffer
(50 mmol/l Tris-HCl, 5 mmol/l CaCl2, 0.02%
NaN3, pH 7.6) for 42 h at 37°C. Following staining with
Coomassie brilliant blue and destaining with methanol and acetic
acid, the gels were scanned using the BioSpectrum Imaging System
(BioSpectrum 510; UVP, Inc., Upland, CA, USA), and the relative
activities of MMP-2 and MMP-9 were quantified by densitometric
analysis of the zymograms using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software
(version 19.0; IBM SPSS, Armonk, NY, USA). A one-way analysis of
variance followed by a Tukey's multiple comparison test was used to
evaluate the differences between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of LASS2 in EJ-M3 cells
following transfection with a LASS2 shRNA construct or a LASS2
overexpression plasmid
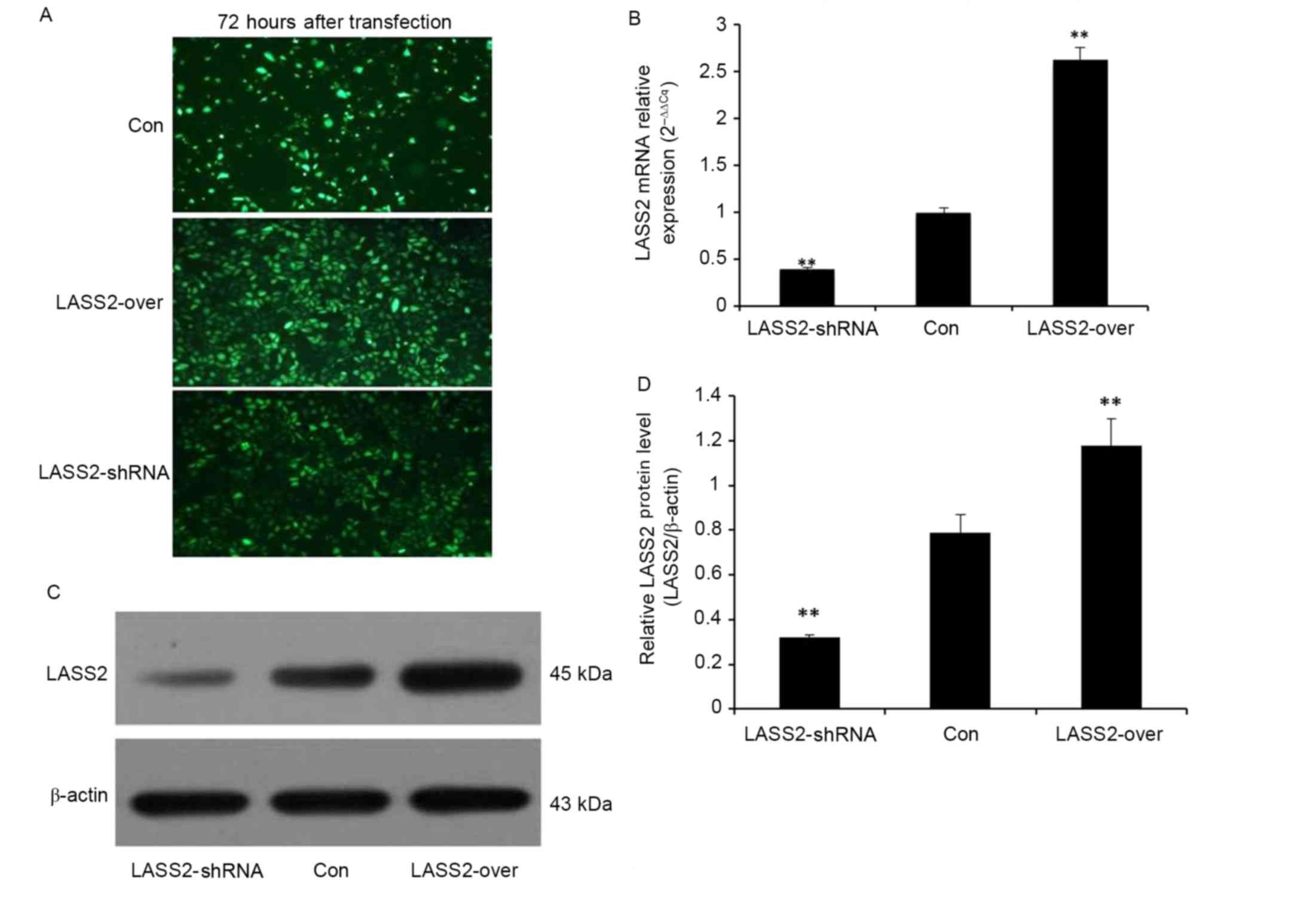
To confirm transfection efficiency, fluorescence
microscopy was used to detect the expression of GFP in EJ-M3 cells
transfected with a LASS2 shRNA construct or a LASS2 overexpression
plasmid 72 h following transfection (Fig.
1A). Non-transfected EJ-M3 cells expressing GFP alone were used
as the Con group. Subsequently, the cells were collected to detect
the expression of LASS2 at the mRNA and protein levels by RT-qPCR
and western blot analysis, respectively. β-Actin was used as the
internal control for normalization. The RT-qPCR results
demonstrated that LASS2 expression in the LASS2-shRNA group was
significantly decreased by 39% (P<0.001), while that in the
LASS2-over group was significantly increased by 263% (P<0.001),
compared with the Con group (Fig.
1B). A western blot analysis demonstrated similar results at
the protein level (Fig. 1C and D).
These results demonstrate that the two different recombinant LASS2
plasmids were successfully transfected into the EJ-M3 cells.
Knockdown or overexpression of LASS2
significantly affects bladder tumor growth in vivo
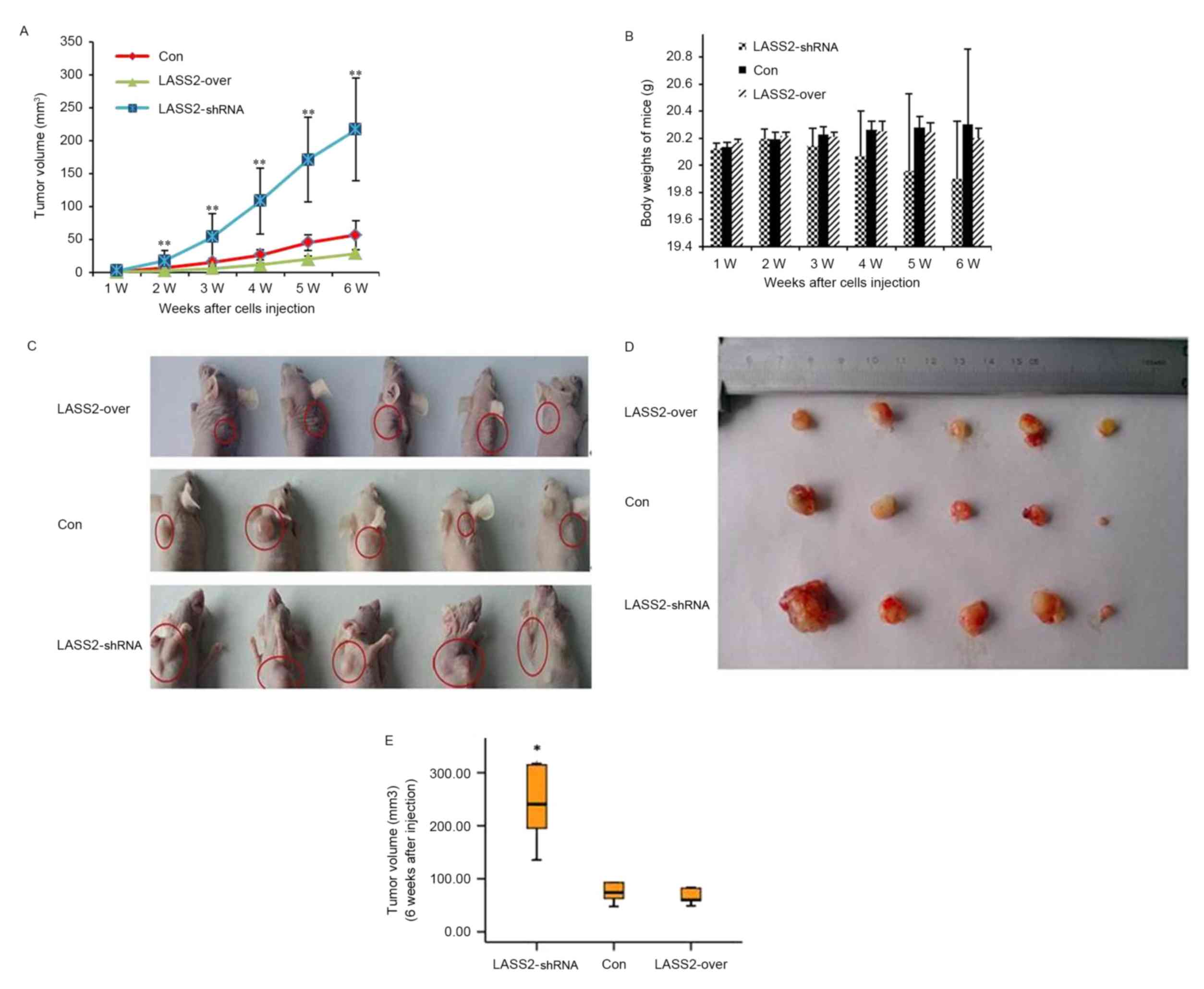
To determine the functional role of LASS2 in human
bladder cancer initiation and progression, EJ-M3 cells transfected
with LASS2 shRNA constructs or LASS2 overexpression plasmids were
subcutaneously injected into the right side of the dorsal aspect of
the neck of nude mice. Non-transfected EJ-M3 cells were injected as
the Con group. Tumor growth and body weight were closely monitored
during the following weeks (Fig. 2).
Ten days following injection, palpable subcutaneous xenografts were
observed in all nude mice, and the tumor incidence rates in the
three groups were 100%. Tumor growth curves demonstrated that the
xenografts grew significantly faster in the LASS2-shRNA group
compared with both the LASS2-over and Con groups (both P<0.001);
however, no significant difference was observed between the
LASS2-over and Con groups (P>0.05) (Fig. 2A). No animals died during the
observation period. Six weeks following injection, all mice were
sacrificed and the tumors were removed. All subcutaneous xenografts
exhibited oval or nodal shapes with a smooth surface (Fig. 2C and D). The tumor volumes of the
LASS2-shRNA, LASS2-over and Con groups were 222.32±124.97,
41.01±15.97 and 69.60±31.37 mm3, respectively. Compared
with the Con group, the TIRs in the LASS2-shRNA and LASS2-over
groups were −219.42 and 41.08%, respectively (data not shown).
These results indicate that LASS2 knockdown significantly increases
tumor volume, while LASS2 overexpression does not significantly
affect tumor growth when compared with the Con group, despite a
trend for LASS2 overexpression to decrease tumor volume (P=0.821;
Fig. 2E). During the experimental
period, LASS2 knockdown had a tendency to reduce the body weight of
nude mice; however, there was no significant difference in body
weight between these three groups (Fig.
2B).
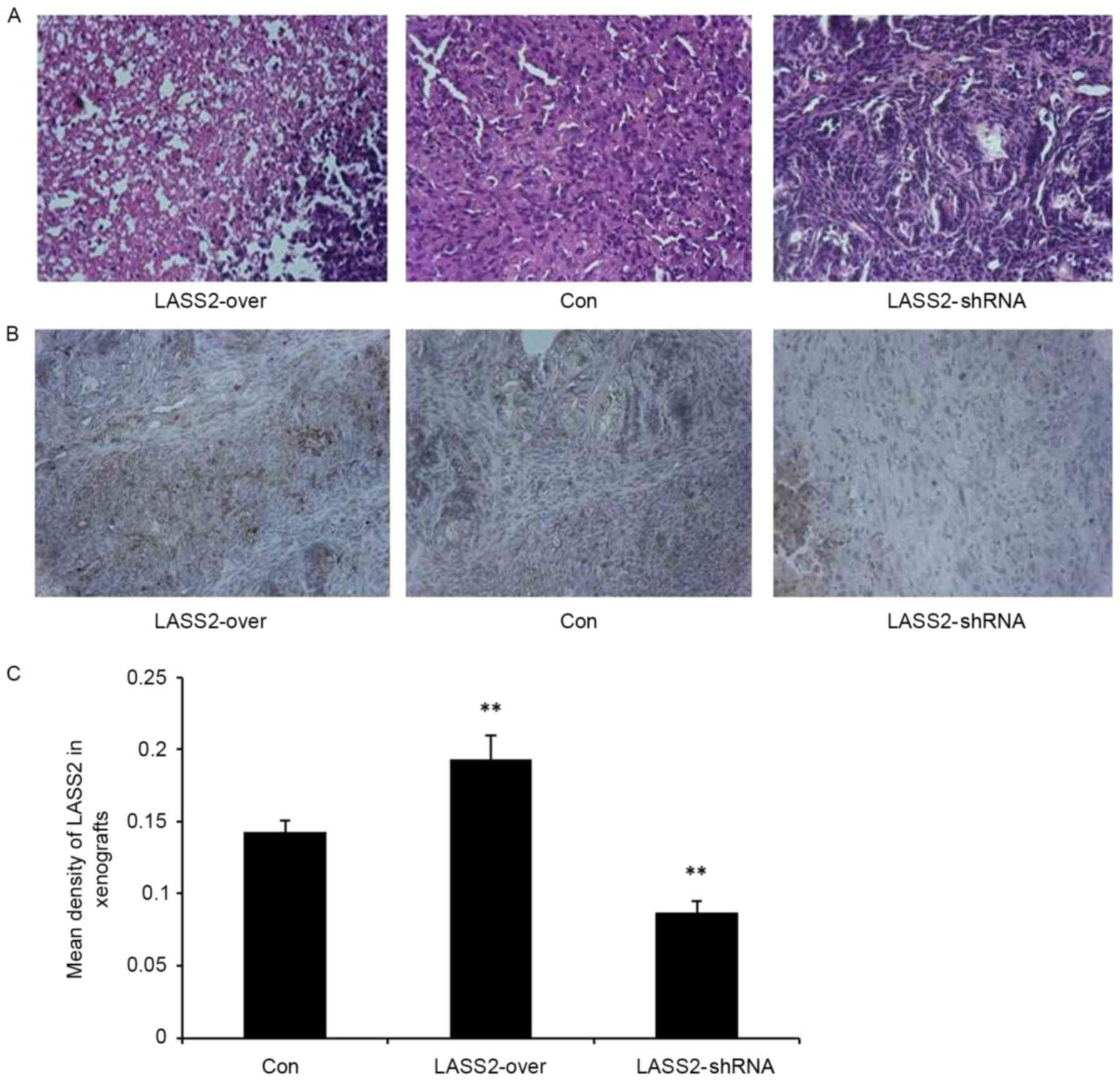
H&E staining confirmed the subcutaneous
xenografts as bladder transitional cell carcinoma. The tumor cells
exhibited a nest-like distribution, apparent heteromorphism, and
large and irregular nuclei with obvious nucleoli, particularly in
the LASS2-shRNA group (Fig. 3A).
Additionally, immunohistochemical analysis was used to detect LASS2
expression levels in the xenografts. There was a significantly
higher mean density of LASS2-positive cells in the LASS2-over group
compared with those in the LASS2-shRNA and Con groups (P<0.001;
Fig. 3B and C). These results suggest
that different LASS2 expression levels regulate the tumorigenicity
of bladder cancer EJ-M3 cells, the growth of xenografts and the
degree of tumor cell heteromorphism in vivo.
Effect of LASS2 expression on MMP-2
and MMP-9 activity in xenografts
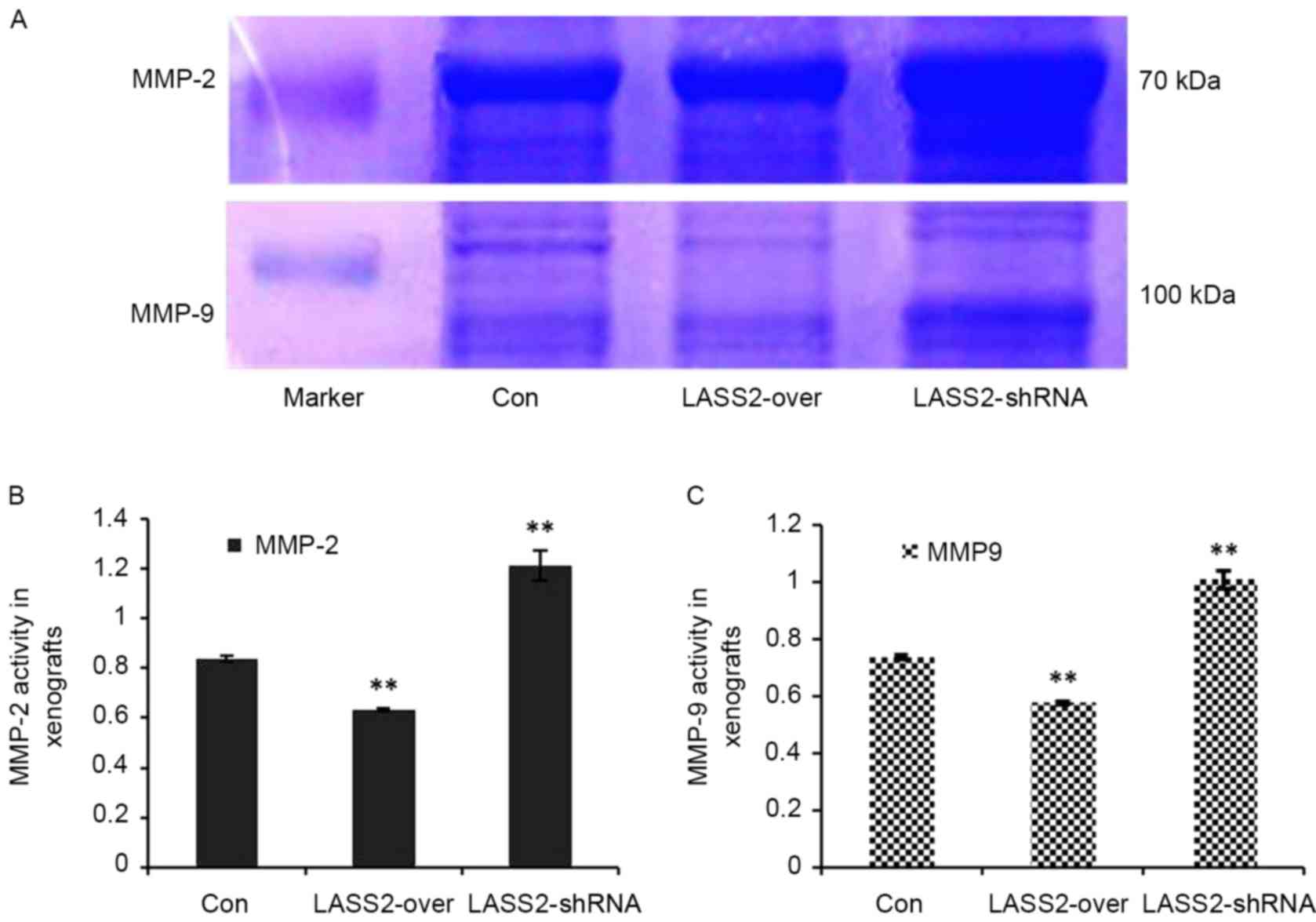
Using gelatin zymography and a quantified analytical
system, MMP-2 and MMP-9 activity in the xenografts with different
LASS2 expression levels was analyzed. MMP-2 and MMP-9 expression
levels were identified as clear bands against a lighter blue
background (Fig. 4A). Compared with
the Con group, the relative activities of MMP-2 (Fig. 4B) and MMP-9 (Fig. 4C) in the LASS2-shRNA group were
significantly higher (P<0.0001), while those in the LASS2-over
group were significantly lower (P<0.0001).
Discussion
LASS2 serves an important role in inhibiting the
proliferation and invasion of a number of tumor cancer cell lines,
including those of the prostate, liver and breast (7,17,18). Previous studies have suggested that
LASS2 inhibits tumor invasion and metastasis through the inhibition
of vacuolar-H(+)-ATPase by LASS2 through its association with the
V-type proton ATPase 16 kDa proteolipid subunit (the C subunit of
V-ATPase) may subsequently suppress the activation of hydrogen
sensitive proteolytic enzymes and the degradation of extracellular
matrix, inducing apoptosis of tumor cells (4–7,19,20).
Previous studies have also revealed that V-ATPase serves a critical
role in the secretion and activation of degradation enzymes, such
as MMPs (21,22).
LASS2 has been demonstrated to promote tumor cell
apoptosis via the synthesis of ceramide (3,23,24). Tang et al (7) transiently transfected HCCLM3 cells with
a pCMV-HA2-LASS2 plasmid in order to increase the expression of
LASS2, and the results suggested that LASS2 overexpression inhibits
the migration and invasion of HCCLM3 cells. Lu et al
(25) demonstrated that the risk of
hepatocellular carcinoma was elevated in 1-month-old mice in which
LASS2 had been knocked out. Previous studies have demonstrated that
LASS2 expression is downregulated and associated with poor clinical
prognosis in advanced human bladder carcinoma (26), and that LASS2 expression is
significantly correlated with diverse proliferation, metastasis and
invasion in bladder cancer cells (13). Another previous study revealed that
LASS2 overexpression downregulated the expression of apoptosis
regulator Bcl-2 and survivin, while LASS2 siRNA upregulated their
expression (27). A recent study by
our group demonstrated that LASS2 overexpression in the bladder
cancer EJ cell line resulted in a decrease in cell proliferation,
metastasis and invasion ability in vitro (28). These results are in agreement with the
previous studies discussed above.
Recent studies have revealed that the silencing of
LASS2 in a number of tumor cell lines increases tumor growth and
lymph node metastases in vivo (20,29). In
the present study, the results of RT-qPCR and Western blot analyses
confirmed that the EJ-M3-LASS2-knockdown cells and
EJ-M3-LASS2-overexpression cells were successfully established by
transfecting LASS2-shRNA or LASS2 overexpression plasmids into the
human bladder cancer EJ-M3 cell line. The cell suspensions were
subcutaneously injected into the right dorsal aspect of the neck of
the nude mice in order to observe xenograft tumor growth. According
to the results of the tumor growth curves produced and the TIRs
calculated, LASS2 knockdown in human bladder cancer EJ-M3 cells
significantly promoted the growth of xenografts in nude mice.
Additionally, H&E staining and the xenograft experiments
suggested that different LASS2 expression levels affect the degree
of tumor cells heteromorphism.
Data from the present study demonstrated that LASS2
overexpression had a tendency to inhibit the growth of xenografts;
however, this result was not statistically significant. Previous
studies have demonstrated that LASS2 overexpression inhibits tumor
growth in vivo; for example, Fan et al (8) demonstrated that the combination of LASS2
overexpression and doxorubicin significantly inhibited the growth
of xenografts in nude mice. Additionally, several previous studies
have revealed that reducing MMP-2 and MMP-9 activity inhibited
tumorigenicity and the growth of xenografts in nude mice. For
example, Xu et al (30)
reported that the silencing of LASS2 may promote invasion of human
prostate cancer cell in vitro by increasing the activity of
secreted MMP-2. Consistently, Mei et al (18) reported that the overexpression of
LASS2 could inhibit the invasion of the human breast cancer cell
line MCF-7 in vitro by suppressing MMP-2 activation and
extracellular matrix degradation. In addition, a recent study has
revealed that MMP-9 knockdown inhibited tumorigenicity in nude mice
(31).
In order to further investigate the influence of
LASS2 overexpression on the growth of bladder tumor xenografts,
LASS2 expression and MMP-2/−9 activity in the xenografts was
detected using immunohistochemistry and gelatin zymography,
respectively. The activities of MMP-2 and MMP-9 were negatively
correlated with LASS2 expression in the xenografts of nude mice.
These results suggest that LASS2 overexpression significantly
suppresses the activities of MMP-2 and MMP-9 in vivo, which
is consistent with the results of previous studies (18,20,30).
The data from the present study revealed that LASS2
overexpression had a tendency to inhibit the growth of bladder
tumor xenografts, though this was not statistically significant.
However, the activities of MMP-2 and MMP-9 were significantly
decreased in LASS2 overexpression xenografts compared with the Con.
Additionally, there was a wide variability in tumor volumes within
the same groups. There are several possible explanations for this,
such as that the mechanisms of tumorigenicity and tumor growth
in vivo are complex, including gene-gene interaction and
multiple factors involved in tumor formation, growth and
metastasis. Another possible explanation is the short observation
period and the small sample size of the present study. Therefore,
further research is required to confirm whether LASS2
overexpression inhibits tumor growth.
In conclusion, the results from the present study
indicate that LASS2 knockdown enhances the heteromorphism of EJ-M3
cell xenograft tumors and promotes the tumorigenicity and growth of
tumors in vivo. In addition, LASS2 overexpression was
identified to have a tendency to inhibit the growth of xenografts,
suggesting that it is a potential therapeutic target for bladder
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81260374 and
81460384), the Yunnan Provincial Department of Education Fund
(grant no. 2014Z072), the Joint Project of Science and Technology
of Yunnan and Kunming Medical Universities (grant nos. 2014FA015
and 2014FZ031), the Yunnan Provincial Health Department Project
(grant no. 2014NS081) and the Yunnan Provincial Science and
Technology Project (grant no. 2015FB196).
Glossary
Abbreviations
Abbreviations:
|
LASS2
|
LAG1 longevity assurance homologue
2
|
|
GFP
|
green fluorescent protein
|
|
RT-qPCR
|
quantitative real-time polymerase
chain reaction
|
|
H&E
|
hematoxylin and eosin
|
|
MMPs
|
matrix metalloproteinases
|
|
HCC
|
hepatocellular carcinoma
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma C, Liu Y, Zheng J, Fang W, You J, Wang
J, Cui X and Wu B: Identification of tumor metastasisrelated gene
TMSG-1 by mRNA differential display. Sci China C Life Sci.
45:553–560. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F and Pei F: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XY, You JF, Pei F and Zhang B:
Silencing of tumor metastasis suppressor gene 1 promotes invasion
of prostate cancer cell in vitro and its molecular mechanisms.
Beijing Da Xue Xue Bao. 43:814–819. 2011.(In Chinese). PubMed/NCBI
|
|
6
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene LASS2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang N, Jin J, Deng Y, Ke RH, Shen QJ, Fan
SH and Qin WX: LASS2 interacts with V-ATPase and inhibits cell
growth of hepatocellular carcinoma. Sheng Li Xue Bao. 62:196–202.
2010.(In Chinese). PubMed/NCBI
|
|
8
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiffmann S, Sandner J, Birod K, Wobst I,
Angioni C, Ruckhäberle E, Kaufmann M, Ackermann H, Lötsch J,
Schmidt H, et al: Ceramide synthases and ceramide levels are
increased in breast cancer tissue. Carcinogenesis. 30:745–752.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Wang J, Zuo Y, Ding M, Yan R, Yang
D and Ke C: Expression and prognostic significance of a new tumor
metastasis suppressor gene LASS2 in human bladder carcinoma. Med
Oncol. 29:1921–1927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang D, Wang H, Wang J, Zhang C and Xu H:
Establishment of a fluorescent implantation metastasis model of
bladder cancer and real-time microscopic detection in nude mice.
Asian Pacific J Cancer Prev. 12:393–396. 2011.
|
|
12
|
Girnita A, All-Ericsson C, Economou MA,
Aström K, Axelson M, Seregard S, Larsson O and Girnita L: The
insulin-like growth factor-I receptor inhibitor picropodophyllin
causes tumor regression and attenuates mechanisms involved in
invasion of uveal melanoma cells. Clin Cancer Res. 12:1383–1391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Q, Wang H, Yang M, Yang D, Zuo Y and
Wang J: Expression of a tumor-associated gene, LASS2, in the human
bladder carcinoma cell lines BIU-87, T24, EJ and EJ-M3. Exp Ther
Med. 5:942–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cumashi A, Tinari N, Rossi C, Lattanzio R,
Natoli C, Piantelli M and Iacobelli S: Sunitinib malate (SU-11248)
alone or in combination with low-dose docetaxel inhibits the growth
of DU-145 prostate cancer xenografts. Cancer Lett. 270:229–233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li
Y, Adell G and Sun XF: Survivin expression quantified by image
Pro-Plus compared with visual assessment. Appl Immunohistochem Mol
Morphol. 17:530–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su J, You JF, Wang JL, Cui XL, Fang WG and
Zheng J: Overexpression of human tumor metastasis-related gene
TMSG-1 suppresses cell proliferation and invasion of a highly
metastatic prostate cancer cell line PC-3M-1E8 in vitro. Zhonghua
Zhong Liu Za Zhi. 30:404–407. 2008.(In Chinese). PubMed/NCBI
|
|
18
|
Mei F, You J, Liu B, Zhang M, Liu J, Zhang
B and Pei F: LASS2/TMSG1 inhibits growth and invasion of breast
cancer cell in vitro through regulation of vacuolar ATPase
activity. Tumour Biol. 36:2831–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu XY, Pei F and You JF: TMSG-1 and its
roles in tumor biology. Chin J Cancer. 29:697–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Liu B, Zou P, Zhang Y, You J and Pei
F: Silencing of LASS2/TMSG1 enhances invasion and metastasis
capacity of prostate cancer cell. J Cell Biochem. 115:731–743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sennoune SR, Luo D and Martinez-Zaguilán
R: Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell
Biochem Biophys. 40:185–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against cancer.
Cancer Res. 67:10627–10630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi MJ, Sundararaj K, Koybasi S,
Phillips MS, Szulc ZM, Bielawska A, Day TA, Obeid LM, Hannun YA and
Ogretmen B: Inhibition of growth and telomerase activity by novel
cationic ceramide analogs with high solubility in human head and
neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg.
132:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su J, Yu W, Gong M, You J, Liu J and Zheng
J: Overexpression of a novel tumor metastasis suppressor gene
TMSG1/LASS2 induces apoptosis via a caspase-dependent mitochondrial
pathway. J Cell Biochem. 116:1310–1317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Chen Y, Zeng T, Chen L, Shao Q and
Qin W: Knockout of the HCC suppressor gene Lass2 downregulates the
expression level of miR-694. Oncol Rep. 32:2696–2702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Wang J, Zuo Y, Ding M, Yan R, Yang
D and Ke C: Expression and prognostic significance of a new tumor
metastasis suppressor gene LASS2 in human bladder carcinoma. Med
Oncol. 29:1921–1927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan
R, Zhan H, Liu J and Wang J: miR-9 promotes cell proliferation and
inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour
Biol. 36:9631–9640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Zuo Y, Ding M, Ke C, Yan R, Zhan
H, Liu J, Wang W, Li N and Wang J: LASS2 inhibits growth and
invasion of bladder cancer by regulating ATPase activity. Oncol
Lett. 13:661–668. 2017.PubMed/NCBI
|
|
29
|
Chen L, Lu X, Zeng T, Chen Y, Chen Q, Wu
W, Yan X, Cai H, Zhang Z, Shao Q and Qin W: Enhancement of
DEN-induced liver tumourigenesis in hepatocyte-specific
Lass2-knockout mice coincident with upregulation of the
TGF-β1-Smad4-PAI-1 axis. Oncol Rep. 31:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene LASS2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo F, Tian J, Cui M, Fang M and Yang L:
Downregulation of matrix metalloproteinase 9 by small interfering
RNA inhibits the tumor growth of ovarian epithelial carcinoma in
vitro and in vivo. Mol Med Rep. 12:753–759. 2015. View Article : Google Scholar : PubMed/NCBI
|