Introduction
Breast cancer is the most common type of malignancy
in women and its incidence rates are increasing (1). It is estimated that ~1100,000 new cases
of female breast cancer are diagnosed worldwide each year, and 37%
of patients (410,000 cases) succumb to the disease each year
(2–4).
Targeted therapy, including RNA interference (RNAi) technology, has
gained interest in recent years as a potential treatment due to its
low toxicity, specificity and efficiency (5). The use of small interfering (si)RNA has
several advantages, including simple sequence design and fewer
adverse effects on cells or tissues. Therefore siRNA could be a
more promising candidate for the diagnosis and treatment of
diseases compared with shRNA (6). A
number of cancer-associated genes, including B-cell lymphoma 2,
tumor protein p53, hypoxia-inducible factor and vascular
endothelial growth factor have previously been identified as
potential targets for RNAi (7–9).
Semaphorin 3C (SEMA3C) is a member of the semaphorin family that
serves important roles in a number of physiological processes,
including axonal growth, immune response, cell adhesion, migration
and bone remodeling (10). Numerous
studies have demonstrated that semaphorins are overexpressed in a
variety of malignant tumors, including glioma, gastric cancer and
lung cancer (11). In addition,
upregulation of semaphorins is associated with cancer metastasis
and angiogenesis, and affects the prognosis and life quality of
patients (12,13). In the present study, siRNA was used to
silence SEMA3C, which resulted in significantly suppressed cell
proliferation and migration in MCF-7 cells. These results suggest
that SEMA3C may be a potential target for breast cancer
therapy.
Materials and methods
Cells and reagents
The human breast cancer cell line MCF-7 was obtained
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Fetal bovine serum (FBS) and
Dulbecco's modified Eagle's medium (DMEM) were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNAiso Plus,
PrimeScript RT Reagent kit, and SYBR Premix Ex Taq II were from
Takara Biotechnology, Co., Ltd. (Dalian, China). A SEMA3C rabbit
polyclonal antibody (catalog number: ARP38906) was purchased from
BD Biosciences (San Jose, CA, USA). GAPDH and α-tubulin mouse
monoclonal antibodies (catalog numbers: ABIN268426 and AB9354) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The horseradish peroxidase (HRP)-conjugated secondary antibodies,
RIPA buffer, SDS-PAGE Gel Preparation kit, BCA Protein Assay kit,
crystal violet, and Cell Counting Kit-8 were obtained from Beyotime
Institute of Biotechnology (Haimen, China). Polyvinylidene
difluoride (PVDF) membranes and Transwell plates were purchased
from EMD Millipore (Billerica, MA, USA). Lipofectamine®
2000 was obtained from Invitrogen (Thermo Fisher Scientific,
Inc.).
siRNA sequences
Three siRNA sequences targeting the SEMA3C gene were
designed using the SEMA3C full-length complementary (c)DNA sequence
(XM_009456869.1) as a template. The SEMA3C siRNA (siRNA-1, siRNA-2
and siRNA-3), fluorescein amidite (FAM)-labeled negative control
siRNA (siRNA-FAM), GAPDH siRNA (siRNA-GAPDH), and negative control
siRNA (siRNA-NC) were synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China) and the sequences are listed in Table I.
 | Table I.Oligonucleotide sequences of the
siRNAs used in the study. |
Table I.
Oligonucleotide sequences of the
siRNAs used in the study.
| Name | Sequence (5′-3′) |
|---|
| siRNA-1 | Sense:
5′-GCCCAGCUUAAUCAAGAAATT-3′ |
|
| Antisense:
5′-UUUGUUGAUUAACCUGGGCTT-3′ |
| siRNA-2 | Sense:
5′-GCGCUACUAAUUGGGAAGATT-3′ |
|
| Antisense:
5′-UCUUCGCAAUUAGUUAGGGCTT-3′ |
| siRNA-3 | Sense:
5′-GGGCUGAGGACCUUGCAGAAGATT-3′ |
|
| Antisense:
5′-UCUUCCGCAAGGUCCUCAGGCCTT-3′ |
| siRNA-FAM | Sense:
5′-UUCUGCGAACGUGUCACGUTT-3′ |
|
| Antisense:
5′-ACGUCACACGUUCGGAGAATT-3′ |
| siRNA-NC | Sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Antisense:
5′-ACGUGACACGUUCGGAGAATT-3′ |
| siRNA-GADPH | Sense:
5′-GUAUCACAACAGCCUCAAGTT-3′ |
|
| Antisense:
5′-CUUGAGGCUGUUGUCAUACTT-3′ |
Cell culture and siRNA
transfection
Human MCF-7 breast cancer cells were cultured in
DMEM containing 10% FBS, 100 µg/ml streptomycin, and 100 U/ml
penicillin, in a humidified 37°C incubator with 5% CO2.
MCF-7 cells (5×104) in the logarithmic growth phase were
seeded into 24-well plates 24 h prior to transfection. Cells were
transfected with siRNA (siRNA-1, siRNA-2, siRNA-3, siRNA-FAM or
siRNA-NC) using Lipofectamine® 2000 reagent according to
the manufacturer's protocol. RNA and protein were isolated at 48
and 72 h following transfection, respectively.
Determination of the optimal siRNA
transfection concentration
MCF-7 cells (5×104) in the logarithmic
growth phase were seeded in 24-well plates. Following 24 h
incubation at 37°C, cells were transfected with increasing
concentrations of siRNA-FAM (0, 10, 25, 50, 75, 100, 150, 200
nmol/l). The fluorescent signal in the cells was detected under
fluorescence microscopy 24 h post-transfection. For the positive
control, cells were transfected with 0, 25, 50, 100, 200 nmol/l
siRNA-GAPDH. At 72 h following transfection, cells were harvested
and protein was collected. The protein level of GAPDH was detected
by western blotting with α-tubulin as an internal control. The band
density was quantified using ImageJ software (version 1.41;
National Institutes of Health, Bethesda, MD, USA). Based on the
fluorescence signal and GAPDH protein level, the optimal siRNA
transfection concentration was defined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The knockdown efficiencies of the SEMA3C-targeting
siRNAs were evaluated by RT-qPCR. Total RNA was isolated from MCF-7
cells using RNAiso Plus reagent 48 h after transfection, and
reverse transcribed to cDNA using the PrimeScript RT Reagent kit.
The total RNA of the cultured cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. A total of 1 µg total RNA was
reverse-transcribed to cDNA using AMV reverse transcriptase (Takara
Biotechnology, Co., Ltd.) and a stem-loop RT primer (Applied
Biosystems; Thermo Fisher Scientific, Inc.), which was performed
with the following conditions: 16°C for 15 min, 42°C for 60 min and
85°C for 5 min. Each cDNA sample was analyzed in triplicate using
SYBR Premix Ex Taq II according to the manufacturer's protocol.
Real-time PCR was performed using a TaqMan PCR kit on an Applied
Biosystems 7500 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The reactions were incubated at
95°C for 5 min followed by 35 cycles of 94°C for 15 sec and 72°C
for 1 min. GAPDH was used as an internal control. The primer
sequences were as follows: GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′, and reverse, 5′-GAAGATGGTGATGGGATTTC-3′;
SEMA3C forward, 5′-GCGAAGCAGCATGAGGTGTATTGGA-3′, and reverse,
5′-CGATGTAGTTGTGGCACTCTGTCTG-3′. Relative mRNA quantification was
assessed using the 2−∆∆Cq method (14).
Western blot analysis
The protein level of SEMA3C was detected by western
blot analysis. MCF-7 cells were harvested and lysed in RIPA buffer
at 72 h post-transfection. The protein concentration was determined
using the BCA Protein Assay kit. Protein (50 µg) was separated
using 8% SDS-PAGE and transferred to PVDF membranes. Following
blocking with 5% bovine serum albumin (Takara Biotechnology, Co.,
Ltd.) for 1 h at room temperature, the membranes were incubated
with primary antibodies (dilutions: SEMA3C, 1:800; GAPDH, 1:1,000)
overnight at 4°C. Following three washes with TBS-Tween-20 (TBST),
the membranes were incubated with HRP-conjugated secondary
antibodies for 2 h at room temperature. Following a second round of
washing with TBST, protein bands were detected with an enhanced
chemiluminescence assay kit (GE Healthcare, Chicago, IL, ISA).
ImageJ software was used for densitometric analysis with GAPDH as
an internal control.
CCK-8 assay
The effect of SEMA3C knockdown on MCF-7 cell
proliferation was accessed using a CCK-8 assay. At 24 h prior to
siRNA transfection, MCF-7 cells in the logarithmic growth phase
were seeded at 2,000 cells/well in 96-well plates containing 100 µl
medium. At the indicated time points (24, 48 and 72 h following
transfection), 10 µl CCK-8 was added into each well and the plates
were incubated at 37°C for another 3 h. The absorbance (optical
density; OD) was measured at a wavelength of 450 nm. The experiment
was performed in triplicate. The relative growth rate was
calculated as follows: Relative growth rate (%)=(OD of treated
cells/OD of control cells)x100.
Migration assay
The effect of SEMA3C knockdown on MCF-7 cell
migration was determined using a Transwell migration assay. MCF-7
cells were plated at 2.5×105/well in 6-well plates.
Following transfection with siRNA for 72 h, cells
(5×104) in 800 µl serum-free medium were seeded in the
upper chamber of 12-well plates and 1 ml DMEM containing 10% FBS
was placed in the lower chamber. Following incubation for 24 h, the
upper chamber was washed twice with PBS and fixed with 4%
paraformaldehyde for 15 min. Cells on the top of the membrane were
wiped off with cotton swabs. Cells that migrated to the bottom were
stained with 0.1% crystal violet for 15 min. Four randomly selected
fields of the membrane were counted under an inverted microscope
(magnification, ×100).
Statistical analysis
All experiments were performed in triplicate.
Statistical analyses were carried out using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). All data are presented as the
mean ± standard deviation. Experiments were performed in
triplicate. Differences between groups were determined by a one-way
analysis of variance. ANOVA was used for comparison between >2
groups and the t-test was used for comparison between 2 groups.
Differences between two groups were accessed by least significant
difference t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Determination of the optimal effective
siRNA concentration
The siRNA transfection efficiency can vary for
different cell types. To achieve the optimal effective siRNA
concentration, the negative fluorescence control siRNA (siRNA-FAM)
and the positive control siRNA (siRNA-GAPDH) were transfected into
MCF-7 cells. Following transfection with siRNA-FAM for 24 h, a
fluorescent signal was observed, which increased in a
dose-dependent manner. Following transfection with 25 nmol/l siRNA,
30% of cells exhibited fluorescence, and ~90% of cells exhibited
fluorescence following transfection with 50 nmol/l siRNA. The
knockdown efficiencies of siRNA-GAPDH were 35.71±5.42% for 25
nmol/l siRNA and 51.6±7.59% for 100 nmol/l siRNA (data not shown).
However, more apoptotic cells were detected with the increased
amount of siRNA. Based on these results, 100 nmol/l siRNA was
selected as the treatment dose for further experiments.
siRNA mediates SEMA3C silencing in
breast cancer cells
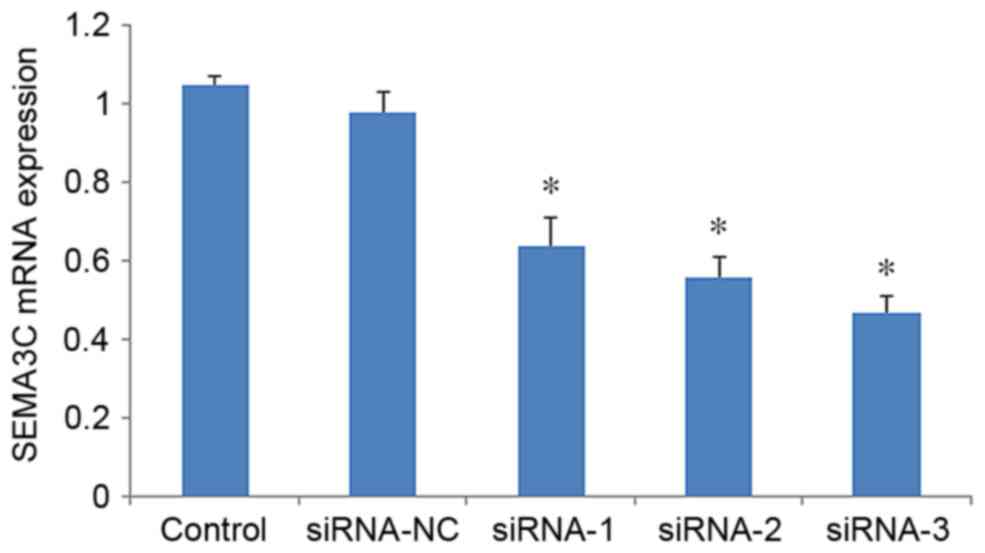
To assess the knockdown efficiency of SEMA3C, SEMA3C
mRNA levels were determined by RT-qPCR 48 h after transfection. The
mRNA level of SEMA3C was significantly decreased in MCF-7 cells
following transfection with SEMA3C siRNAs compared with
non-transfected cells (P<0.05; Fig.
1). The mRNA levels of SEMA3C were 71.13±3.15% (siRNA-1),
58.26±2.04% (siRNA-2) and 37.11±2.53% (siRNA-3) of the level in the
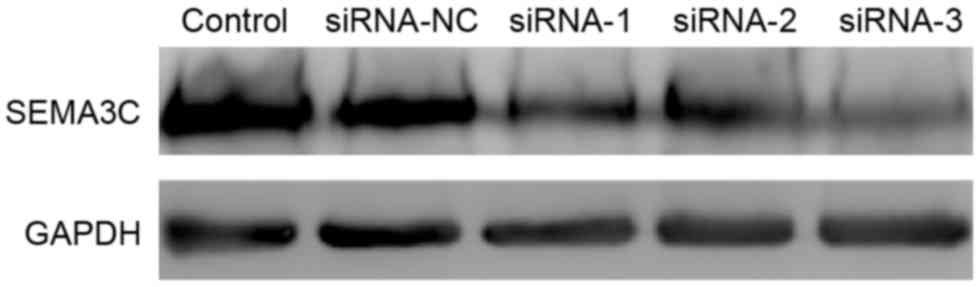
control group. In agreement this result, western blot analysis
demonstrated that the protein levels of SEMA3C were significantly
decreased following siRNA knockdown (Fig.
2). The knockdown efficiencies were 47.37±6.02, 50.87±4.61 and
65.27±3.15% for siRNA-1, siRNA-2, and siRNA-3, respectively,
relative to the control (data not shown). Together, these results
suggest that all three siRNAs effectively suppress SEMA3C
expression. siRNA-3, which exhibited the highest knockdown
efficiency, was selected for further experiments.
SEMA3C knockdown inhibits MCF-7 cell
proliferation
To understand the function of SEMA3C in breast
cancer, the effect of SEMA3C knockdown on breast cancer cell
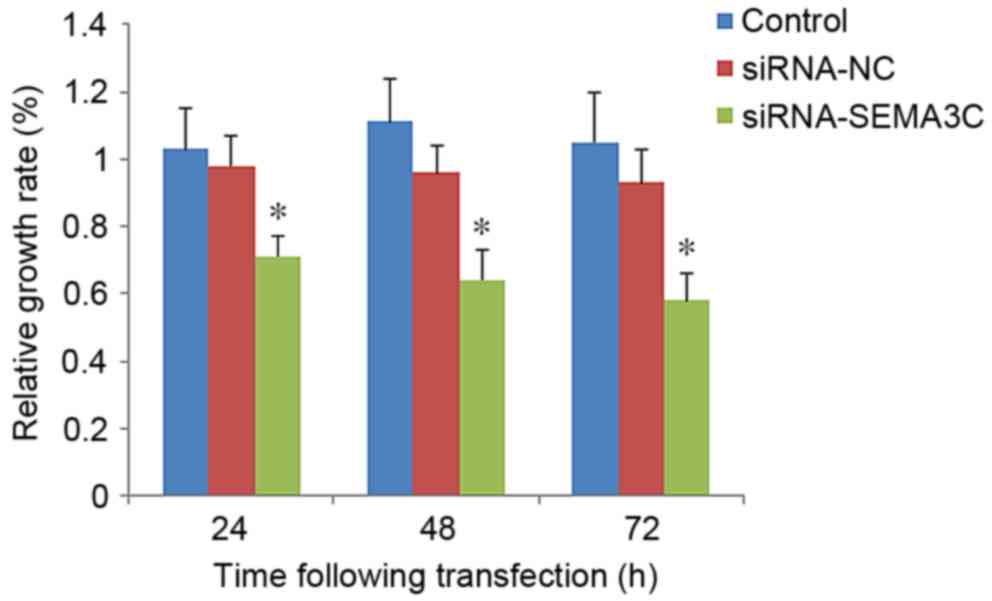
proliferation was examined. A CCK-8 assay demonstrated that the
relative growth rate [(OD of treated cells/OD of control
cells)x100] was significantly decreased in SEMA3C siRNA-transfected
cells compared with that of control cells (control or siRNA-NC;
P<0.05; Fig. 3). The numbers of
cells in the SEMA3C-siRNA group were 81.06±9.43, 63.47±7.81 and
55.12±5.03% relative to the siRNA-NC group at 24, 48 and 72 h
post-transfection (data not shown). No significant differences were
observed between the growth rates of the control cells and cells
transfected with siRNA-NC (P>0.05; Fig. 3). These results suggest that SEMA3C
knockdown inhibits MCF-7 cell proliferation.
SEMA3C knockdown suppresses MCF-7 cell
migration
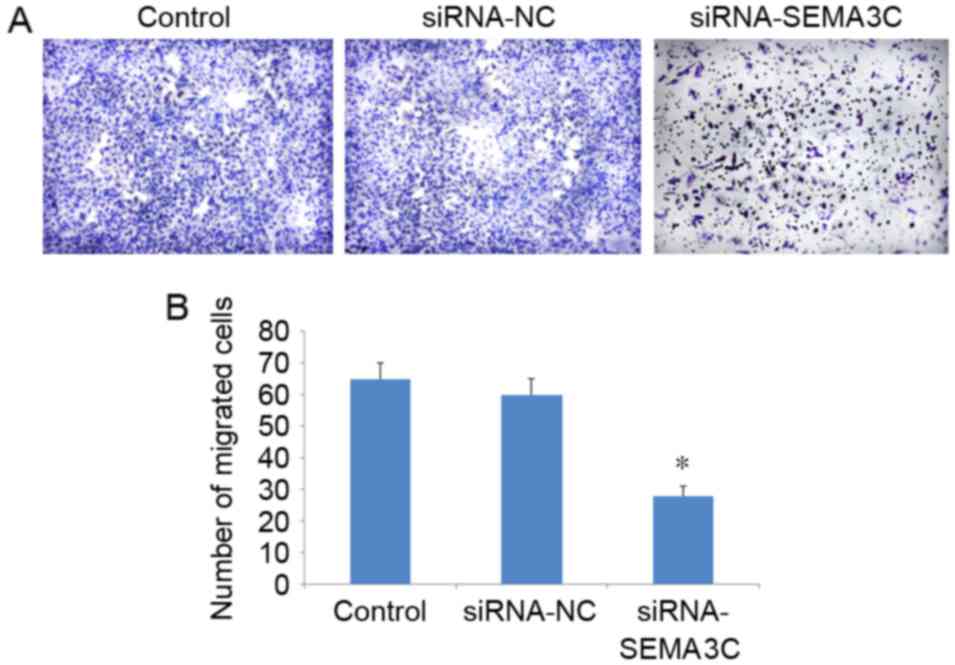
A Transwell migration assay demonstrated that the
number of migrated cells was significantly decreased following
SEMA3C knockdown (104.71±3.01) compared with the number of migrated
cells in the control (198.16±9.07) and siRNA-NC groups
(179.34±6.48) (P<0.05; Fig. 4). No
significant differences in the number of migrated cells were
observed between the control and siRNA-NC groups (P>0.05). These
results suggest that SEMA3C knockdown inhibits MCF-7 breast cancer
cell migration.
Discussion
Semaphorins are a family of secreted proteins that
have been identified as novel tumor-associated factors (15). Based on their structural similarity,
semaphorins are divided into eight classes that contain ~25
proteins. Semaphorins serve crucial roles in the nervous system,
immune system, bone remodeling and cancer (8,16).
Previous studies have demonstrated that in the tumor
microenvironment, SEMA3C promotes endotheliocyte migration, cancer
metastasis and angiogenesis (17–20). By
contrast, deletion of SEMA3C suppresses tumorigenesis and
angiogenesis (21). However, the
functions of SEMA3C in breast cancer cell growth and migration
remain unknown. In the present study, the expression of SEMA3C in
MCF-7 breast cancer cells was suppressed using RNAi. Treating MCF-7
cells with SEMA3C siRNA significantly inhibited cell proliferation
and migration. These results, together with previous studies,
suggest that SEMA3C serves an important role in breast
tumorigenesis and metastasis, indicating that SEMA3C may be a
potential target for breast cancer therapy.
The combination of specific molecular targeted
therapy and conventional chemotherapy may allow a reduction of the
chemotherapy dose, increase the sensitivity of tumors to therapy,
and reduce adverse reactions (22,23). Data
from the present study demonstrated that an siRNA sequence
effectively suppresses SEMA3C expression in vitro. Knockdown
of SEMA3C significantly inhibited breast cancer cell growth and
migration. These findings suggest that SEMA3C may be a promising
target for breast cancer therapy and SEMA3C siRNA may be a novel
therapy for the treatment of breast cancer. However, further
studies are required to investigate the molecular mechanisms
underlying the effect of SEMA3C on breast cancer cell growth and
migration.
Acknowledgements
The current study was supported by the Science &
Technology Action Plan for Prevention and Control of Major
Diseases: Special Fund for Wound Repair (The First Affiliated
Hospital of Wenzhou Medical University, Wenzhou, China; grant no.
ZX-01-C2015051).
References
|
1
|
Eccles SA, Aboagye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Karim-Kos HE, Coebergh JW,
Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D and
Soerjomataram I: Recent trends in incidence of five common cancers
in 26 European countries since 1988: Analysis of the European
Cancer Observatory. Eur J Cancer. 51:1164–1187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart BW and Kleihues Paul P: World
Cancer Report. Lyon, France: International Agency Research on
Cancer; 2003
|
|
5
|
Shao S and Zhao X, Zhang X, Luo M, Zuo X,
Huang S, Wang Y, Gu S and Zhao X: Notch1 signaling regulates the
epithelial-mesenchymal transition and invasion of breast cancer in
a Slug-dependent manner. Mol Cancer. 14:282015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barrio-Real L, Benedetti LG, Engel N, Tu
Y, Cho S, Sukumar S and Kazanietz MG: Subtype-specific
overexpression of the Rac-GEF P-REX1 in breast cancer is associated
with promoter hypomethylation. Breast Cancer Res. 16:4412014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Cai Q, Lu J, Jha HC and Robertson
ES: Upregulation of cellular Bcl-2 by the KSHV encoded rta promotes
virion production. PLoS One. 6:e238922011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie L, Gazin C, Park SM, Zhu LJ, Debily
MA, Kittler EL, Zapp ML, Lapointe D, Gobeil S, Virbasius CM and
Green MR: A synthetic interaction screen identifies factors
selectively required for proliferation and TERT transcription in
p53-deficient human cancer cells. PLoS Genet. 8:e10031512012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harper SJ and Bates DO: VEGF-A splicing:
The key to anti-angiogenic therapeutics? Nat Rev Cancer. 8:880–887.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizzolio S and Tamagnone L: Semaphorin
signals on the road to cancer invasion and metastasis. Cell Adh
Migr. 1:62–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyato H, Tsuno NH and Kitayama J:
Semaphorin 3C is involved in the progression of gastric cancer.
Cancer Sci. 103:1961–1966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin-Satué M and Blanco J:
Identification of semaphorin E gene expression in metastatic human
lung adenocarcinoma cells by mRNA differential display. J Surg
Oncol. 72:18–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rieger J, Wick W and Weller M: Human
malignant glioma cells express semaphorins and their receptors,
neuropilins and plexins. Glia. 42:379–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neufeld G, Mumblat Y, Smolkin T, Toledano
S, Nir-Zvi I, Ziv K and Kessler O: The semaphorins and their
receptors as modulators of tumor progression. Drug Resist Updat.
29:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feiner L, Webber AL, Brown CB, Lu MM, Jia
L, Feinstein P, Mombaerts P, Epstein JA and Raper JA: Targeted
disruption of semaphorin 3C leads to persistent truncus arteriosus
and aortic arch interruption. Development. 128:3061–3070.
2001.PubMed/NCBI
|
|
17
|
Brown CB, Feiner L, Lu MM, Li J, Ma X,
Webber AL, Jia L, Raper JA and Epstein JA: PlexinA2 and semaphorin
signaling during cardiac neural crest development. Development.
128:3071–3080. 2001.PubMed/NCBI
|
|
18
|
Herman JG and Meadows GG: Increased class
3 semaphorin expression modulates the invasive and adhesive
properties of prostate cancer cells. Int J Oncol. 30:1231–1238.
2007.PubMed/NCBI
|
|
19
|
Liao YL, Sun YM, Chau GY, Chau YP, Lai TC,
Wang JL, Horng JT, Hsiao M and Tsou AP: Identification of SOX4
target genes using phylogenetic footprinting-based prediction from
expression microarrays suggests that overexpression of SOX4
potentiates metastasis in hepatocellular carcinoma. Oncogene.
27:5578–5589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esselens C, Malapeira J, Colomé N, Casal
C, Rodríguez-Manzaneque JC, Canals F and Arribas J: The cleavage of
semaphorin 3C induced by ADAMTS1 promotes cell migration. J Biol
Chem. 285:2463–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mishra R, Kumar D, Tomar D, Chakraborty G,
Kumar S and Kundu GC: The potential of class 3 semaphorins as both
targets and therapeutics in cancer. Expert Opin Ther Targets.
19:427–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong CA and Nam YS: Functional
nanostructures for effective delivery of small interfering RNA
therapeutics. Theranostics. 4:1211–1232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burnett JC, Rossi JJ and Tiemann K:
Current progress of siRNA/shRNA therapeutics in clinical trials.
Biotechnol J. 6:1130–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|