Introduction
A significant proportion (15–20%) of patients with
lung adenocarcinoma harbor epidermal growth factor receptor
(EGFR) activating mutations (1) and can benefit from first-line treatment
with tyrosine kinase inhibitors (TKI), including gefitinib
(2) or erlotinib (3) (first generation TKI) and afatinib
(second generation TKI) (4). However,
after 12–16 months of TKI treatment almost all patients develop
acquired resistance and experience tumour progression (5,6).
The most common resistance mechanism, detectable in
~50% of TKI resistant tumors, is the emergence of a secondary T790M
mutation in exon 20 of EGFR (5,7). Other
well-known resistance mechanisms, in patients with non-small cell
lung cancer treated with EGFR-TKI, include the amplification of
MET proto-oncogene tyrosine kinase receptor (MET)
(20%) (8), the development of small
cell lung cancer transformation (14%) (9,10) and the
presence of acquired phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit alpha (PI3KCA) mutations (5%) (6). Less common resistance mechanisms (30%)
include the activation of Insulin-Like Growth Factor-1 Receptor
(11), the epithelial to mesenchymal
transition (12), and more rarely
squamous cell transformation (13–16).
Different resistance mechanisms may be observed in the same patient
(6) due to intratumor and
intrametastatic heterogeneity (17),
which strongly influence the patient's response to treatment.
The identification of molecular alterations
responsible for acquired TKI resistance is crucial for patient
management, as multiple novel treatment strategies are available to
overcome this issue (18). For
instance, in cases of T790M-mediated resistance, the use of a
third-generation TKI, which irreversibly and selectively blocks
T790M mutant clones, has been demonstrated to increase the potency
of EGFR-TK inhibition (19,20).
A second tumour biopsy is recommended in case of TKI
resistance; however, a single biopsy specimen may not mirror all
the biological properties of a tumour. Combining re-biopsy analysis
with molecular characterization of circulating cell-free tumour
(ct) DNA represents a good strategy to describe the molecular
landscape of a tumour (21–23). In addition, temporal changes to
EGFR activating and resistance mutations in plasma DNA are
directly linked to treatment efficacy (24,25).
In the present report the case of a patient who
developed two resistance mechanisms in response to first-line
afatinib, the T790M mutation and the rare squamous cell
transformation, is described. To the best of our knowledge, only a
few similar cases have previously been described and they focused
on patients with lung adenocarcinoma who were treated with
erlotinib and gefitinib (13–15).
Case report
Written informed consent for the publication of this
report was obtained from the patient. In October 2014, a
44-year-old female with an 8 pack/year smoking history presented at
the University Hospital of Pisa (Pisa, Italy) with back pain. A few
weeks later the patient underwent magnetic resonance imaging of the
vertebral column, which revealed a number of osteoblastic bone
lesions (S1-3; D2-3 and D7-10 laminas). A computed tomography (CT)
scan revealed a left lower lobe mass, and pleural and pericardial
effusions (PE). The patient underwent endobronchial ultrasound
biopsy and pleural fluid analyses. Histological and cytological
samples examination identified an adenocarcinoma, further
characterized using cell-block (stained with 10% buffered formalin
at room temperature for 24 h) paraffin-embedded sections
(thickness, 2 µm) by immunohistochemical staining using the
ultraView Universal DAB Detection kit (Ventana Medical Systems.
Inc., Tucson, AZ, USA), according to the manufacturer's protocol,
with anti-thyroid transcription factor (TTF-1) antibody (mouse
monoclonal primary antibody; clone 8G7G3/1; ready-to-use; catalog
no. 790-438; Ventana Medical Systems, Inc.) for 44 min at 37°C,
which demonstrated a strong positive nuclear stain. An Olympus BX51
light microscope (Olympus Italia Srl; Segrate, Italy) was used for
the analysis. The final diagnosis was adenocarcinoma (26), consistent with a lung primary cancer
with bone metastases.
An extensive molecular analysis was performed on the
PE. Fluorescent In Situ Hybridization (FISH) was performed
to evaluate translocations of anaplastic lymphoma kinase
(ALK) (using a Vysis ALK Dual-Color Break Apart FISH probe
kit; Abbott Laboratories, Abbott Park, IL, USA), proto-oncogene
tyrosine-protein kinase ROS (ROS1; using a ROS1 6q22
Break Probe; Kreatech; Leica Microsystems, Ltd., Milton Keynes, UK)
and RET proto-oncogene (RET; using a RET 10q11
Break Probe; Kreatech; Leica Microsystems, Ltd.), and to assess the
presence of MET amplification (Vysis MET Spectrum Red and
CEP7 D7Z1 Spectrum Green; Abbott Laboratories). FISH analysis was
performed according to the manufacturers'protocols. All FISH tests
were negative: ALK, 4% of neoplastic rearranged cells
(cut-off 15%); ROS1, 0% of neoplastic rearranged cells
(cut-off 15%); RET, 5% of neoplastic rearranged cells
(cut-off 15%); and MET, MET/CEP7=1,1 (cut-off ≥2).
Mutational analysis of KRAS, BRAF, NRAS, PIK3CA,
ALK, ERBB2, DDR2, MAP2K1, EGFR, RET was performed using a
Sequenom Mass-Array (matrix assisted laser desorption
ionization-time of flight mass spectrometry) using the Myriapod
Lung Status kit (Diatech Pharmacogenetics SRL, Jesi, Italy)
together with the analysis software MASSARRAY® TYPER 4.0
(Diatech Pharmacogenetics, Jesi SRL) according to the
manufacturer's protocol (limit of detection: 2.5–5% for EGFR and
2.5–10% for all other genes). The PE demonstrated a deletion in
EGFR exon 19 (ex19del).
In November 2014, the patient started treatment with
afatinib (40 mg/day, orally). The bone lesions required a radio
therapeutic approach due to nerve peduncle compression, therefore
the patient received radiation (30 Gy in five fractions on S1-3; 25
Gy in five fractions on D7-10 and 30 Gy in five fractions on D2-3).
In addition, the patient was treated with denosumab (120 mg every
28 days, intravenously). After 4 weeks of afatinib, a CT scan
revealed a partial response in the lung mass, and a total response
for the effusions and bone lesions.
The patient tolerated the therapy well, with mild
diarrhea and post-actinic pneumonia, which was treated with
antibiotics and anti-inflammatory therapy. Foci of post-actinic
pneumonia were observed, primarily on paravetebral and medium lobe
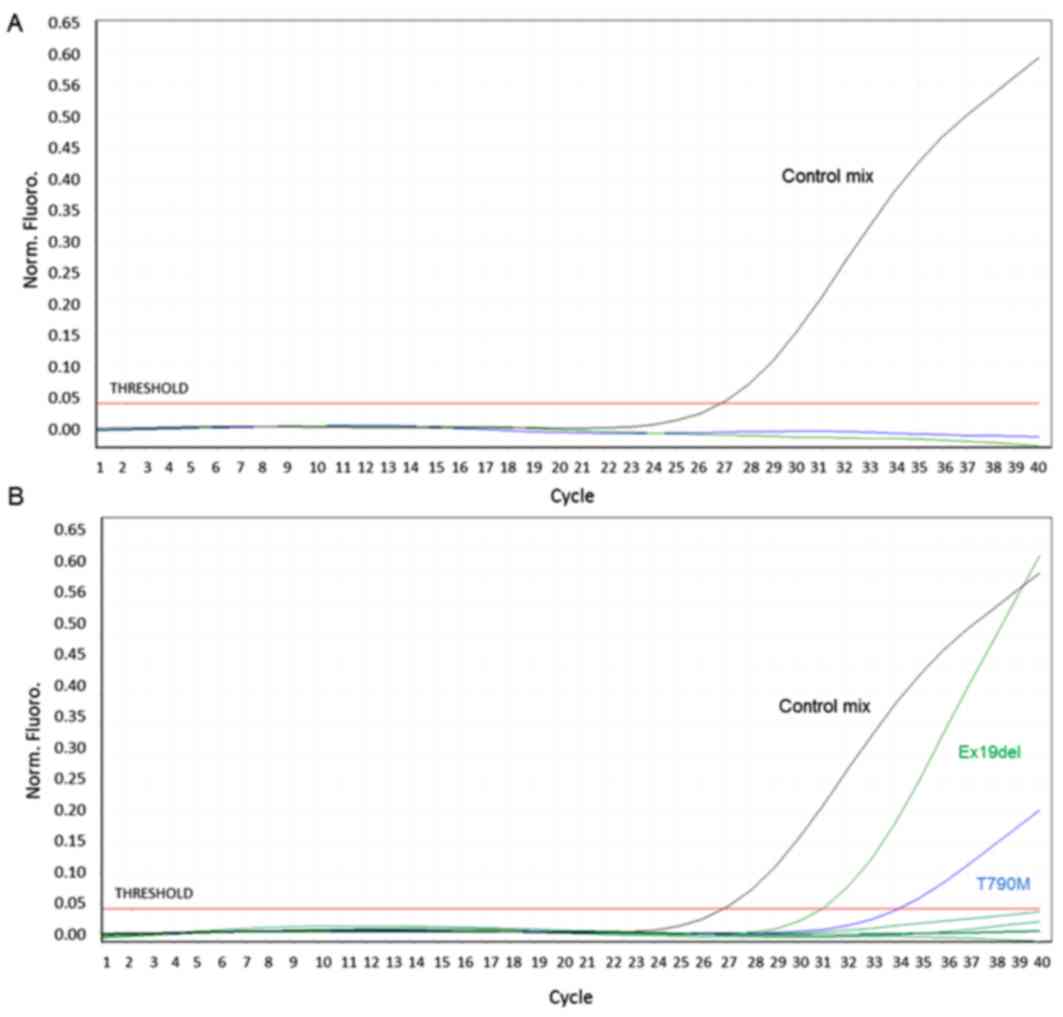
sites. At subsequent medical examinations, after 5, 7 and 9 months
of treatment, the patient was stable and no EGFR mutations
were detected on ctDNA from plasma collected at each visit
(Fig. 1A). ctDNA was purified from 4
ml of plasma using a QIAmp Circulating Nucleic Acid kit (Qiagen,
Inc., Valencia, CA, USA) and EGFR mutational analysis was
performed using an Easy®EGFR Quantitative Real Time PCR
kit (Diatech Pharmacogenetics SRL) according to the manufacturer's
protocol. The Easy®EGFR quantitative Real Time PCR kit
is validated for use on liquid biopsy and its limit of detection
ranges from 0.5 to 2%.
In February 2016, a CT scan control detected an
almost complete response on the primitive lung mass and bone
lesions. The patient carried on the therapy with afatinib and
denosumab. Three months later (May 2016), a CT scan demonstrated a
large area of atelectasia of the upper left lung lobe partially
involving the lower lobe with PE. The cytological examination of PE
confirmed an adenocarcinoma with a positive immunohistochemical
stain for TTF-1. Cytological samples from pleural effusion were
positive for EGFR ex19del and T790M; both mutations were
concomitantly detected in ctDNA (Fig.
1B). Broncospic investigation of the upper left bronchus
revealed a partial obstruction and infiltration from a whitish
neoformation. This lesion was biopsied. The obtained tissue was
fixed in 10% buffered formalin (room temperature, 24 h),
paraffin-embedded and cut into 5 µm thick sections. The
hematoxylin-eosin stain (room temperature, 1 h 26 min) revealed a
keratinizing squamous cell carcinoma confirmed by
immunohistochemical examination, performed as aforementioned, using
a p40 antibody (mouse monoclonal primary antibody; clone BC28;
ready-to-use; catalog no. 790-4950; Ventana Medical System, Tucson,
AZ, USA) for 40 min at 42°C, the results of which were strongly
positive, and for TTF-1 antibody, the result of which was negative.
The two lesions harboured the ex19del mutation. The same FISH and
mutational tests as those executed on pre-TKI specimen were
performed on the post-TKI adenocarcinoma and squamous cell
carcinoma samples. Again, all FISH tests gave negative results:
ALK, 0% of neoplastic rearranged cells; ROS1, 0% of
neoplastic rearranged cells; RET, 0% of neoplastic
rearranged cells; and MET, MET/CEP7=1 in the adenocarcinoma.
In the squamous cell carcinoma: ALK, 2% of neoplastic
rearranged cells; ROS1, 0% of neoplastic rearranged cells;
RET, 0% of neoplastic rearranged cells; and MET,
MET/CEP7=1. In addition, HER2 amplification (Vysis HER-2/neu
SpectrumOrange/CEP17 SpectrumGreen Probes; Abbott Laboratories) was
evaluated in post-TKI samples, for which the results were negative:
HER2/CEP17=0.9 (cut-off ≥2) in the adenocarcinoma, HER2/CEP17=1
(cut-off ≥2) in the squamous cell carcinoma.
The patient underwent osimertinib (80 mg/day,
orally) therapy and radiotherapy for squamous lesions. At a 2-month
follow-up T790M positive lesions exhibited a partial regression.
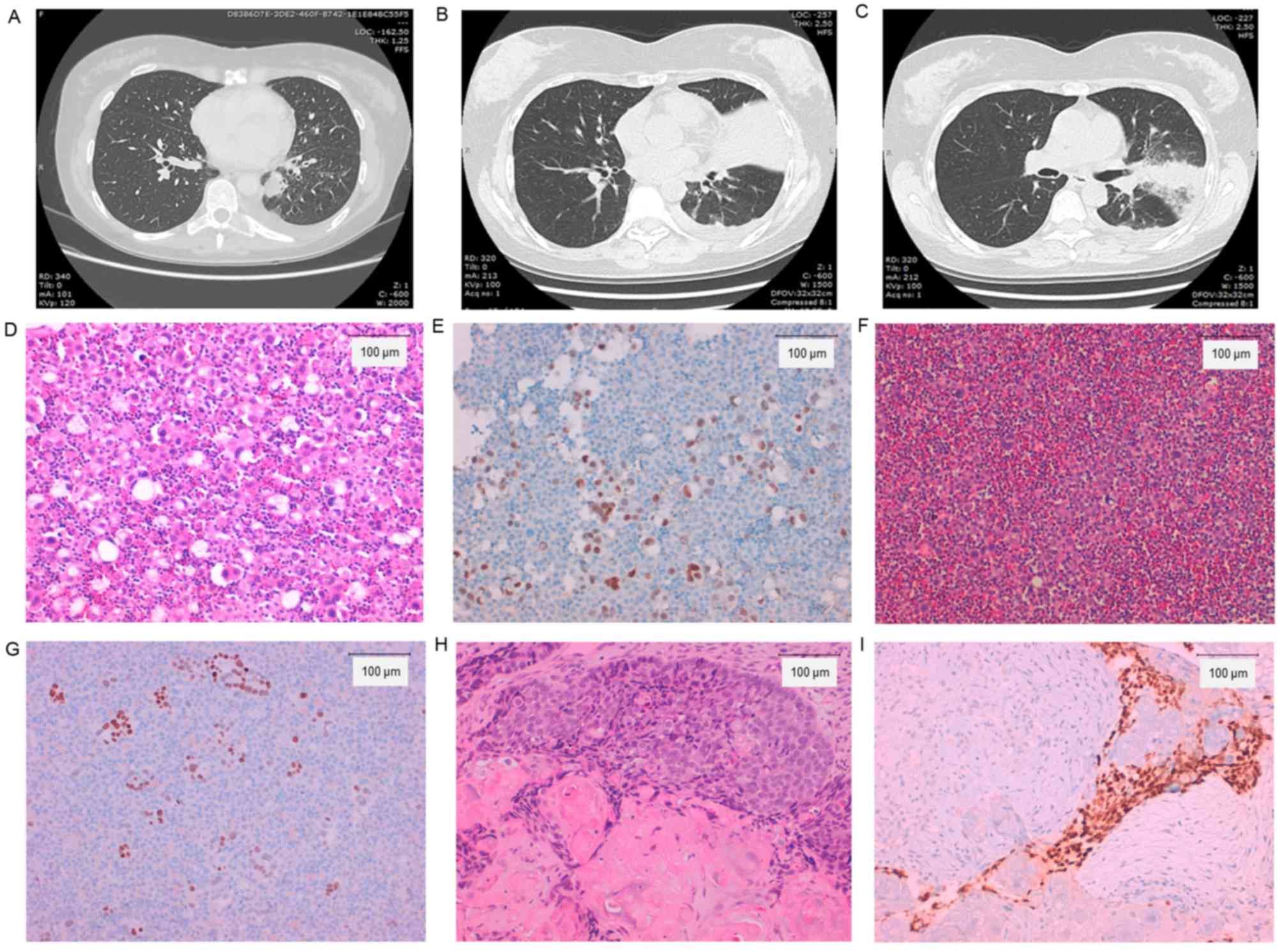
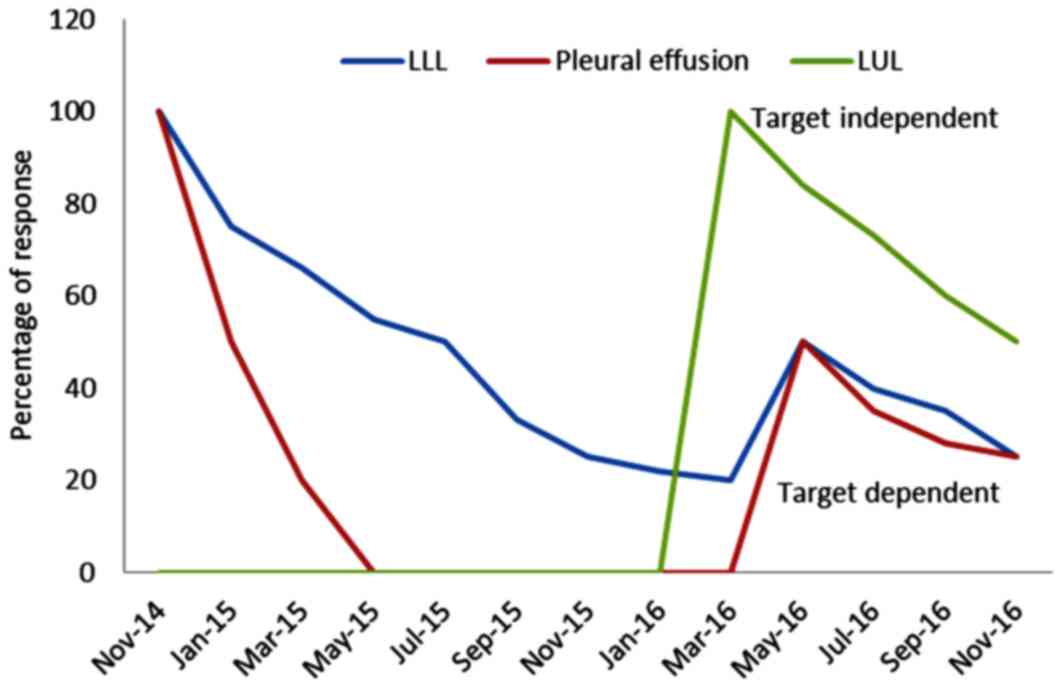
Fig. 2 presents the patient's
CT-images and histological examinations, and Fig. 3 presents the patient's clinical
response.
Discussion
Patients with EGFR mutant lung adenocarcinoma
treated with TKI typically develop resistance within 1 year of
treatment (5). The existence of
different acquired TKI resistance mechanisms together with tumour
heterogeneity constitutes a major challenge for clinical practice
(6).
The present report describes the case of a patient
with lung adenocarcinoma treated with afatinib who developed the
T790M mutation and squamous cell transformation. To date, only a
few cases of squamous cell transformation, with (13,15) and
without (14) concomitant T790M, have
been reported in response to erlotinib and gefitinib, and no
similar cases have been reported in response to afatinib (27). Longo et al (16) recently reported a case of lung cell
adenocarcinoma positive for the EGFR exon 21 L858R mutation,
who, following TKI treatment, developed squamous cell carcinoma
change together with an EGFR exon 20 S768I secondary
mutation.
In our case, histological transformation may have
been a consequence of TKI treatment or it could have been enhanced
by radiotherapy, as reported in other types of cancer, including
prostate cancer (28).
All the histological evaluations have been performed
on specimens obtained by needle biopsy, and although there were
different morphological and immunohistochemical characteristics in
pre and post-TKI lesions, the presence of the squamous cell
carcinoma prior to EGFR-TKI therapy in form of an adenosquamous
carcinoma cannot be excluded. However, lung adenocarcinoma exhibits
a different molecular landscape compared with squamous cell
carcinoma, for instance EGFR mutations are present in 10–40%
of cases of adenocarcinoma, but rarely in squamous cell carcinoma
(29). The presence of ex19del in
both lesions in the present study suggests that the adenocarcinoma
and squamous carcinoma components share the same clonal origin and
a mixed tumour is unlikely on the basis of the different location
of the two lesions. Furthermore, all the tumour lesions were
extensively characterized from a molecular point of view and the
only difference was the presence of the T790M mutation, which was
detected only in the post-TKI adenocarcinoma specimen.
The reported case highlights the role of
intra-tumour heterogeneity, defined as the presence within the same
tumour of distinct cellular populations with specific phenotypic
features, in response to TKI treatment, which selects clones with
intrinsic or acquired resistance that drive disease progression
(30–32). However, a complete characterization of
the mechanisms of response and resistance is essential to provide
patients with the greatest clinical benefit and several studies and
case reports confirm this issue (5,6,9,10,13–16,18).
In the present context, a single tumour biopsy,
limited by the presence of geographic heterogeneity, may be
inadequate to detect all cancer gene mutations, explaining the lack
of a direct correlation between molecular alteration and clinical
efficacy of treatment (23). The
liquid biopsy and analysis of ctDNA furthers understanding of
intra-tumour heterogeneity, since it detects contributions from
multiple tumour sites. In the present case report, as soon as the
patient experienced clinical progression, activating and resistance
mutations became detectable on ctDNA, supporting its value in
agreement with previously published data (24,25).
However, according to current knowledge and reported
cases, neither liquid biopsy nor solid biopsy on their own can
suffice for the monitoring of cancer therapy. In spite of the
non-invasiveness of liquid biopsy and its high informative value,
resistance mechanisms, including phenotypic changes, cannot be
evaluated without a histopathological analysis of tumour tissues;
for this reason tissue biopsy should be performed whenever
possible.
In conclusion, the present case report underlines
the complementarity of tumour re-biopsies and analysis of ctDNA in
order to have a more complete view of temporal evolution and
molecular diversity of TKI-resistant disease, thus improving
therapeutic regimens.
References
|
1
|
Ileana EE, Wistuba II and Izzo JG: From
uniplex to multiplex molecular profiling in advanced non-small cell
lung carcinoma. Cancer J. 21:413–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumour specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris M and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furugen M, Uechi K, Hirai J, Aoyama H,
Saio M, Yoshimi N, Kinjo T, Miyagi K, Haranaga S, Higa F, et al: An
autopsy case of two distinct, acquired drug resistance mechanisms
in epidermal growth factor receptor-mutant lung adenocarcinoma:
Small cell carcinoma transformation and epidermal growth factor
receptor T790M mutation. Intern Med. 54:2491–2496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alì G, Bruno R, Giordano M, Prediletto I,
Marconi L, Zupo S, Fedeli F, Ribechini A, Chella A and Fontanini G:
Small-cell lung cancer transformation and the T790M mutation: A
case report of two acquired mechanisms of TKI resistance detected
in a tumor rebiopsy and plasma sample of EGFR-mutant lung
adenocarcinoma. Oncol Lett. 12:4009–4012. 2016.PubMed/NCBI
|
|
11
|
Peled N, Wynes MW, Ikeda N, Ohira T,
Yoshida K, Qian J, Ilouze M, Brenner R, Kato Y, Mascaux C and
Hirsch FR: Insulin-like growth factor-1 receptor (IGF-1R) as a
biomarker for resistance to the tyrosine kinase inhibitor gefitinib
in non-small cell lung cancer. Cell Oncol (Dordr). 36:277–288.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomson S, Buck E, Petti F, Griffin G,
Brown E, Ramnarine N, Iwata KK, Gibson N and Haley JD: Epithelial
to mesenchymal transition is a determinant of sensitivity of
non-small-cell lung carcinoma cell lines and xenografts to
epidermal growth factor receptor inhibition. Cancer Res.
65:9455–9462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scher KS, Saldivar JS, Fishbein M,
Marchevsky A and Reckamp KL: EGFR-mutated lung cancer with
T790M-acquired resistance in the brain and histologic
transformation in the lung. J Natl Compr Canc Netw. 11:1040–1044.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levin PA, Mayer M, Hoskin S, Sailors J,
Oliver DH and Gerber DE: Histologic transformation from
adenocarcinoma to squamous cell carcinoma as a mechanism of
resistance to EGFR inhibition. J Thorac Oncol. 10:e86–e88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jukna A, Montanari G, Mengoli MC, Cavazza
A, Covi M, Barbieri F, Bertolini F and Rossi G: Squamous cell
carcinoma ‘Transformation’ concurrent with secondary T790M mutation
in resistant EGFR-mutated adenocarcinomas. J Thorac Oncol.
11:e49–e51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Longo L, Mengoli MC, Bertolini F, Bettelli
S, Manfredini S and Rossi G: Synchronous occurrence of squamous
cell carcinoma ‘transformation’ and EGFR exon 20 S768I mutation as
a novel mechanism of resistance in EGFR-mutated lung
adenocarcinoma. Lung Cancer. 103:24–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jamal-Hanjani M, Quezada SA, Larkin J and
Swanton C: Translational implications of tumor heterogeneity. Clin
Cancer Res. 21:1258–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piotrowska Z and Sequist LV: Epidermal
growth factor receptor-mutant lung cancer: New drugs, New
resistance mechanisms, and future treatment options. Cancer J.
21:371–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Song Z, Jin Y, Tang Z, Kang J and Ma
X: Novel selective and potent EGFR inhibitor that overcomes
T790M-Mediated resistance in non-small cell lung cancer. Molecules.
21:pii: E1462. 2016. View Article : Google Scholar
|
|
20
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pantel K, Diaz LA Jr and Polyak K:
Tracking tumor resistance using ‘liquid biopsies’. Nat Med.
19:676–677. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imamura F, Uchida J, Kukita Y, Kumagai T,
Nishino K, Inoue T, Kimura M, Oba S and Kato K: Monitoring of
treatment responses and clonal evolution of tumor cells by
circulating tumor DNA of heterogeneous mutant EGFR genes in lung
cancer. Lung Cancer. 94:68–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chabon JJ, Simmons AD, Lovejoy AF,
Esfahani MS, Newman AM, Haringsma HJ, Kurtz DM, Stehr H, Scherer F,
Karlovich CA, et al: Circulating tumor DNA profiling reveals
heterogeneity of EGFR inhibitor resistance mechanisms in lung
cancer patients. Nat Commun. 7:118152016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chia PL, Do H, Morey A, Mitchell P,
Dobrovic A and John T: Temporal changes of EGFR mutations and T790M
levels in tumor and plasma DNA following AZD9291 treatment. Lung
Cancer. 98:29–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchida J, Imamura F, Kukita Y, Oba S,
Kumagai T, Nishino K, Inoue T, Kimura M and Kato K: Dynamics of
circulating tumor DNA represented by the activating and resistant
mutations in epidermal growth factor receptor tyrosine kinase
inhibitor treatment. Cancer Sci. 107:353–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to the 2015 World Health
Organization Classification of tumors of the lung, Pleura, Thymus
and heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ,
Yang PC, Yang JC, Wen YF and Shih JY: The mechanism of acquired
resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib
in lung adenocarcinoma patients. Oncotarget. 7:12404–12413. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller VA, Reuter V and Scher HI: Primary
squamous cell carcinoma of the prostate after radiation seed
implantation for adenocarcinoma. Urology. 46:111–113. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devarakonda S, Morgensztern D and Govindan
R: Genomic alterations in lung adenocarcinoma. Lancet Oncol.
16:e342–e351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu JY, Yu SF, Wang SH, Bai H, Zhao J, An
TT, Duan JC and Wang J: Clinical outcomes of EGFR-TKI treatment and
genetic heterogeneity in lung adenocarcinoma patients with EGFR
mutations on exons 19 and 21. Chin J Cancer. 35:302016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hata A, Yoshioka H, Fujita S, Kunimasa K,
Kaji R, Imai Y, Tomii K, Iwasaku M, Nishiyama A, Ishida T and
Katakami N: Complex mutations in the epidermal growth factor
receptor gene in non-small cell lung cancer. J Thorac Oncol.
5:1524–1528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morgillo F, Corte CM Della, Fasano M and
Ciardiello F: Mechanisms of resistance to EGFR-targeted drugs: Lung
cancer. ESMO Open. 1:e0000602016. View Article : Google Scholar : PubMed/NCBI
|