Introduction
Rhabdomyosarcoma (RMS) is the most common type of
soft tissue sarcoma in children. However, overall survival times
for patients with RMS have remained unchanged since the 1970s
(1). Despite the use of systemic
chemotherapy, metastatic or unresectable RMS remains an unmet
clinical challenge. The cure rate of this disease is particularly
poor for those patients, with a 3-year event-free survival rate of
only 27% (2).
Immunotherapy has been proposed as a treatment for
this RMS (3,4). Adoptive T-cell therapy utilizing
cytotoxic T lymphocytes (CTLs) directed against tumor-associated
antigens represents a promising immunotherapy approach. A major
limitation, however, is that CTLs recognize and kill tumor cells in
a major histocompatibility complex (MHC)-restricted manner, but
almost half of the bone and soft tissue sarcoma cases have
developed to evade immune recognition by decreasing MHC expression
(5). To this end, alternative
approaches using MHC-independent immune effectors may circumvent
this problem and allow for more universal application.
γδ T cells express T cell receptors (TCRs) composed
of γ and δ chains (6). Unlike tumor
antigen-specific αβ T cells, identification of tumor target
antigens is not required for γδ T cells. A previous study focused
primarily on peripheral Vδ2-positive γδ T cells (Vδ2 T cells) with
potential antitumor reactivity (7).
This subset typically accounts for between 50 and 95% of the total
γδ T cells in peripheral blood, and contributes to the cytotoxic
response against a broad range of tumors. Vδ2 T cells recognize
isopentenyl pyrophosphate (IPP) as phosphoantigens without
MHC-restriction (8,9). In tumor cells, the mevalonate signaling
pathway is frequently dysregulated, leading to the upregulation of
an intermediate IPP. Cumulative evidence indicates that γδ T cells
are capable of lysis of a broad range of tumor cells, including
ovarian, breast, renal cell cancer, glioblastoma and other solid
tumors (6). Most noteworthy,
nitrogen-containing bisphosphonates (N-BPs), including zoledronic
acid (Zol), sensitize tumor cells to the Vδ2 T cell cytotoxicity
(10,11). Recently, we also characterized the
cytotoxicity of γδ T cells against osteosarcoma and chondrosarcoma
cells in a preclinical setting (12,13).
The known potential of γδ T cells in anticancer
surveillance suggests their possible role in cellular
immunotherapy. Although the effectiveness of γδ T cells is
increasingly well-described, further studies are required in the
area of sarcoma in particular. In the present study, the antitumor
activity of γδ T cells against RMS cells was demonstrated for the
first time, to the best of our knowledge. Furthermore, it was
demonstrated that Zol enhances this cytolytic effect mediated by
human γδ T cells. The potential underlying molecular mechanism of
the interaction between γδ T cells and Zol-treated RMS cells is
also discussed.
Materials and methods
Cell lines
The RMS cell lines RD and A-673 were purchased from
the Cell Collection of Chinese Academy of Science (Shanghai,
China). The firefly luciferase-expressing RD cell line RD-LUC was
purchased from Invabio Biotechnology, Ltd. (Shanghai, China). RMS
cells were cultured in complete Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100
U/ml streptomycin and 1% L-glutamine (all from Invitrogen; Thermo
Fisher Scientific, Inc.).
Ex vivo expansion and phenotype of γδ
T cells
γδ T cells were expanded from peripheral blood
collected from healthy donors (n=5). Informed written consent was
obtained from all donors, and the research was approved by the
Human Research Ethics Committees of the Second Affiliated Hospital,
College of Medicine, Zhejiang University (Hangzhou, China).
Peripheral blood mononuclear cells (PBMCs) were separated from
peripheral blood by density gradient centrifugation (Cedarlane
Laboratories, Burlington, ON, Canada) and seeded on 24-well culture
plates at a concentration of 1.5×106 cells/ml in
RPMI-1640 medium (Gibco), supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin, 100 U/ml streptomycin and 1%
L-glutamine. Zol (Zometa®; Novartis International AG,
Basel, Switzerland) at 1 µM and 400 IU/ml interleukin 2 (IL-2;
PeproTech, Inc., Rocky Hill, NJ, USA) were added on day 0. After 3
days, half of the culture medium was replaced with fresh medium
containing 400 IU/ml IL-2. Fresh medium and IL-2 (400 U/ml) were
added every 3 days during culture. At day 14, γδ T cells were
purified by negative magnetic-activated cell sorting isolation
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The phenotype
of γδ T cells was analyzed using standard flow cytometric assays.
Briefly, the cells were stained with the indicated fluorescently
labeled antibodies for 30 min at 4°C, washed, and analyzed by flow
cytometry according to the manufacturer's instructions. The
following monoclonal antibodies (mAbs) were obtained from BioLegend
(San Diego, CA, USA): anti-Vδ2-fluorescein isothiocyanate (FITC)
(cat. no. 331406; clone B6), anti-cluster of differentiation (CD)
3-FITC (cat. no. 344804; clone SK7), anti-interferon γ (IFN-γ)-FITC
(cat. no. 506504; clone B27), anti-CD69-FITC (cat. no. 310904;
clone FN50) and anti-TCR-γ/δ-phycoerythrin (cat. no. 331210; clone
B1). Flow cytometry was performed using a FACS CantoII instrument
(BD Biosciences, San Jose, CA, USA) and the data were analyzed
using FlowJo software (version 9.3.2; Tree Star, Inc., Ashland, OR,
USA).
Intracellular staining of IFN-γ
γδ T cells were co-cultured with tumor cells for 4 h
at 37°C in the presence of 20 µg/ml brefeldin A (BD Biosciences).
γδ T cells stimulated with phorbol-12-myristate-13-acetate (PMA;
2.5 mg/ml; Sigma-Aldrich; Merck KGaA) and ionomycin (0.5 mg/ml;
Sigma-Aldrich; Merck KGaA) for 2 h was used as positive control.
Cells were re-suspended in PBS with 1% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and stained with specific monoclonal antibody
TCR-γ/δ for 30 min in the dark at 4°C. Following surface staining
of TCR-γ/δ, cells were fixed and permeabilized using
Cytofix/Cytoperm buffer (BD Biosciences). γδ T cells were washed
with Perm/Wash buffer (BD Biosciences) and stained with
FITC-labeled anti-human IFN-γ mAb for 30 min in the dark at
4°C.
Cytotoxicity assays
The cytotoxicity γδ T cells was determined using a
CellTiter 96 cytotoxicity MTS assay (Promega Corporation, Madison,
WI, USA) as described previously (12). Briefly, 5×103 tumor cells
were seeded in 96-well flat-bottomed plates in triplicate. In
certain experiments, Zol was used to sensitize tumor cells for 24 h
after cell attachment. Subsequently, γδ T cells were added at the
indicated effector/target (E:T) ratio and co-cultured with tumor
cells for 4 h at 37°C. The plates were washed gently three times,
and the residual viable tumor cells were quantified using the MTS
assay according to the manufacturer's protocol. In blocking
studies, γδ T cells were incubated with 10 µg/ml (saturating
concentrations) anti-human natural killer group 2, member D (NKG2D;
clone 149810; R&D Systems, Inc., Minneapolis, MN, USA),
anti-pan-γδ TCR (clone B1; BD Biosciences), anti-tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) (clone RIK-2; BD
Biosciences) or anti-human Fas ligand (FasL; clone NOK-2; BD
Biosciences) for 30 min before co-culture to block the relevant
cytotoxic pathways. All experiments were performed in triplicate.
Cytotoxicity at each E:T ratio was calculated according to the
following formula: Cytotoxicity (%) = 100 – 100× (optical density
at 490 nm for co-culture well/optical density at 490 nm for target
cell well).
ELISA
RMS cells (1×104) were co-cultured with
2×105 γδ T cells in triplicate in 96-well flat-bottomed
plates for 4 h. Supernatants were harvested and assayed for IFN-γ
using a Human IFN-γ ELISA kit (Dakewe Biotech Co., Ltd., Shenzhen,
China), according to the manufacturer's protocol.
Adoptive immunotherapy with human γδ T
cells
Female 4-week-old athymic nude mice weighing ~20 g
were purchased from the Experimental Animal Center of the Zhejiang
Traditional Medical University (Hangzhou, China), and were housed
under specific pathogen-free conditions at 25°C in an atmosphere
with 50% humidity and at 12/12 h light/dark cycle with free access
to food and water. RD-LUC tumor cells (5×106) were
implanted subcutaneously (s.c.) into the upper right flank of mice
under anesthesia. Mice were randomized into four groups (6
mice/group) 7 days after tumor implantation: i) Control mice,
treated with PBS; ii) mice treated with intraperitoneal (i.p.)
injections of Zol (50 µg/kg); iii) mice treated with intravenous
(i.v.) injections of γδ T cells through the tail vein
(5×106 cells/mouse in 100 µl serum-free culture medium);
and iv) mice treated with Zol followed by γδ T cells 1 day later.
All treatments were performed once a week for 4 weeks. The survival
and general status of mice was monitored daily. Tumor
bioluminescence was observed using an IVIS Lumina Series III
Imaging platform (PerkinElmer, Inc., Waltham, MA, USA) as described
previously (13). Tumor size was
measured and calculated according to the formula: Volume = 1/2 ×
length × width2. All animal procedures and protocols
followed the guidelines of the Institutional Authority for
Laboratory Animal Care of the Zhejiang University and were approved
by the Ethics Committee of the Second Affiliated Hospital, School
of Medicine, Zhejiang University (Hangzhou, China).
Statistical analysis
Comparison of quantitative data between two groups
was performed using Student's t-test. Analysis of variance was used
to determine the difference among three or more groups. Differences
between paired samples were tested by Wilcoxon's tests. All data
were analyzed using SPSS software (version 11.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Zol and IL-2 induced the ex vivo
expansion and activation of γδ T cells
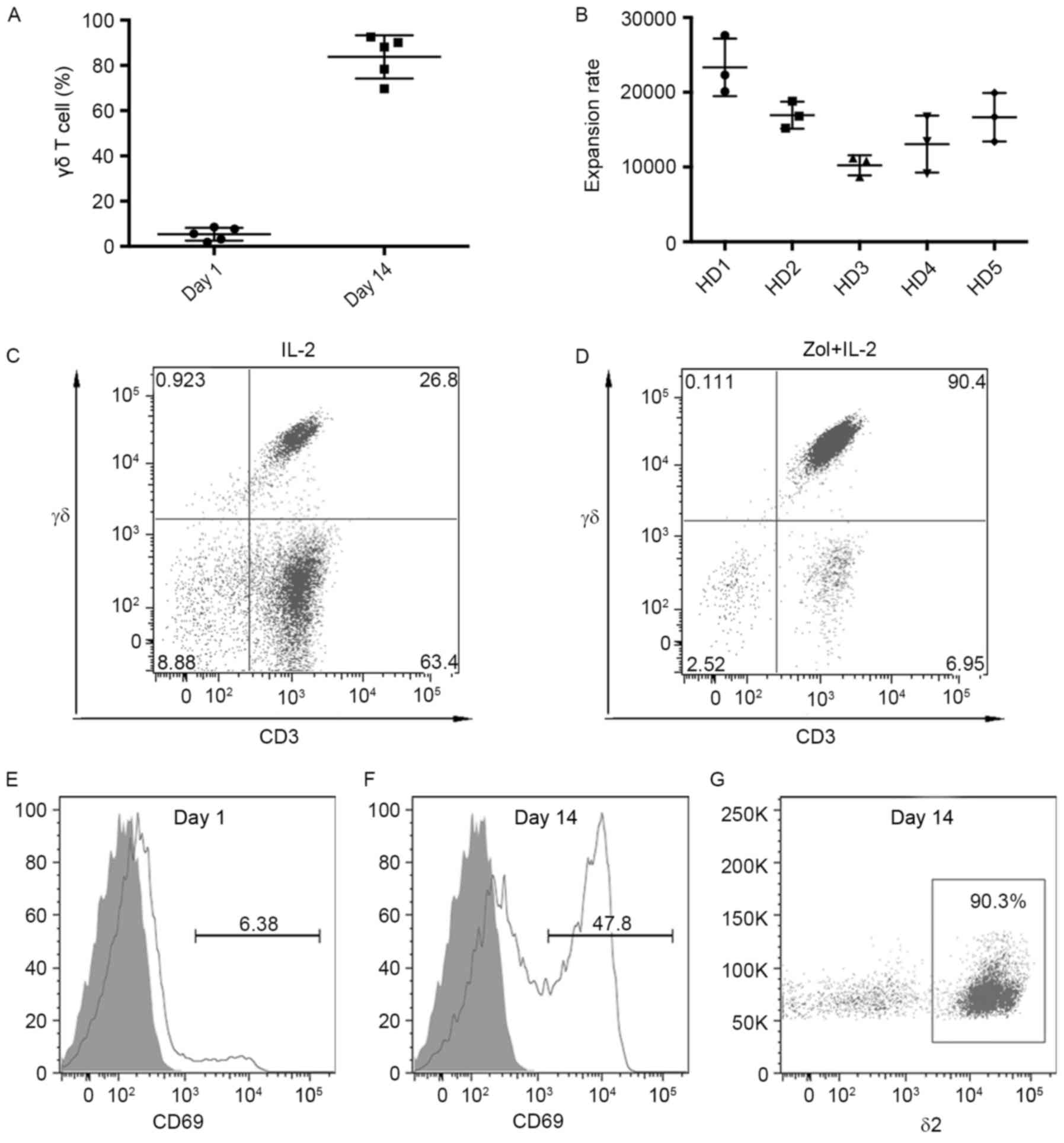
According to flow cytometric analysis, the
proportion of γδ T cells in the T-cell population from healthy
donors was low, ranging between 1.9 and 8.5% (Fig. 1A). However, Zol and IL-2 induced the
robust expansion of γδ T cells in peripheral blood. At the end of
the 14-day culture, γδ T cells were successfully expanded. Although
the expansion varied between donors, stimulation of PBMCs with Zol
and IL-2 resulted in >104-fold higher levels of the
numbers of γδ T cells for all the donors tested (Fig. 1B). Notably, the preferential expansion
of γδ T cells was dependent on Zol stimulation, because in culture
with addition of IL-2 alone, the percentage of γδ T cells averaged
only 25.7% on day 14 (range, 12.5–34.2%; Fig. 1C), whereas the median percentages of
γδ T cells on day 14 was 88.1% (range, 69.7–92.5%; Fig. 1D). With the expansion of γδ T cells,
the expression of activation marker CD69 was also upregulated. At
the onset of culture, γδ T cells expressed little CD69 (Fig. 1E). By contrast, on day 14, ~45% of the
γδ T cells were observed to express CD69 (Fig. 1F). In accordance with previous
reports, a subset of δ2-positive γδ T cells was preferentially
expanded. The majority of the expanded γδ T cells were δ2-positive
T cells (Fig. 1G).
Zol pretreatment enhances the in vitro
tumor-killing activity of γδ T cells against RMS cells
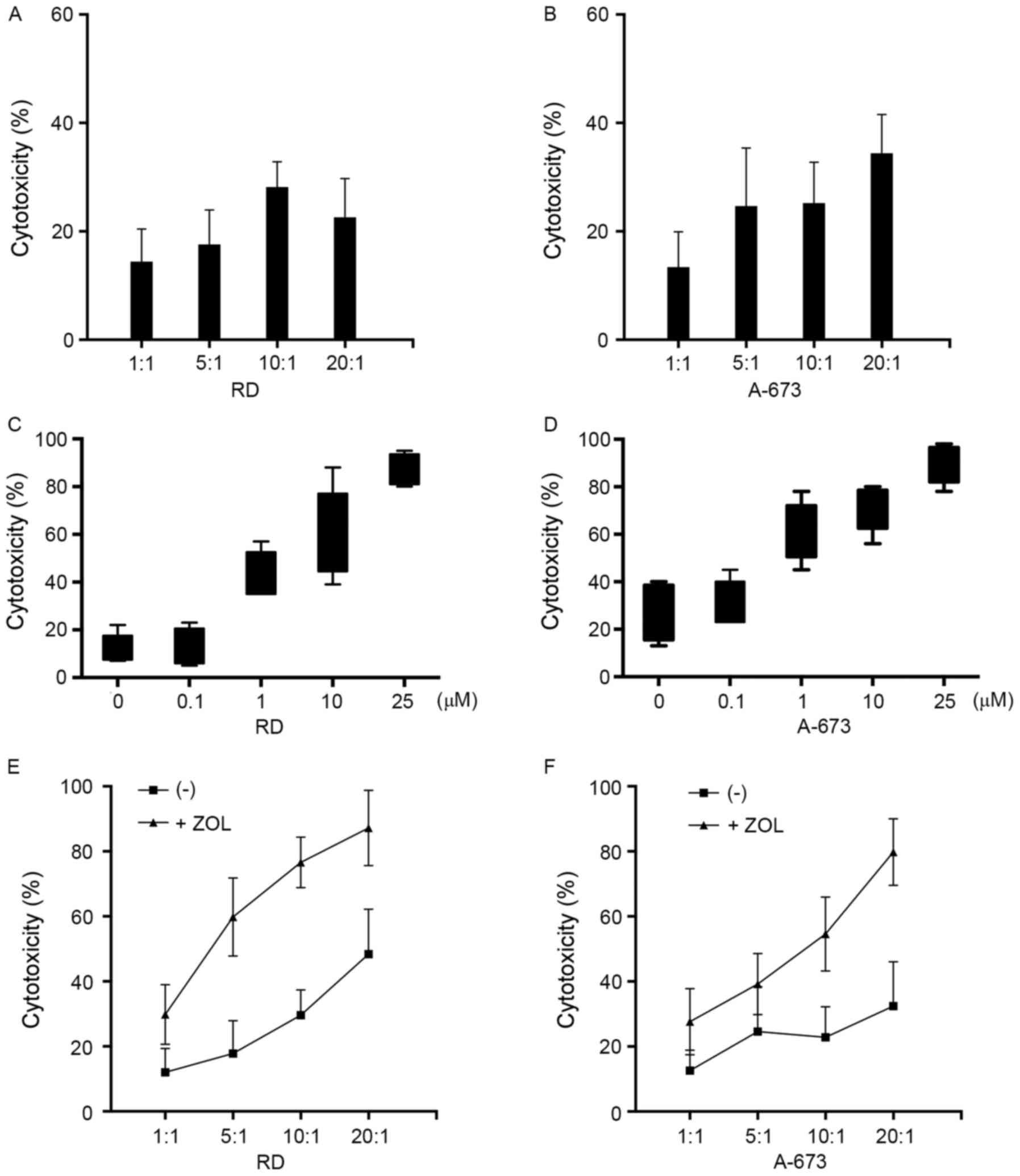
The sensitivity of RMS cell lines RD and A-673 to
lysis by γδ T cells was determined using an MTS assay. Results
presented in Fig. 2A and B indicated
that γδ T cells exhibited only moderate cytotoxicity towards RMS
cells, with 28.2 and 25.2% lysis for RD and A-673, respectively, at
an E:T ratio of 10:1. The effect of Zol pretreatment on the
susceptibility of the RMS cells to γδ T cell-mediated cytotoxicity
was determined. Target cells were cultured in medium supplemented
with a graded concentration of Zol for 24 h before a 4 h MTS assay
at an E:T ratio 10:1. When Zol was used at 0.1 µM, no appreciable
increase in cytotoxicity against the RD cell line was observed
(P>0.05; Fig. 2C). γδ T cells
began to exhibit enhanced levels of cytotoxicity with 1 µM Zol.
Increased cytotoxicity was detected with an increase in Zol
concentration, and peaked at a concentration of 25 µM. This
experiment revealed that the sensitization effect of Zol was
dose-dependent. Similarly, γδ T cells demonstrated comparable
cytotoxic activity with that towards A-673 cells (Fig. 2D). A detectable increase was already
observed when target cells were treated with 1 µM Zol, therefore a
concentration of 1 µM was used in the subsequent experiments. The
increase in cytotoxicity towards Zol-treated tumor cells was
consistently observed at all E:T ratios used (Fig. 2E and F). Not unexpectedly, a
ratio-dependent increase in cytotoxicity was observed, and almost
complete killing could be achieved at an E:T ratio of 20:1,
suggesting that optimal cytotoxicity requires sufficient effector
cells. Notably, no apparent tumor cell death was observed using the
MTS assay when cultured for 24 h in medium supplemented with the
indicated concentration of Zol, indicating that Zol alone did not
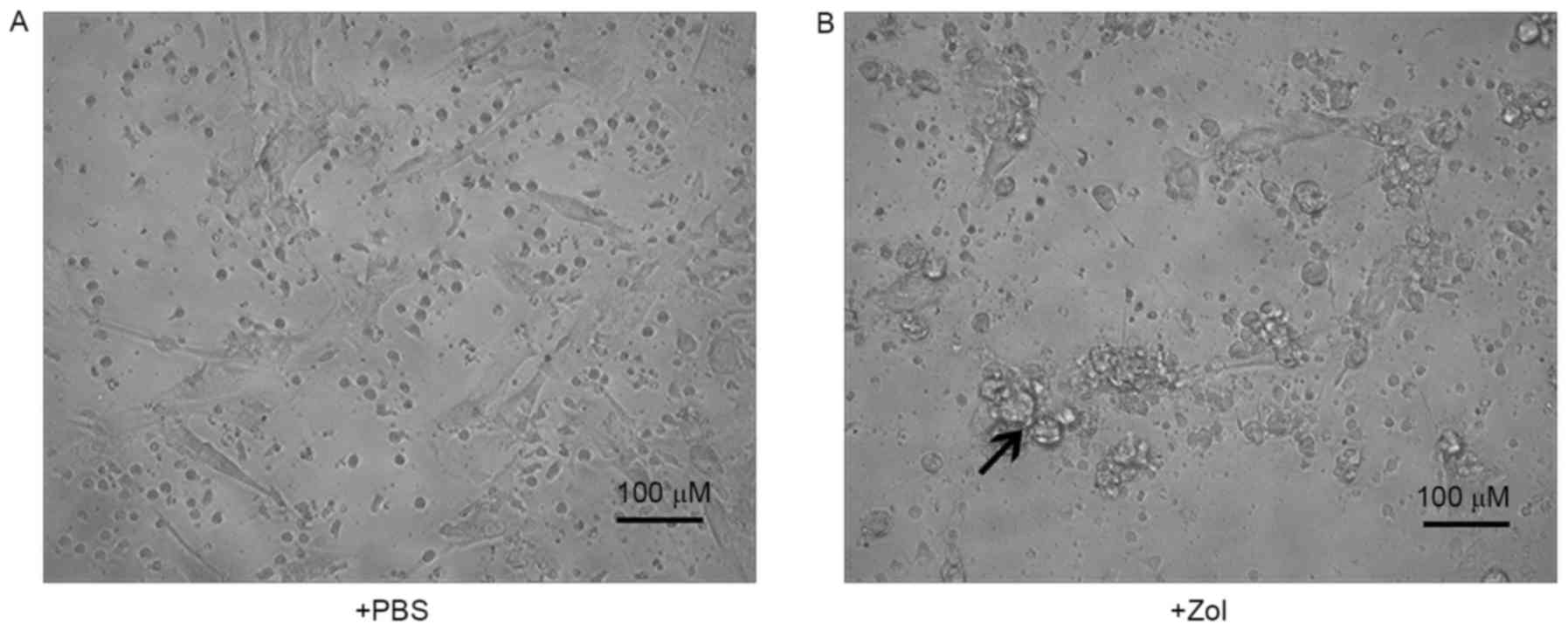
induce direct tumor cell lysis (data not shown). To further
investigate the effect of Zol on the lysis of RMS cells by γδ T
cells, target cells were treated with or without Zol, the cell
lines were co-cultured and visualized microscopically. As presented
in Fig. 3A, Zol-treated RMS cells
were surrounded by γδ T cells, leading to cell death induced by γδ
T cells. By contrast, fewer T cells were bound to untreated RMS
cells, many of which remained intact throughout the 4-h co-culture
period (Fig. 3B). Overall, these data
suggest that Zol pre-treatment sensitized the γδ T cell-mediated
cytotoxicity to RMS cells.
RMS cells treated with Zol induce γδ T
cells to produce IFN-γ
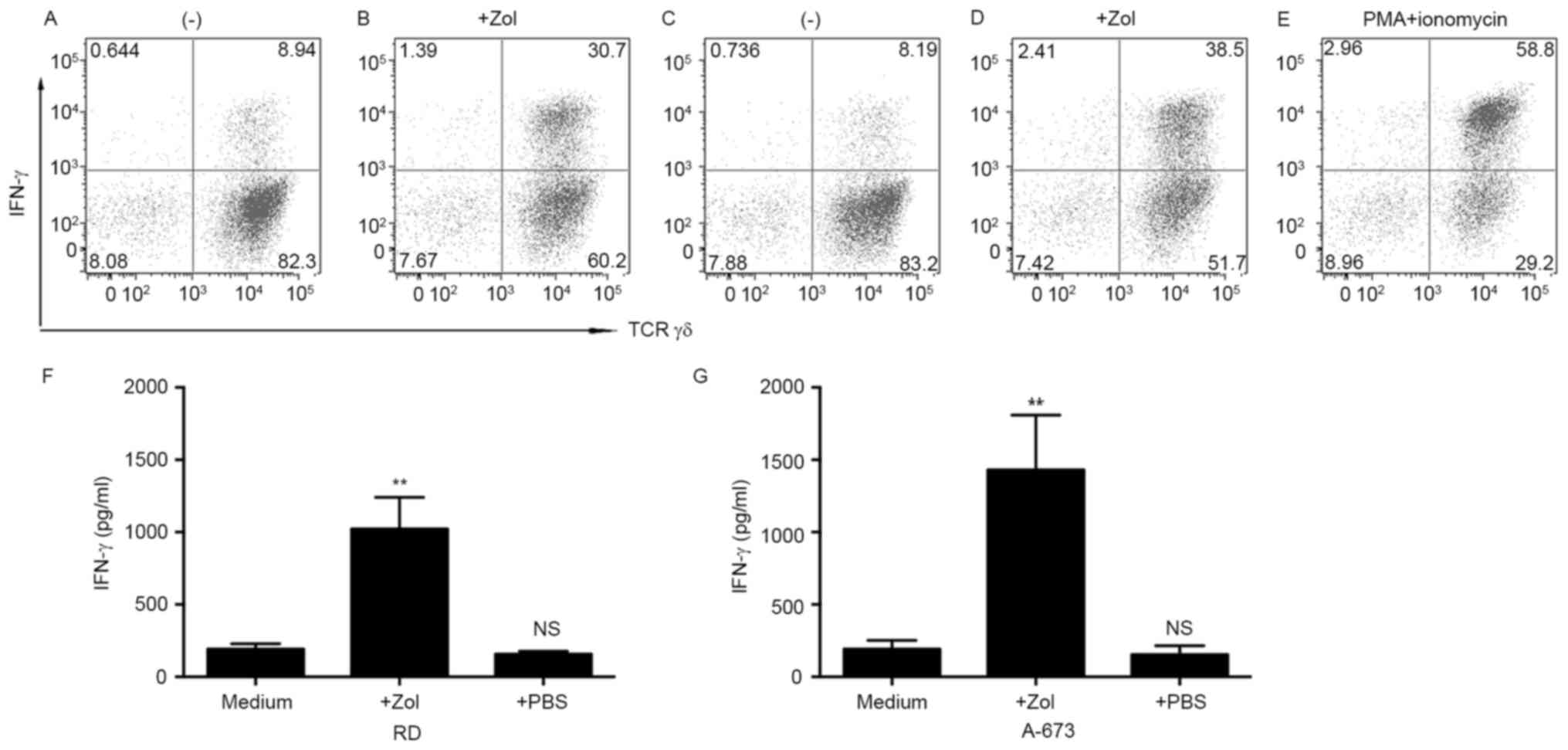
IFN-γ production in γδ T cells was examined in
response to RMS cells. Flow cytometry of the intracellular staining
of IFN-γ was performed. Culture of γδ T cells with untreated tumor
cells resulted in relatively low levels of IFN-γ (Fig. 4A and B). The poor response towards
human RMS cells was not an intrinsic property of the γδ T cells,
because a markedly increased level of IFN-γ was observed in γδ T
cells stimulated with phorbol myristate acetate and ionomycin
(Fig. 4C). The Zol-sensitized immune
response of γδ T cells was evaluated. Pretreatment of RD cells with
Zol led to marked levels of intracellular IFN-γ within γδ T cells
(Fig. 4D). Likewise, γδ T cells
displayed increased intracellular IFN-γ levels in response to
Zol-treated A-673 cells (Fig.
4E).
To confirm the ability of γδ T cells to secrete
IFN-γ, the supernatants of co-culture was determined by ELISA. γδ T
cells were cultured with tumor cells as aforementioned. After 4 h
of co-culture, supernatants were harvested and analyzed for IFN-γ
content. In line with the flow cytometric data, γδ T cells produced
moderate levels of IFN-γ when co-cultured with either untreated RD
or A-673 cells. However, Zol pretreatment increased IFN-γ protein
production. The results presented in Fig.
4F and G demonstrated that γδ T cells secreted significantly
increased amounts of IFN-γ in the Zol-sensitized RMS cell lines
(P<0.01). These results indicate that Zol enhanced γδ T cell
responsiveness compared with that in untreated target cells.
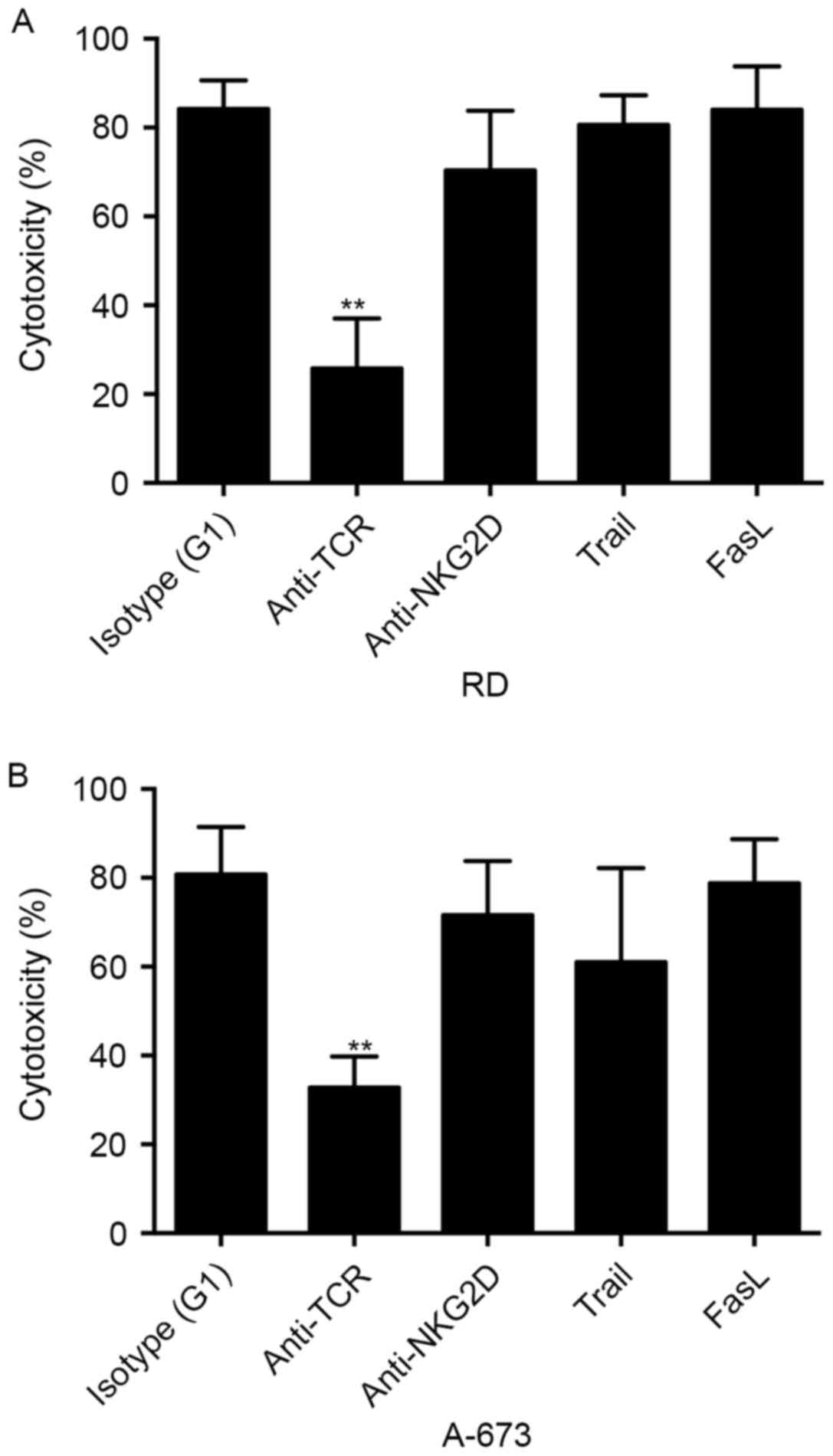
Zol sensitizes RMS cells susceptible
to the γδ T cell-mediated cytotoxicity in a TCR-dependent
manner
To study the molecular mechanisms involved in the
interaction between γδ T cells and Zol-treated RMS cells, a
blocking assay was used to test the effect of surface molecules on
γδ T cell cytotoxicity. γδ T cells were incubated with anti-pan-γδ
TCR, anti-NKG2D and anti-human TRAIL mAbs for 30 min before
co-culture. Pre-incubation of γδ T cells with anti-pan-γδ TCR
antibody significantly inhibited the cytotoxicity against the RMS
cell lines (P<0.01; Fig. 5),
whereas anti-TRAIL antibody did not result in an appreciable
decrease in γδ T cell cytotoxicity. Previous study has indicated
the role of NKG2D pathway in the lysis of distinct tumors (14). However, as presented in Fig. 5, anti-NKG2D mAb blockade had no
discernible effect on the cytolysis of Zol-treated RD or A-673 cell
line by γδ T cells. These results suggest that γδ T cell-mediated
cytolysis of RMS cells was dependent on TCR pathways.
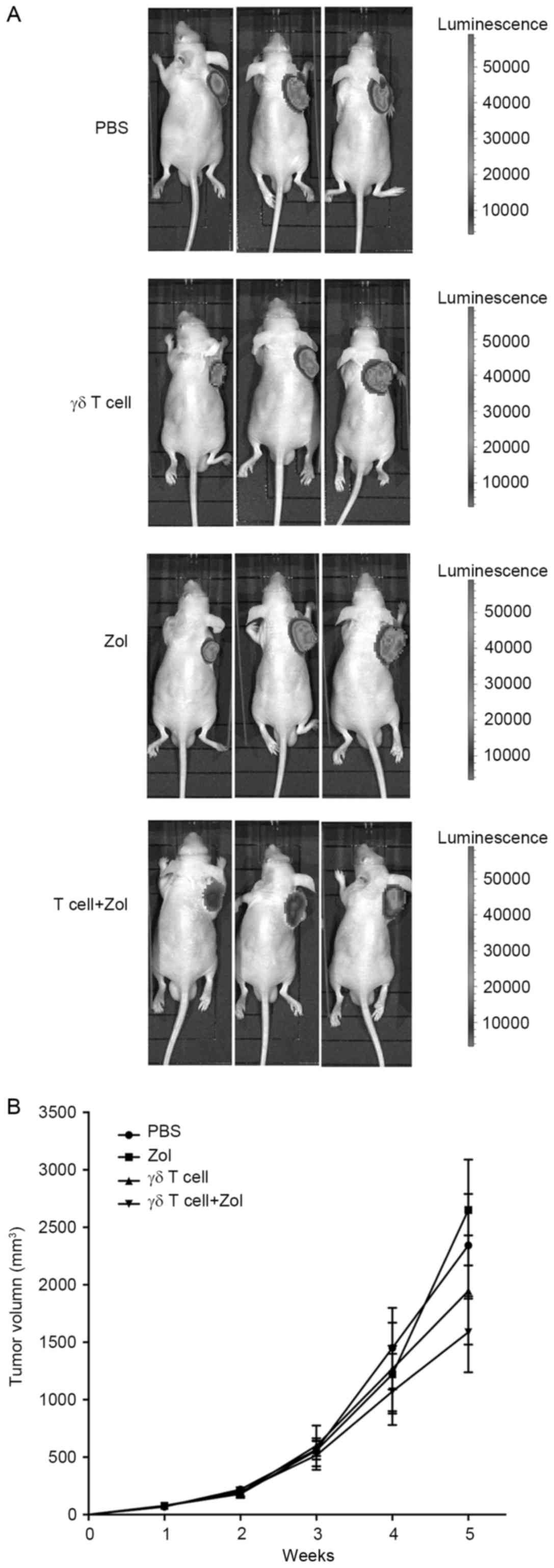
In vivo antitumor effect of infused γδ
T cells against RD xenograft tumors
To examine the in vivo immunotherapeutic
effects of γδ T cells, a RMS xenograft nude mouse model was
established by subcutaneous injection into mice with established
firefly luciferase-expressing RD cell line RD-LUC cells (Fig. 6A). At 1 week after tumor inoculation,
mice were treated weekly with γδ T cells (5×106
cells/mouse, i.v.), or Zol (50 µg/kg/mouse, i.p.), or a combination
of γδ T cells and Zol (injection of Zol and then γδ T cells 1 day
later) for 4 weeks. PBS treatment was set as control. As presented
in Fig. 6B, all untreated control
mice demonstrated progressive tumor growth. The volume of single
treatment alone was not significantly different from that of
control mice, whereas a significant decrease in the tumor volume in
mice injected with combined treatment was observed (P<0.01).
These results demonstrated that a combination of γδ T cells and Zol
significantly inhibited the growth of RMS cells in vivo.
Discussion
Current immunotherapeutic approaches that target RMS
cells mainly focus on natural killer (NK) cells or CTLs. It remains
challenging to expand NK cells or antigen-specific αβ T cells ex
vivo to the amount required for efficacious adaptive
immunotherapy. Conversely, the results of the present study
confirmed that γδ T cells could be sufficiently obtained from PBMCs
of healthy donors in short-term culture. Additionally, the
cytotoxic activity of γδ T cells against RMS cells is, to the best
of our knowledge, reported for the first time. The results of the
present study revealed that γδ T cells had direct cytotoxic
activity towards RMS cell lines. Importantly, Zol sensitization
markedly increased the susceptibility of RMS cells to γδ T
cells.
Zol is an N-BP and exerts pharmacological effects by
specifically inhibiting farnesyl pyrophosphate synthase, a key
enzyme in the mevalonate signaling pathway (15). This process leads to the accumulation
of the upstream metabolite of the mevalonate signaling pathway,
including IPP, which is sensed by γδ T cells as stimulating
antigens. The use of Zol may represent a double strategy for
adoptive γδ T cell-based immunotherapy (16). On one hand, when Zol is internalized
by monocytes and dendritic cells, γδ T cells in PBMCs are expanded
and activated to an effector phenotype (17). On the other hand, tumor cells
pretreated by Zol are sensitized to the cytolysis mediated by human
γδ T cells (10). Zol is a clinically
approved drug widely used in the treatment of bone
resorption-associated disease for patients with cancer (18). It has been demonstrated that Zol may
also exert direct antitumor effects in vitro and in animal
models (16). Therefore, this
therapeutic strategy is of practical value in a clinical scenario,
particularly in settings in which there are limited options for
treating metastatic RMS.
The second major result is that the γδ T cell
response to Zol-treated RMS cells was primarily through the
TCR-mediated signaling pathway. NKG2D was originally described as a
stimulatory receptor for NK cells. Several lines of evidence
indicate that tumor cell lysis by γδ T cell may be modulated by TCR
and NKG2D ligation (14,19). However, the results of the antibody
blockade assay in the present study indicated that the NKG2D
signaling pathway may serve a lesser role in the recognition of
Zol-treated RMS cells, because blocking NKG2D on γδ T cells was
largely ineffective. This result is not unexpected. As reported
previously, NKG2D activates γδ T cells in an antigen-independent
manner (19), whereas Zol-produced
IPP on the tumor surface is mainly recognized by γδ TCR. The
underlying molecular mechanisms mediating the cytotoxic effect of
γδ T cells to Zol-treated RMS cells were investigated in the
present study. Zol treatment caused γδ T cells to secrete increased
levels of IFN-γ. It coincides with the results by Rincon-Orozco
et al (19) that IFN-γ
production may not be induced by NKG2D ligation. Mattarollo et
al (20) also observed that NKG2D
interactions did not significantly contribute to the cytotoxicity
of Zol-sensitized tumor cells, but did not develop this issue
further in their study.
The present study has some limitations. Only PBMCs
from healthy donors were used to expand γδ T cells. Whether the
expansion efficiency of γδ T cells from patients with RMS is
comparable with that of healthy donors remains to be determined. In
previous studies, γδ T cells have been successfully expanded from
patients with lung cancer (21),
neuroblastoma (22) and follicular
lymphoma (23). Therefore, the
proliferative responses of γδ T cells from certain patients with
RMS are presumably not impaired. Owing to the lack of
alloreactivity of γδ T cells, for patients with impaired autologous
γδ T cell expansion capacities, it is possible to transfer
sufficient allogeneic γδ T lymphocytes expand from normal donors.
As only the cytotoxic activity of γδ T cells against RD and A-673
cells was examined, other RMS cell lines and autologous tumor cells
are required to confirm the results of the present study. Finally,
the in vivo results indicate that a combination of Zol and
γδ T cells yielded marked antitumor responses compared with other
single treatment, and it was consistent with in vitro
studies. Considering the promising results of the present study,
further studies including immunohistochemical analysis of
localization and kinetics of infused γδ T cells are warranted to
explore the mechanism of the synergistic antitumor activity of
human γδ T cells in combination with Zol.
The results of the present study confirmed that Zol
is able to sensitize RMS cells to γδ T cell cytotoxicity. Adoptive
γδ T cell therapy combined with Zol may serve as a novel approach
for the treatment of RMS and therefore warrants further scientific
investigation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30973444 and 81172547).
Glossary
Abbreviations
Abbreviations:
|
Zol
|
zoledronic acid
|
|
mAb
|
monoclonal antibody
|
|
IPP
|
isopentenyl pyrophosphate
|
|
E:T ratio
|
effector/target ratio
|
|
N-BP
|
nitrogen-containing bisphosphonate
|
References
|
1
|
Malempati S and Hawkins DS:
Rhabdomyosarcoma: Review of the Children's Oncology Group (COG)
soft-tissue sarcoma committee experience and rationale for current
COG studies. Pediatr Blood Cancer. 59:5–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oberlin O, Rey A, Lyden E, Bisogno G,
Stevens MC, Meyer WH, Carli M and Anderson JR: Prognostic factors
in metastatic rhabdomyosarcomas: Results of a pooled analysis from
United States and European cooperative groups. J Clin Oncol.
26:2384–2389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schilbach K, Alkhaled M, Welker C, Eckert
F, Blank G, Ziegler H, Sterk M, Müller F, Sonntag K, Wieder T, et
al: Cancer-targeted IL-12 controls human rhabdomyosarcoma by
senescence induction and myogenic differentiation. Oncoimmunology.
4:e10147602015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuçi S, Rettinger E, Voss B, Weber G,
Stais M, Kreyenberg H, Willasch A, Kuçi Z, Koscielniak E, Klöss S,
et al: Efficient lysis of rhabdomyosarcoma cells by
cytokine-induced killer cells: Implications for adoptive
immunotherapy after allogeneic stem cell transplantation.
Haematologica. 95:1579–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mesiano G, Leuci V, Giraudo L, Gammaitoni
L, Schianca Carnevale F, Cangemi M, Rotolo R, Capellero S,
Pignochino Y, Grignani G, et al: Adoptive immunotherapy against
sarcomas. Expert Opin Biol Ther. 15:517–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrarini M, Ferrero E, Dagna L, Poggi A
and Zocchi MR: Human gammadelta T cells: A nonredundant system in
the immune-surveillance against cancer. Trends Immunol. 23:14–18.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiplunkar S, Dhar S, Wesch D and Kabelitz
D: gammadelta T cells in cancer immunotherapy: Current status and
future prospects. Immunotherapy. 1:663–678. 2009.PubMed/NCBI
|
|
8
|
Hayday AC: [gamma][delta] cells: A right
time and a right place for a conserved third way of protection.
Annu Rev Immunol. 18:975–1026. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gober HJ, Kistowska M, Angman L, Jenö P,
Mori L and De Libero G: Human T cell receptor gammadelta cells
recognize endogenous mevalonate metabolites in tumor cells. J Exp
Med. 197:163–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato Y, Tanaka Y, Miyagawa F, Yamashita S
and Minato N: Targeting of tumor cells for human gammadelta T cells
by nonpeptide antigens. J Immunol. 167:5092–5098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gogoi D and Chiplunkar SV: Targeting gamma
delta T cells for cancer immunotherapy: Bench to bedside. Indian J
Med Res. 138:755–761. 2013.PubMed/NCBI
|
|
12
|
Liu M, Sun LL, Li YJ, Li HY, Zhang J, Li
BH and Ye ZM: Trastuzumab enhanced the cytotoxicity of Vγ9Vδ2 T
cells against zoledronate-sensitized osteosarcoma cells. Int
Immunopharmacol. 28:160–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Li Y, Jiang Z, Zhang J, Li H, Li B
and Ye Z: Vγ9Vδ2 T cells and zoledronate mediate antitumor activity
in an orthotopic mouse model of human chondrosarcoma. Tumor Biol.
37:7333–7344. 2016. View Article : Google Scholar
|
|
14
|
Wrobel P, Shojaei H, Schittek B, Gieseler
F, Wollenberg B, Kalthoff H, Kabelitz D and Wesch D: Lysis of a
broad range of epithelial tumour cells by human gamma delta T
cells: Involvement of NKG2D ligands and T-cell receptor-versus
NKG2D-dependent recognition. Scand J Immunol. 66:320–328. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clézardin P, Benzaïd I and Croucher PI:
Bisphosphonates in preclinical bone oncology. Bone. 49:66–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stresing V, Daubiné F, Benzaid I,
Mönkkönen H and Clézardin P: Bisphosphonates in cancer therapy.
Cancer Lett. 257:16–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunzmann V, Bauer E and Wilhelm M:
Gamma/delta T-cell stimulation by pamidronate. N Engl J Med.
340:737–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rincon-Orozco B, Kunzmann V, Wrobel P,
Kabelitz D, Steinle A and Herrmann T: Activation of V gamma 9V
delta 2 T cells by NKG2D. J Immunol. 175:2144–2151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mattarollo SR, Kenna T, Nieda M and Nicol
AJ: Chemotherapy and zoledronate sensitize solid tumour cells to
Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother.
56:1285–1297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakamoto M, Nakajima J, Murakawa T, Fukami
T, Yoshida Y, Murayama T, Takamoto S, Matsushita H and Kakimi K:
Adoptive immunotherapy for advanced non-small cell lung cancer
using zoledronate-expanded γδTcells: A phase I clinical study. J
Immunother. 34:202–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Carlo E, Bocca P, Emionite L, Cilli M,
Cipollone G, Morandi F, Raffaghello L, Pistoia V and Prigione I:
Mechanisms of the antitumor activity of human Vγ9Vδ2 T cells in
combination with zoledronic acid in a preclinical model of
neuroblastoma. Mol Ther. 21:1034–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braza MS, Klein B, Fiol G and Rossi JF: γδ
T-cell killing of primary follicular lymphoma cells is dramatically
potentiated by GA101, a type II glycoengineered anti-CD20
monoclonal antibody. Haematologica. 96:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|