Introduction
Gastric cancer (GC) is a major public health issue,
and is the second leading cause of cancer-associated mortality
worldwide, particularly in East Asia (1). Current treatment modalities for GC
include surgery, radiotherapy, chemotherapy and their combinations.
New therapies, including molecule-targeted therapy, have been
prescribed for gastric cancer due to their marked benefits in
reducing disease recurrence and increasing long-term survival
(2,3).
Tumor invasion and metastasis, which are primary causes for
treatment failure or mortality among cancer patients, involve
multiple steps. The process involves regulation at the molecular
level of adhesive molecules, proteolytic enzymes and cell growth
and angiogenesis factors, and its mechanism is not yet fully
understood (4). Therefore, searching
for tumor-specific biomarkers for invasion and metastasis has
become necessary for the treatment of GC.
The homeobox (HOX) proteins are transcription
factors with roles in development, including regulating the
patterning during embryogenesis and the control of cell
differentiation (5,6). In mammals, the HOX genes are organized
into clusters named A, B, C and D on four separate chromosomes
(7). The HOXA cluster contains 12
genes (11 HOX genes and EVX1) and is located in a 155 kb-long
genomic region on chromosome 7p15-7p14.2 (8). HOXA9 is normally expressed during
development of the female reproductive tract, and its expression is
tightly regulated in the adult tract (9,10).
A previous study revealed that deregulated
expression of HOX genes is found in cancers (11). However, another study demonstrated
that HOX proteins function in a context-dependent manner (5). HOXA9 was revealed to exert a
tumor-suppressive effect in breast cancer, reported by Gilbert
et al (12). Uchida et
al also demonstrated that HOXA9 acts as a tumor suppressor in
oral cancer (13). Furthermore,
methylation and loss of expression of HOXA9 was reported in oral
cavity (14), breast (12,15,16), lung
(17), ovarian (18) and bladder (19) cancers.
In contrast to the tumor suppressor role, several
studies have considered the oncogenic role of HOXA9 in human cancer
(11,20). Ko et al (20) identified that high expression of HOXA9
is associated with poor overall survival (OS) in ovarian cancer,
and HOXA9 could promote ovarian tumor growth in vivo. In
addition, a previous study also demonstrated that HOXA9 may act as
an oncogene in leukemia (11).
Therefore, HOXA9 appears to exert its function by interacting with
different types of proteins in a tissue-specific manner. However,
the role of HOXA9 in gastric cancer is poorly understood.
PBX3 is a member of the PBX family of three-amino
acid loop extension HOX genes. PBX proteins are well known for
their interaction with HOX proteins that increases the DNA-binding
affinity of HOX proteins, thereby enhancing the transcription of
the downstream target genes (11,21). A
study by Li et al indicated that HOXA/PBX3 interaction is
critical for mixed lineage leukemia-induced leukemia (22). The identification of this HOXA/PBX3
gene signature triggered the present study to investigate whether a
synergistic effect exists between HOXA9 and PBX3 in GC. The aim of
the present study was to evaluate the clinical significance of
HOXA9 and PBX3 in the progression and prognosis of GC, and to
explore the potential association between HOXA9 and PBX3 in GC
progression.
Materials and methods
Patients and tissue samples
The project was approved by the ethics committee on
the use of human subjects of Zhejiang Provincial People's Hospital
(ZPPH; Hangzhou, China) and written informed consent was obtained
from each patient. A total of 24 fresh specimens from patients with
GC were acquired from ZPPH between January 2013 and December 2013,
and stored at −80°C prior to use. Surrounding normal gastric mucosa
samples were also obtained and studied.
In addition, 128 paraffin-embedded specimens of GC
were collected at ZPPH between January 2006 and December 2009. All
cases were diagnosed clinically at the Department of
Gastrointestinal Surgery, and histopathologically at the Department
of Pathology of ZPPH. The patient cohort consisted of 104 males and
24 females (Table I), with a median
age of 54 years (range, 17–87 years) at the time of surgery.
 | Table I.Association of HOXA9 and PBX3
expression with clinicopathological features of patients with
gastric cancer. |
Table I.
Association of HOXA9 and PBX3
expression with clinicopathological features of patients with
gastric cancer.
|
|
| Positive HOXA9
expression | Positive PBX3
expression |
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Total | Patients, n
(%) | χ2 | P-value | Patients, n
(%) | χ2 | P-value |
|---|
| Gender |
|
| 0.060 | 0.807 |
| 0.777 | 0.378 |
|
Male | 104 | 72 (69.2) |
|
| 73 (70.2) |
|
|
|
Female | 24 | 16 (66.7) |
|
| 19 (79.2) |
|
|
| Age range |
|
| 0.180 | 0.671 |
| 0.020 | 0.887 |
| <60
years | 83 | 56 (67.5) |
|
| 60 (72.3) |
|
|
| ≥60
years | 45 | 32 (71.1) |
|
| 32 (71.1) |
|
|
|
Differentiation |
|
| 14.896 | 0.001 |
| 1.890 | 0.420 |
|
Well | 16 | 8 (50.0) |
|
| 10 (62.5) |
|
|
|
Moderate | 51 | 28 (54.9) |
|
| 35 (68.6) |
|
|
|
Poor | 61 | 52 (85.2) |
|
| 47 (77.0) |
|
|
| Lymph node
metastasis |
|
| 6.390 | 0.011 |
| 17.264 | <0.001 |
|
Negative | 62 | 36 (58.1) |
|
| 34 (54.8) |
|
|
|
Positive | 66 | 52 (78.8) |
|
| 58 (87.9) |
|
|
| Distant
metastasis |
|
| 0.924 | 1.000 |
| 0.795 | 1.000 |
|
Negative | 126 | 86 (68.3) |
|
| 90 (71.4) |
|
|
|
Positive | 2 | 2 (100.0) |
|
| 2 (100.0) |
|
|
| TNM stage |
|
| 14.396 | 0.0001 |
| 29.692 | <0.001 |
|
I+II | 61 | 32 (52.5) |
|
| 30 (49.2) |
|
|
|
III+IV | 67 | 56 (83.6) |
|
| 62 (92.5) |
|
|
All cases were classified according to the World
Health Organization pathological classification of tumors. Among
the 128 cases of GC, 16 were well differentiated, 51 were
moderately differentiated and 61 were poorly differentiated. There
were 62 cases without lymph node metastasis, 66 cases with lymph
node metastasis, 2 cases with distant metastasis and 126 cases
without distant metastasis. According to TNM stage classification,
61 cases were categorized as stage I+II and 67 cases were
categorized as stage III+IV. None of the patients had received any
radiotherapy or chemotherapy prior to surgery.
All patients were followed for >5 years, and the
survival time was calculated from the date of surgery to the
deadline for follow-up, or to the date of mortality.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the fresh specimens
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and RNA concentration was determined using a Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). A total of 2 µg
of RNA was reverse transcribed using the SuperScript II reverse
transcriptase system (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. The cDNA was then subjected
to RT-PCR using specific primers with the SYBR Premix ExTaq kit
(Takara Bio, Inc., Otsu, Japan). The forward and reverse primers
for HOXA9 (NM_152739) were 5′-GTGATGCCATTTGGGCTTATT-3′ and
5′-GGTTTAGAGCCGCTTTGTGC-3′, respectively. Those for PBX3
(NM_001134778) were 5′-CTGTTTGCCTATCCCTGTT-3′ (forward) and
5′-GCAGCAAGTATCTTCGTCTC-3′ (reverse). GAPDH was used as an internal
control using the following primers: Forward,
5′-TGAAGGTCGGAGTCAACGG-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′.
The relative amount of mRNA level to GAPDH was calculated as the
average 2−ΔΔCq, where ΔCq=Cq-CqGAPDH
(23).
Immunohistochemical staining
Each tissue section was baked at 60°C for 2 h,
deparaffinized with xylene and rehydrated in graded alcohol.
Antigen retrieval was then performed by autoclaving in 0.01 M
citrate buffer (pH 6.0) for 3 min. Subsequently, sections were
incubated with 3% (v/v) H2O2 for 10 min to
block endogenous peroxidase.
To reduce nonspecific reactions, sections were then
incubated with 10% (vol/vol) normal goat serum (Histostain-Plus
kit; cat. no. 859043; Invitrogen; Thermo Fisher Scientific, Inc.)
for 15 min at room temperature. Subsequently, the slides were
incubated overnight at 4°C with rabbit polyclonal antibody against
human HOXA9 (dilution, 1:500; cat. no. bs6667R; BIOSS, Beijing,
China) or rabbit polyclonal antibody to human PBX3 (dilution,
1:500; cat. no. bs12295R; BIOSS). Subsequent to rinsing with PBS,
tissue sections were incubated for 20 min at room temperature with
biotin-labeled secondary antibody (Histostain-Plus kit; cat. no.
859043; Invitrogen; Thermo Fisher Scientific, Inc.) followed by
horseradish peroxidase-linked goat anti-rabbit antibody
(Histostain-Plus kit; cat. no. 859043; Invitrogen; Thermo Fisher
Scientific, Inc.) for 20 min at room temperature. Sections were
then stained with 3,3-diaminobenzidine (ZSGB-BIO, Beijing, China).
Finally, the sections were counterstained with hematoxylin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), dehydrated and
mounted with a coverslip. Phosphate buffer was used to replace the
primary antibody as a negative control.
Evaluation of immunohistochemical
staining
Immuno-histochemical staining showed that HOXA9 and
PBX3 positive staining were mainly located in the nucleus and
cytoplasm. The degree of immunostaining was reviewed under a light
microscope (5 fields were viewed with magnification ×200) by two
expert pathologists without knowledge of the clinical data and
scored independently. The HOXA9 and PBX3 expression level was based
on the intensity of cellular staining and the proportion of stained
tumor cells.
Staining intensity was scored according to the
following criteria: 0, no staining; 1, weak staining (light
yellow); 2, moderate staining (yellow brown); and 3, strong
staining (brown). The proportion of stained tumor cells was scored
according to the proportion of positively stained tumor cells, as
follows: 0, <5% positive tumor cells; 1, 6–25% positive tumor
cells; 2, 26–50% positive tumor cells; and 3, >51% positive
tumor cells. The staining intensity and proportion immunoreactivity
scores were then multiplied to obtain a composite score. The values
of the composite score ranged from 0 to 9. For additional
evaluation, a staining index score of ≤4 was defined as HOXA9 or
PBX3 negative expression, and a staining index score of >5 was
regarded as HOXA9 or PBX3 positive expression. In cases of
discrepancy, a consensus score was chosen for evaluation.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Differences between
HOXA9 and PBX3 mRNA expression levels of cancer and normal tissues
were determined using the Mann-Whitney U test. χ2 test
or Fisher's exact test was used to evaluate the associations
between the expression of HOXA9 or PBX3 and the clinicopathological
features of the patients with GC. Univariate survival analysis was
performed using the Kaplan-Meier method, accompanying the log-rank
test to calculate differences among the curves. Multivariate
survival analysis was performed to assess predictors associated
with prognosis using Cox proportional hazards regression model.
Additionally, association between HOXA9 expression, PBX3 expression
and clinicopathological features was estimated using Spearman's
rank correlation coefficient. All P-values were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Detection of HOXA9 and PBX3 mRNA
expression level
To detect HOXA9 and PBX3 mRNA expression level, a
total of 24 paired fresh specimens of GC and surrounding normal
mucosa were analyzed using RT-PCR. The results revealed that the
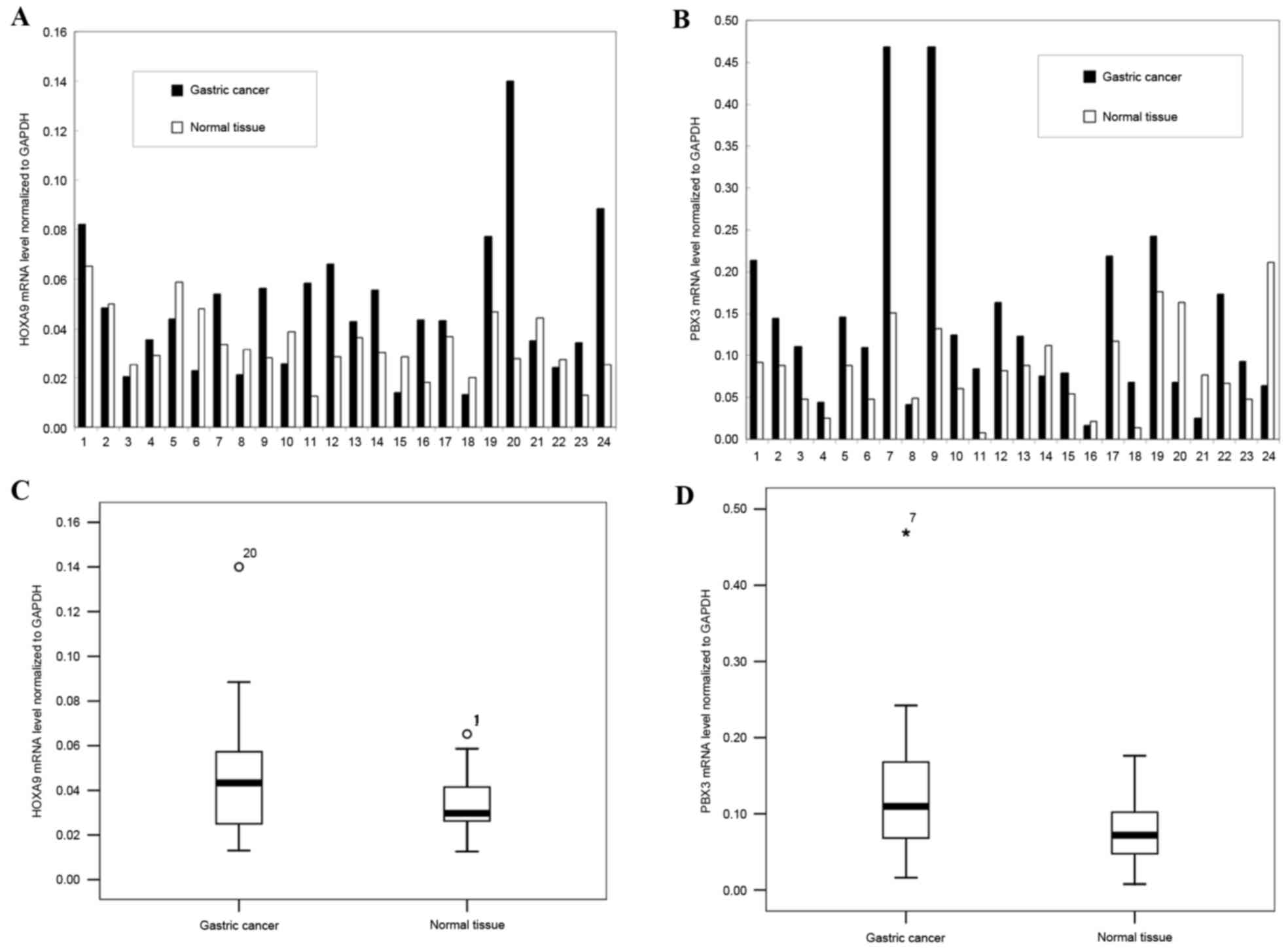
HOXA9 mRNA level was upregulated in 62.5% of GCs (15/24), and
downregulated in 37.5% of the GCs (9/24), and the mean mRNA level
of HOXA9 was upregulated in GC tissues compared with normal tissues
(P=0.032; Fig. 1).
Similarly, PBX3 was significantly upregulated in
79.2% of GCs (19/24) and downregulated in the remainder of GCs
(5/24, 20.8%), and the mean mRNA level of PBX3 was upregulated in
GC tissues compared with normal tissues (P=0.031; Fig. 1).
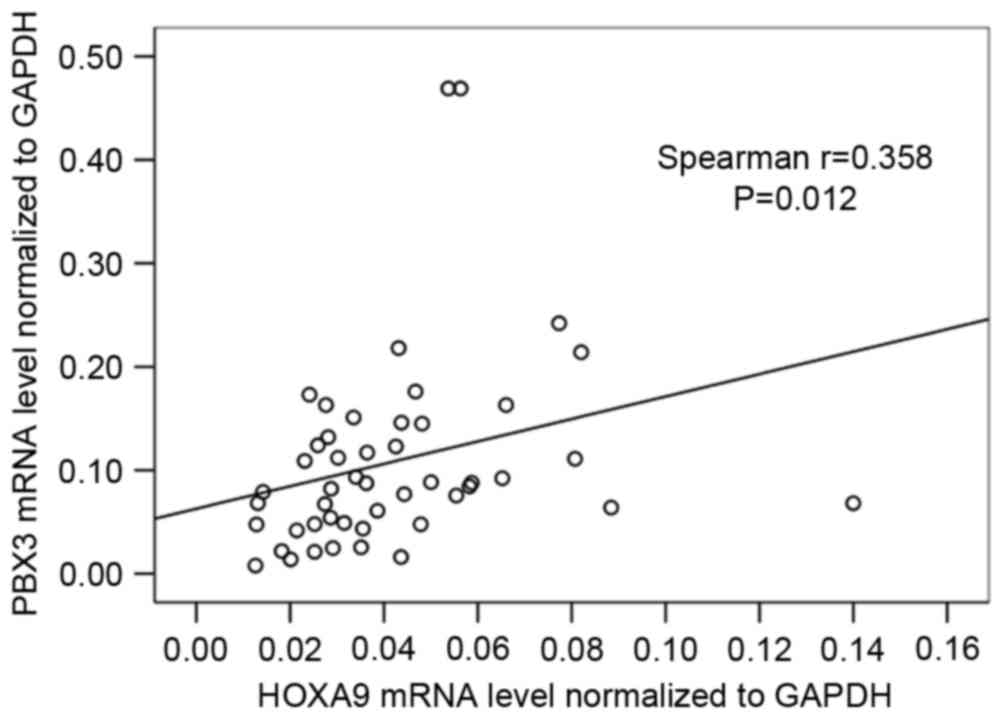
Further analysis of the association between HOXA9
and PBX3 mRNA level was also performed, and the result showed that
the HOXA9 mRNA level was significantly correlated with PBX3 mRNA
level (r=0.358; P=0.012; Fig.
2).
Association between HOXA9 and PBX3
expression with clinicopathological features of GC
In order to detect the presence and distribution of
HOXA9 and PBX3 expression in GC, immunohistochemical staining was
performed, and the association between HOXA9 and PBX3 expression
with clinicopathological features of GC was analyzed. The results
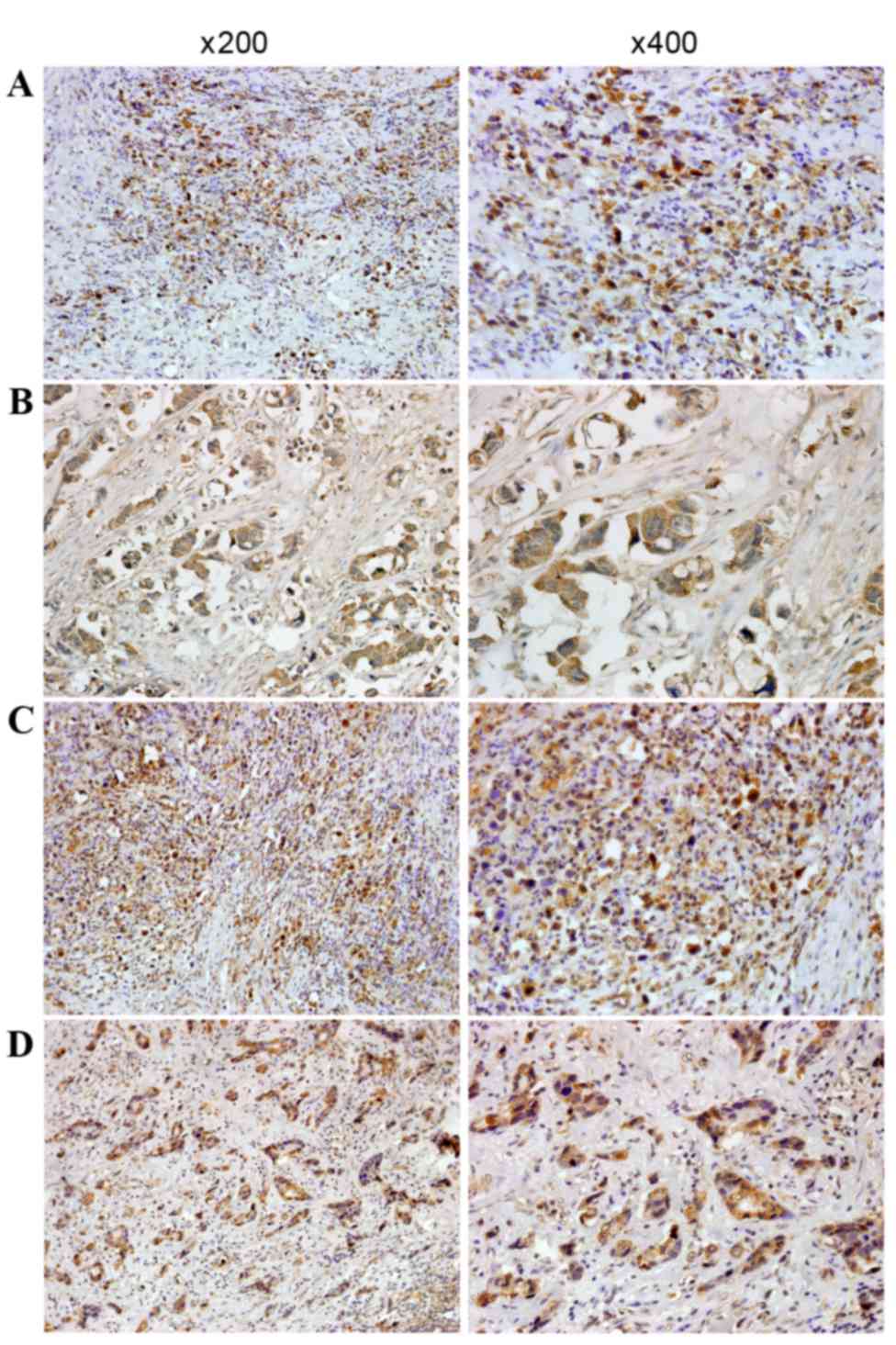
revealed that immunostaining of HOXA9 was mainly located in the
nucleus and cytoplasm of the tumor cells (Fig. 3), and positive expression of HOXA9 was
detected in 88 of the 128 patients with GC (68.8%). Additional
analysis demonstrated that HOXA9 expression was associated with
differentiation, lymph node metastasis and TNM stage (Table I). Gastric cancer patients with poor
differentiation, lymph node metastasis and high TNM stage (stages
III+IV) had significantly increased expression of HOXA9 compared
with those with well or moderate differentiation (P=0.001), no
lymph node metastasis (P=0.011) and low TNM stage (stages I+II)
(P=0.0001; Table I). The Spearman's
rank correlation coefficient of HOXA9 expression with
differentiation, lymph node metastasis and TNM stage was 0.185
(P=0.037), 0.298 (P=0.001) and 0.439 (P<0.001),
respectively.
Immunostaining of PBX3 was predominantly distributed
in the nucleus and cytoplasm of the tumor cells (Fig. 3), and positive expression of PBX3 was
detected in 92 of the 128 patients with GC (71.9%). PBX3 expression
was associated with lymph node metastasis and TNM stage (Table I). Positive expression of PBX3 was
detected in 87.9% (58/66) of GC patients with lymph node
metastasis, which was increased compared with the expression rate
in patients without lymph node metastasis (34/62, 54.8%)
(χ2=17.264; P<0.001). The detection rate of PBX3
expression was 92.5% (62/67) in GC patients with TNM stage III+IV,
which revealed a significant difference from TNM stage I+II (49.2%;
χ2=26.692; P<0.001). Furthermore, the Spearman's rank
correlation coefficients of PBX3 expression with lymph node
metastasis and TNM stage were 0.438 (P<0.001) and 0.579
(P<0.001), respectively.
Association between expression of
HOXA9 and PBX3 in GC
In order to investigate the synergistic effect
between HOXA9 and PBX3 in GC, the association between HOXA9 and
PBX3 in the development of gastric cancer was analyzed. High
coincidental expression of the HOXA9 and PBX3 proteins was observed
in gastric cancer. Of the 88 patients that were found to express
HOXA9, 69 (78.4%) also expressed PBX3. The correlation between the
expression of HOXA9 and PBX3 expression in patients with gastric
cancer was statistically significant (r=0.391; P<0.001).
Clinical significance of HOXA9 and
PBX3 expression in prognosis of GC
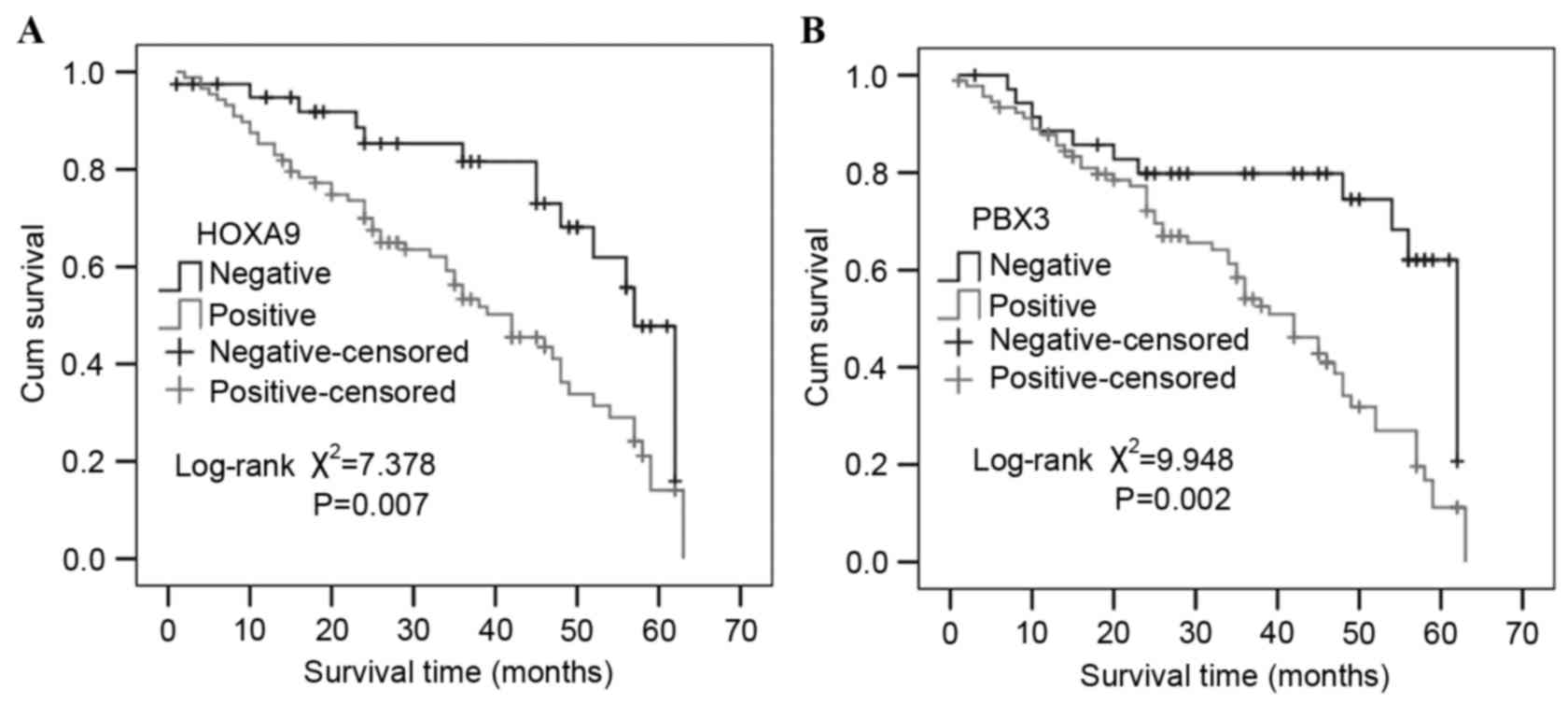
Univariate survival analysis revealed the 3- and
5-year cumulative survival rates were 81.6 and 47.8% in patients
with negative HOXA9 expression, and 56.3 and 14.1% in those with
positive HOXA9 expression. The mean survival time in patients of GC
with positive HOXA9 expression was 37.96±2.22 months, and
50.69±3.02 months for those with negative HOXA9 expression.
Evidently, GC patients with positive expression of HOXA9 have a
poorer prognosis than those with negative expression
(χ2=7.378; P=0.007; Fig.
4).
Similarly, the 3- and 5-year cumulative survival
rates were 79.8 and 62.1% in patients with negative expression of
PBX3, which were increased compared with patients with positive
expression of PBX3 (58.4 and 11.2%, respectively). The mean
survival time in patients of GC with positive expression of PBX3
was 38.13±2.11 months and 50.59±3.46 months for those with negative
expression of PBX3. Notably, GC patients with positive expression
of PBX3 had a poorer prognosis than those with negative expression
(χ2=9.948; P=0.002; Fig.
4). Multivariate analysis using the Cox regression model
demonstrated that survival was independently associated with lymph
node metastasis (P=0.032) and PBX3 expression (P=0.024; Table II).
 | Table II.Multivariate analysis of the
correlation between clinicopathological parameters and prognosis in
patients with gastric cancer. |
Table II.
Multivariate analysis of the
correlation between clinicopathological parameters and prognosis in
patients with gastric cancer.
| Covariates | Coefficient | Standard error | HR | 95% CI | P-value |
|---|
| Gender | −0.073 | 0.340 | 0.929 | 0.477–1.810 | 0.829 |
| Age | 0.001 | 0.299 | 1.001 | 0.557–1.799 | 0.998 |
|
Differentiation | −0.192 | 0.189 | 0.825 | 0.570–1.196 | 0.310 |
| TNM stage | −1.048 | 0.616 | 0.350 | 0.105–1.171 | 0.089 |
| Lymph node
metastasis | 1.166 | 0.542 | 3.209 | 1.109–9.287 | 0.032 |
| Distant
metastasis | 0.741 | 0.794 | 2.097 | 0.442–9.942 | 0.351 |
| HOXA9
expression | 0.637 | 0.351 | 1.890 | 0.951–3.758 | 0.069 |
| PBX3
expression | 0.885 | 0.393 | 2.424 | 1.123–5.233 | 0.024 |
Discussion
HOX genes are an important class of patterning
regulators that modulate tumor progression and alter tumor cell
growth in vitro (24–26). HOX proteins can form heterodimers or
heterotrimers with members of the 3-amino-acid loop extension
family of cofactors, including PBX and Meis proteins, which may
directly regulate the transcription of downstream target genes.
Previous studies showed that HOXA9 appears to exert its function by
interacting with different types of proteins in a tissue-specific
manner (5,11,12,20).
However, the role of HOXA9 in gastric cancer has not been fully
elucidated.
To understand the clinicopathological significance
of HOXA9 in GC, the expression of HOXA9 mRNA level was analyzed in
34 paired fresh GC tissue and 128 paraffin-embedded GC tissues. In
the present study, HOXA9 and PBX3 mRNA levels were revealed to be
significantly upregulated in GC tissue compared with adjacent
normal tissue. Immunohistochemical staining also revealed that
gastric cancer patients with poor differentiation, lymph node
metastasis and high TNM stage (stages III+IV) had significantly
increased expression of HOXA9 compared with patients with well or
moderate differentiation (P=0.001), no lymph node metastasis
(P=0.011) and low TNM stage (stages I+II; P=0.0001). These results
showed that HOXA9 overexpression was involved in the progression of
GC. HOXA9 has been implicated in carcinogenesis, since it acts as a
transcription factor with roles in development, regulating
patterning during embryogenesis and controlling cell
differentiation (5,6). Studies also revealed that HOXA9
increases endothelial cell migration and tube formation in human
myeloid leukemia cells (27,28). In addition, HOXA9 promotes tumor
metastasis by enhancing the adhesion of circulating tumor cells to
endothelial cells (29). HOXA9 was
also reported to increase cell proliferation and inhibit apoptosis
in human glioblastoma (30). These
findings indicate that HOXA9 may be involved in tumor progression
by modulating interactions between tumor cells and host cells.
However, a number of studies revealed that HOXA9 exerted a
tumor-suppressive effect in breast cancer, lung cancer, ovarian
cancer and bladder cancer (12,17–19). HOXA9
is frequently deregulated in a variety of human cancers, in which
it acts as a tumor suppressor or as an oncogene. Although HOXA9
seems to exert its function by interacting with different types of
proteins in a tissue-specific manner, the mechanisms underlying
these differential functions remain to be identified.
PBX proteins are also well known for their
interaction with HOX proteins, which increase the DNA-binding
affinity of HOX proteins and thereby enhance the transcription of
the downstream target genes (11,31,32). The
cooperation between PBX3 proteins and HOXA9 in GC progression is
unclear. The present study revealed hat HOXA9 mRNA level was
associated with that of PBX3. Immunohistochemical staining also
revealed a high coincidental expression of the HOXA9 and PBX3
proteins in GC. Additional analysis revealed hat PBX3 expression
was associated with lymph node metastasis and TNM stage. Positive
expression rates of PBX3 were increased in GC patients with lymph
node metastasis and TNM stage III+IV compared with patients without
lymph node metastasis and TNM stage I+II. Therefore, the present
data suggest that PBX3 may be a critical cofactor of HOXA9 in GC
carcinogenesis and development.
Survival analysis also revealed that high expression
of HOXA9 or PBX3 was associated with poor survival of GC, and
multivariate analysis using the Cox regression model showed that
PBX3 expression was an independent prognostic factor in GC. High
HOXA9 expression was reported to be associated with poor OS of
epithelial ovarian carcinoma patients (20). Li et al also demonstrated that
increased expression of a 4-HOX gene signature (composed of HOXA7,
HOXA9, HOXA11 and PBX3) is an independent predictor of shortened OS
in patients with cytogenetically abnormal acute myeloid leukemia
(33). The present study showed that
the HOXA9/PBX3 gene signature has a prognostic value for GC.
On the basis of these findings, it is suggested that
PBX3 is a critical cofactor of HOXA9, and that cross-talk between
HOXA9 and PBX3 may perform an important role in the mechanism
underlying the carcinogenesis, development and progression of GC.
Therefore, targeting the interaction of these genes is a feasible
strategy for the therapy of GC; however, the mechanisms underlying
the regulation of HOXA9/PBX3 in GC development remain to be
identified.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LQ16H160017 to
Ying-Yu Ma and grant no. LY15H160051 to Xiao-Zhou Mou).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mullen JT and Ryan DP: Neoadjuvant
chemotherapy for gastric cancer: What are we trying to accomplish?
Ann Surg Oncol. 21:13–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: European Society for Medical Oncology
(ESMO); European Society of Surgical Oncology (ESSO); European
Society of Radiotherapy and Oncology (ESTRO): Gastric cancer:
ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis,
treatment and follow-up. Eur J Surg Oncol. 40:584–591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pearson JC, Lemons D and McGinnis W:
Modulating Hox gene functions during animal body patterning. Nat
Rev Genet. 6:893–904. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samuel S and Naora H: Homeobox gene
expression in cancer: Insights from developmental regulation and
deregulation. Eur J Cancer. 41:2428–2437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krumlauf R: Hox genes in vertebrate
development. Cell. 78:191–201. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rauch T, Wang Z, Zhang X, Zhong X, Wu X,
Lau SK, Kernstine KH, Riggs AD and Pfeifer GP: Homeobox gene
methylation in lung cancer studied by genome-wide analysis with a
microarray-based methylated CpG island recovery assay. Proc Natl
Acad Sci USA. 104:5527–5532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng W, Liu J, Yoshida H, Rosen D and
Naora H: Lineage infidelity of epithelial ovarian cancers is
controlled by HOX genes that specify regional identity in the
reproductive tract. Nat Med. 11:531–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vitiello D, Kodaman PH and Taylor HS: HOX
genes in implantation. Semin Reprod Med. 25:431–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilbert PM, Mouw JK, Unger MA, Lakins JN,
Gbegnon MK, Clemmer VB, Benezra M, Licht JD, Boudreau NJ, Tsai KK,
et al: HOXA9 regulates BRCA1 expression to modulate human breast
tumor phenotype. J Clin Invest. 120:1535–1550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchida K, Veeramachaneni R, Huey B,
Bhattacharya A, Schmidt BL and Albertson DG: Investigation of HOXA9
promoter methylation as a biomarker to distinguish oral cancer
patients at low risk of neck metastasis. BMC Cancer. 14:3532014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guerrero-Preston R, Soudry E, Acero J,
Orera M, Moreno-López L, Macía-Colón G, Jaffe A, Berdasco M,
Ili-Gangas C, Brebi-Mieville P, et al: NID2 and HOXA9 promoter
hypermethylation as biomarkers for prevention and early detection
in oral cavity squamous cell carcinoma tissues and saliva. Cancer
Prev Res (Phila). 4:1061–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reynolds PA, Sigaroudinia M, Zardo G,
Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF
and Tlsty TD: Tumor suppressor p16INK4A regulates polycomb-mediated
DNA hypermethylation in human mammary epithelial cells. J Biol
Chem. 281:24790–24802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Song CX, Huang H, Frankenberger CA,
Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C and Rosner
MR: HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer
growth and metastasis. Proc Natl Acad Sci USA. 110:9920–9925. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Son JW, Jeong KJ, Jean WS, Park SY, Jheon
S, Cho HM, Park CG, Lee HY and Kang J: Genome-wide combination
profiling of DNA copy number and methylation for deciphering
biomarkers in non-small cell lung cancer patients. Cancer Lett.
311:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Q, Lothe RA, Ahlquist T, Silins I,
Tropé CG, Micci F, Nesland JM, Suo Z and Lind GE: DNA methylation
profiling of ovarian carcinomas and their in vitro models
identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol
Cancer. 6:452007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reinert T, Borre M, Christiansen A,
Hermann GG, Ørntoft TF and Dyrskjot L: Diagnosis of bladder cancer
recurrence based on urinary levels of EOMES, HOXA9, POU4F2, TWIST1,
VIM and ZNF154 hypermethylation. PLoS One. 7:e462972012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko SY, Barengo N, Ladanyi A, Lee JS,
Marini F, Lengyel E and Naora H: HOXA9 promotes ovarian cancer
growth by stimulating cancer-associated fibroblasts. J Clin Invest.
122:3603–3617. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CP, Brocchieri L, Shen WF, Largman C
and Cleary ML: Pbx modulation of Hox homeodomain amino-terminal
arms establishes different DNA-binding specificities across the Hox
locus. Mol Cell Biol. 16:1734–1745. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhang Z, Li Y, Arnovitz S, Chen P,
Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H, et al: PBX3 is an
important cofactor of HOXA9 in leukemogenesis. Blood.
121:1422–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muratovska A, Zhou C, He S, Goodyer P and
Eccles MR: Paired-Box genes are frequently expressed in cancer and
often required for cancer cell survival. Oncogene. 22:7989–7997.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan Y, Cheung M, Pei J, Menges CW, Godwin
AK and Testa JR: Upregulation of DLX5 promotes ovarian cancer cell
proliferation by enhancing IRS-2-AKT signaling. Cancer Res.
70:9197–9206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trinh BQ, Ko SY, Barengo N, Lin SY and
Naora H: Dual functions of the homeoprotein DLX4 in modulating
responsiveness of tumor cells to topoisomerase II-targeting drugs.
Cancer Res. 73:1000–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruhl T, Urbich C, Aicher D, Acker-Palmer
A, Zeiher AM and Dimmeler S: Homeobox A9 transcriptionally
regulates the EphB4 receptor to modulate endothelial cell migration
and tube formation. Circ Res. 94:743–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura T, Largaespada DA, Lee MP,
Johnson LA, Ohyashiki K, Toyama K, Chen SJ, Willman CL, Chen IM,
Feinberg AP, et al: Fusion of the nucleoporin gene NUP98 to HOXA9
by the chromosome translocation t(7;11)(p15;p15) in human myeloid
leukaemia. Nat Genet. 12:154–158. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bandyopadhyay S, Ashraf MZ, Daher P, Howe
PH and DiCorleto PE: HOXA9 participates in the transcriptional
activation of E-selectin in endothelial cells. Mol Cell Biol.
27:4207–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Costa BM, Smith JS, Chen Y, Chen J,
Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, et
al: Reversing HOXA9 oncogene activation by PI3K inhibition:
Epigenetic mechanism and prognostic significance in human
glioblastoma. Cancer Res. 70:453–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang CP, Shen WF, Rozenfeld S, Lawrence
HJ, Largman C and Cleary ML: Pbx proteins display
hexapeptide-dependent cooperative DNA binding with a subset of Hox
proteins. Genes Dev. 9:663–674. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milech N, Kees UR and Watt PM: Novel
alternative PBX3 isoforms in leukemia cells with distinct
interaction specificities. Genes Chromosomes Cancer. 32:275–280.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Huang H, Li Y, Jiang X, Chen P,
Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X, et al:
Up-regulation of a HOXA-PBX3 homeobox-gene signature following
down-regulation of miR-181 is associated with adverse prognosis in
patients with cytogenetically abnormal AML. Blood. 119:2314–2324.
2012. View Article : Google Scholar : PubMed/NCBI
|