Introduction
The prevalence of primary malignant bone tumors is
markedly lower than the prevalence of benign bone tumors in
children, with malignant bone tumors accounting for only 6% of all
bone tumors (1). Over 90% of cases of
primary malignant bone tumors are osteosarcomas or Ewing sarcomas
(2). Owing to their rapid and
invasive growth, malignant bone tumors are a major cause of
mortality and disability in children, despite their low incidence
(3). Compared with the elderly and
young adults, children and adolescents with malignant bone tumors
have a lower survival rate and poorer prognosis; as such, malignant
bone tumors in children are the focus of research (3).
Early diagnosis and treatment are important for
improving the quality of life and survival rate of children with
malignant bone tumors. If the tumor is diagnosed and treated early,
limb salvage can be achieved during the tumor is resected. Motor
function can also be reconstructed to improve quality of life and
extend survival time (4,5). However, diagnosis is frequently delayed
for weeks to months, partly because the rarity of such tumors and
that the presence of a malignancy in an otherwise healthy
adolescent is unexpected (6).
Therefore, performing an accurate diagnosis of malignant bone
tumors in children is extremely important.
Radiography is the prime imaging modality for
evaluation of primary bone tumors (7). The role of cross-sectional imaging,
including magnetic resonance imaging (MRI), computed tomography
(CT), and nuclear medicine (NM) technetium bone scan, plays an
integral part in characteristics of the extent of tumors (8). In addition, dynamic MRI, diffusion
weighted MRI and 18F-fluorodeoxyglucose positron
emission tomography-computed tomography (18FDG-PET/CT)
to monitor tumor necrosis (1,8). These imaging modalities revolutionized
diagnostic and therapeutic approaches to musculoskeletal oncology,
which has a major role in evaluating metastases, guiding surgery
and radiation and detecting response to treatment and tumor
recurrence (5).
In the present study, 34 cases of pathologically
confirmed primary malignant bone tumors in children were
retrospectively analyzed. The aim of the present study was to
investigate the imaging characteristics of primary malignant bone
tumors in children.
Patients and methods
Patients
The study procedures were approved by the Ethics
Committee of Shengjing Hospital (China Medical University,
Shenyang, Liaoning, China) and were conducted in accordance with
the Declaration of Helsinki. Patient records were anonymized and
de-identified prior to analysis. The imaging results of 34
children, aged <18 years, who were diagnosed with a primary
malignant bone tumor that was confirmed by histopathological
diagnosis in Shengjing Hospital (China Medical University,
Shenyang, Liaoning, China) between March 2008 to January 2014 were
collected. Of the 34 patients, 18 were male and 16 were female,
with a mean age of 10.8 years (range, 4–17 years). A total of 25
patients presented with osteosarcoma, 5 with Ewing sarcoma, 3 with
chondrosarcoma and 1 with lymphoma. The main clinical symptoms
included pain, swelling, gradually enlarging soft-tissue mass and
pathological fractures.
Imaging
Anterior-posterior and lateral plain radiographs
were performed using a digital radiography system (Kodak DirectView
DR7500; Kodak, Rochester, NY, USA). Axial CT was performed using
multislice CT scanners (Somatom Sensation 64/Definition 64; both
Siemens Medical Solutions, Forchheim, Germany; and Brilliance 64;
Philips Healthcare, Cleveland, OH, USA). The coronal and sagittal
images were subject to multiplanar reconstruction. The scanning
parameters were as follows: Tube voltage, 120 kV; tube current, 200
mA; and slice thickness, 1 mm. Non-ionic iodinated contrast agent
(80–100 ml) injected intravenously at a rate of 3.0 ml/sec, was
used for contrast enhancement. Whole-body bone scintigraphy was
performed using a single-photon emission computed tomography
imaging system (Infinia Hawkeye; GE Healthcare, Chicago, IL, USA).
At 3 h after the intravenously administration of 725 MBq (25 mCi)
of Technetium-99 m methylene diphosphonate (99mTc-MDP),
anterior and posterior views of whole-body bone scintigraphy was
obtained. 18F-FDG PET/CT was performed using a PET/CT
imaging system (Discovery ST, GE Healthcare, Chicago, IL, USA).
18F-FDG was intravenously administered at a dose of 3.7
MBq/kg. MRI was performed using superconducting MR systems (Signa
HDxt3.0T; GE Healthcare; or Achieva 3.0T/Gyroscan Intera 1.5T;
Philips Healthcare, Best, the Netherlands). A combination of axial,
sagittal, and coronal images were obtained using T1-weighted
spin-echo (SE) sequence, T2-weighted fast spin-echo (FSE) sequence,
and T2-weighted fat suppression sequences, including short-time
inversion recovery (STIR), spectral-presaturation inversion
recovery (SPIR), and iterative Dixon water-fat separation with echo
asymmetry and least-squares estimation (IDEAL). The following
acquisition parameters were employed: Slice thickness, 3.5–5 mm;
field of view, 300–400 mm; and matrix, 512×512. A total of 0.1
mmol/kg gadolinium-diethylene triamine pentacetate acid, infused
intravenously at 2 ml/sec, was used for enhancement.
Imaging features of the tumors, including location,
size, boundary, number and tumor matrix, type of bony destruction,
soft-tissue mass and aggressive periosteal reaction, among others,
were independently evaluated by two radiologists with 9 and 20
years of bone tumor MRI experience, respectively). If the judgment
was inconsistent, the result was determined by a consensus after
discussion.
Histology
All histological samples were reviewed by
pathologist. All specimens were fixed in 10% formalin for 4 h at
room temperature, embedded in paraffin, sectioned at a thickness of
5 µm for hematoxylin-eosin (HE) staining and 3 µm for
immunohistochemical staining, followed by observed under light
microscope.
Immunohistochemical staining performed with primary
monoclonal antibodies targeted at: B-cell lymphoma 2, cluster of
differentiation 99 (CD99), S-100, vimentin, LCA, neuron-specifc
enolase, ALK tyrosine kinase receptor, CD3, CD10, CD20, CD30, CD68,
CD117, epithelial membrane antigen, myeloperoxidase and T-cell
intracellular antigen-1 (cat. nos. ZM-0010, ZM-0296, ZM-0224,
ZM-0260, ZM-0183, ZM-0203, ZM-0248, ZA-0503, ZA-0526, ZM-0039,
ZA-0591, ZM-0060, ZA-0523, ZA-0197 and ZM-0457, respectively;
ready-to-use; Origene Technologies, Inc., Beijing, China).
The Bond Polymer Refine Detection kit (cat. no.
DS9800; Leica Microsystems, Inc., Buffalo Grove, IL, USA) was
applied using the primary monoclonal antibody. The incubation time
for the primary antibody was 15 min. The incubation time for the
horseradish peroxidase conjugates was 8 min at 37°C, and
diaminobenzidine (DAB) incubation time was 10 min. The procedure
was performed by a professional pathologist using the Bond-Max
autostainer (Leica Microsystems, Inc.) according to the
manufacturer's instructions.
Results
The specific imaging characteristics of the 34
pediatric cases of malignant bone tumors are listed in the
subsections that follow.
Osteosarcoma
There were 25 cases of osteosarcoma (12 males and 13
females), with patient ages ranging from 4 to 17 years, which were
all located in the long bones, including 14 involving the distal
femur, 7 in the proximal tibia and 4 in the proximal humerus. The
types of bone destruction included 4 osteolytic, 4 osteoblastic and
17 mixed cases. The histopathology included 24 conventional
osteosarcoma cases and 1 telangiectatic osteosarcoma case. A
pathological fracture was present in 4 cases, skip lesions in 2
cases and lung metastasis in 5 cases.
X-ray (22/25) and CT (21/25) revealed osteolytic
and/or osteoblastic bone destruction. The size of the tumors ranged
from 6.1–15.2 cm. A total of 24 cases had fluffy, sunburst,
spiculated and other aggressive periosteal reactions; of which 19
showed a Codman triangle. Of the 23 cases with a soft-tissue mass,
21 had punctate or irregular cancerous bone in the area of bone
destruction and in the soft-tissue mass. A single case with lesions
in the femur only showed a small shadow, indicating patchy bone
sclerosis, in addition to thickening of the neighboring bone cortex
and a pathological fracture. Another case showed inhomogeneous
enhancement of the tumor.
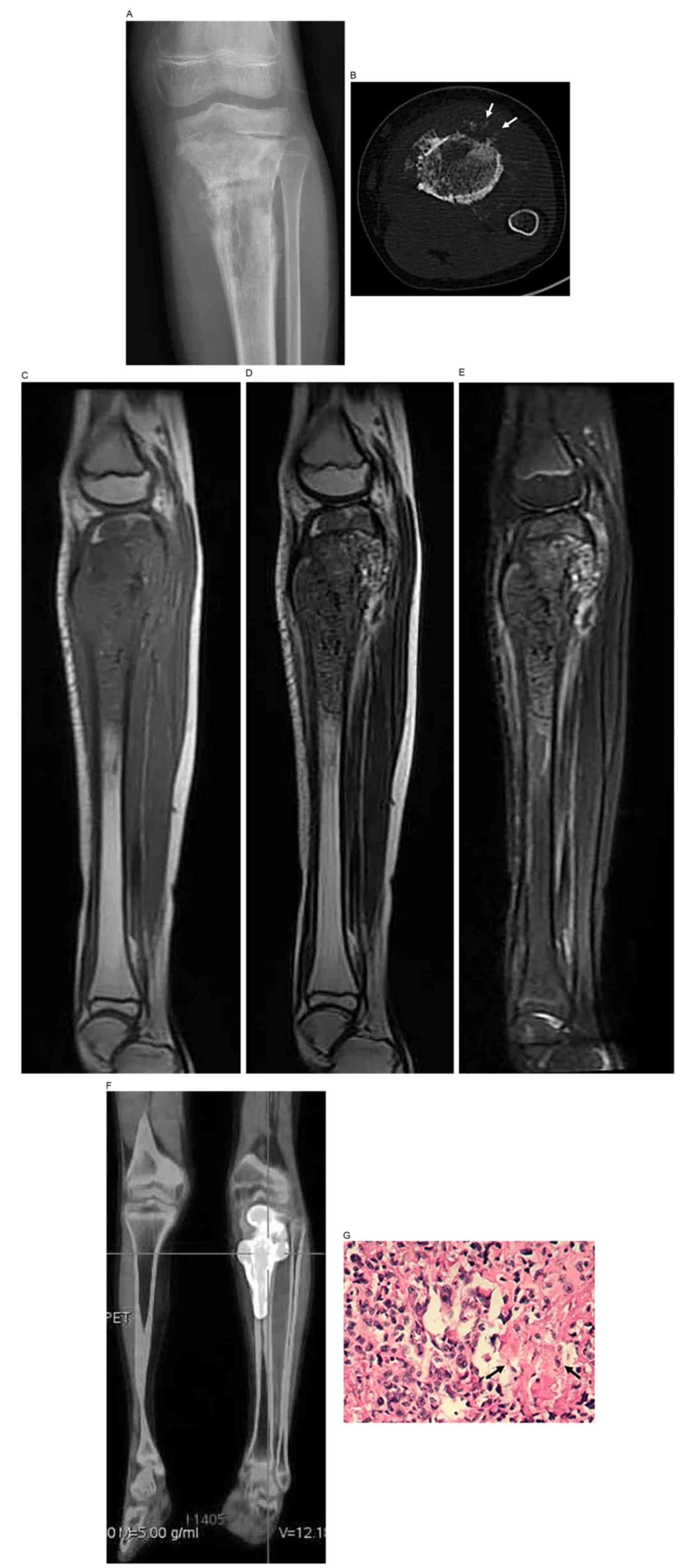
MRI manifestations of osteosarcoma often consisted
of low mixed signals on T1WI and high mixed signals on T2WI. The
surrounding soft-tissue masses appeared as equally long T1and
slightly long T2 mixed signals. The epiphysis and epiphyseal plate
were invaded in 17 cases, and fat-suppressed T2WI provided the
clearest images. Enhanced MRI (2/25) showed markedly inhomogeneous
enhancement in the areas of bone destruction and the soft-tissue
mass (Fig. 1). Bone scintigraphy
(19/25) showed abnormal radionuclide uptake by the lesions. PET-CT
(1/25) showed increased 18F-FDG metabolism in the tibial
bone marrow [standardized uptake value (SUV), 20.77]. A case
assessed by ultrasonography showed a mixed-echoic soft-tissue mass
adjacent to the humerus, and color Doppler flow imaging detected
abundant blood flow signals.
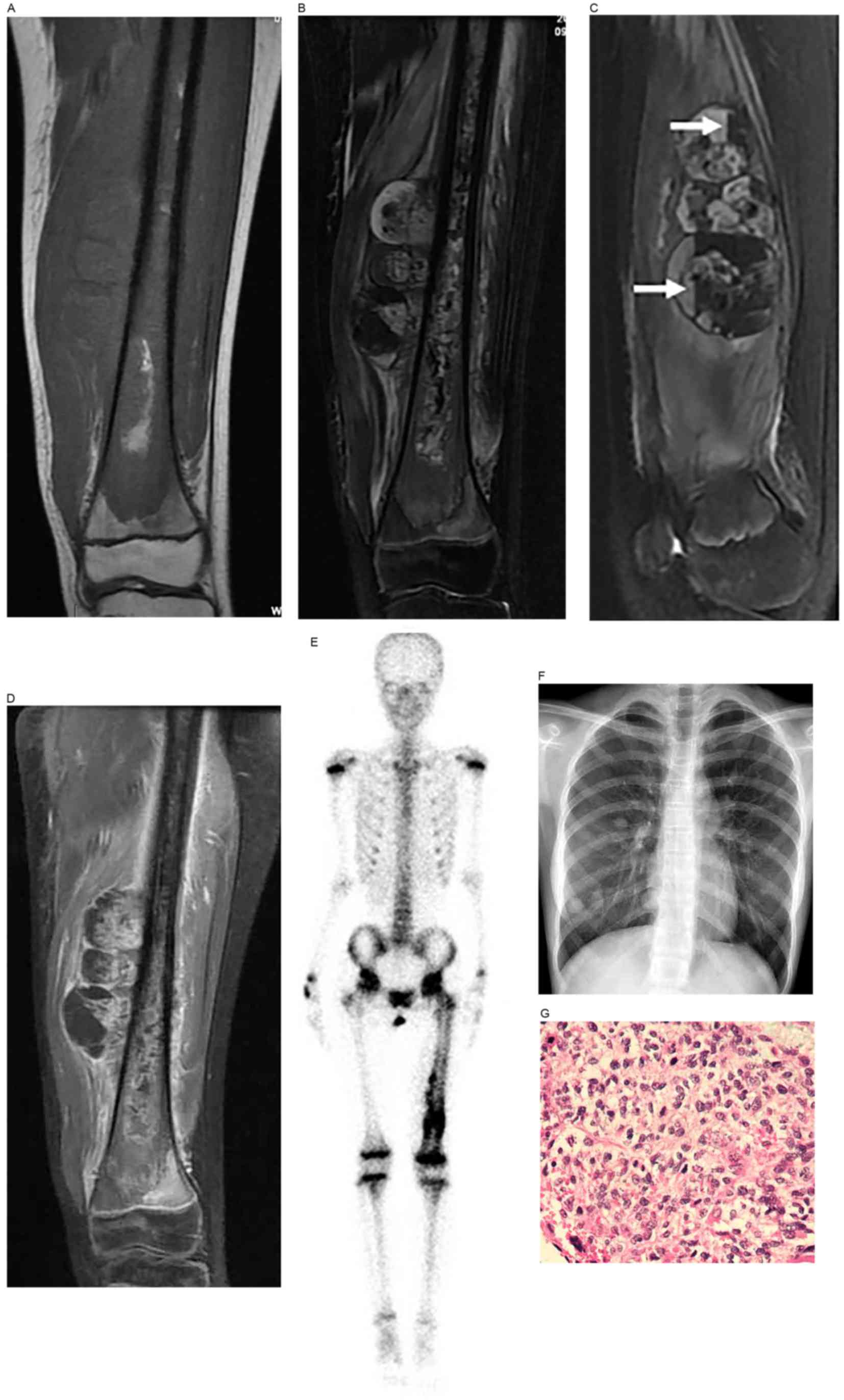
MRI showed inhomogeneous bone signals of the lesions
produced in the case of telangiectatic osteosarcoma, with low
signals on T1WI and high mixed signals on T2WI. The surrounding
soft tissues showed mixed signals and contained multiple cysts
showing fluid-fluid levels (Fig.
2).
Ewing sarcoma
There were 5 cases of Ewing sarcoma (3 males and 2
females), with ages ranging from 4 to 17 years. The lesions were
located in the right proximal femur of 3 cases, the sacral
vertebrae of 1 case and the rib of 1 case. The size of the tumors
ranged from 6.3–19.7 cm. A pathological fracture was found in one
case, whereas another case exhibited lung metastasis and multiple
vertebral metastases.
Radiography (2/5) and CT (3/5) showed infiltrative
bone destruction and soft-tissue masses. Lesions in the long bone
showed a laminar periosteal reaction in 2 cases (Fig. 3), and the case with the pathological
fracture showed a ruptured periosteum. Enhanced CT (1/5) showed
marked inhomogeneous enhancement of the tumor, with enhanced
vessels present within.
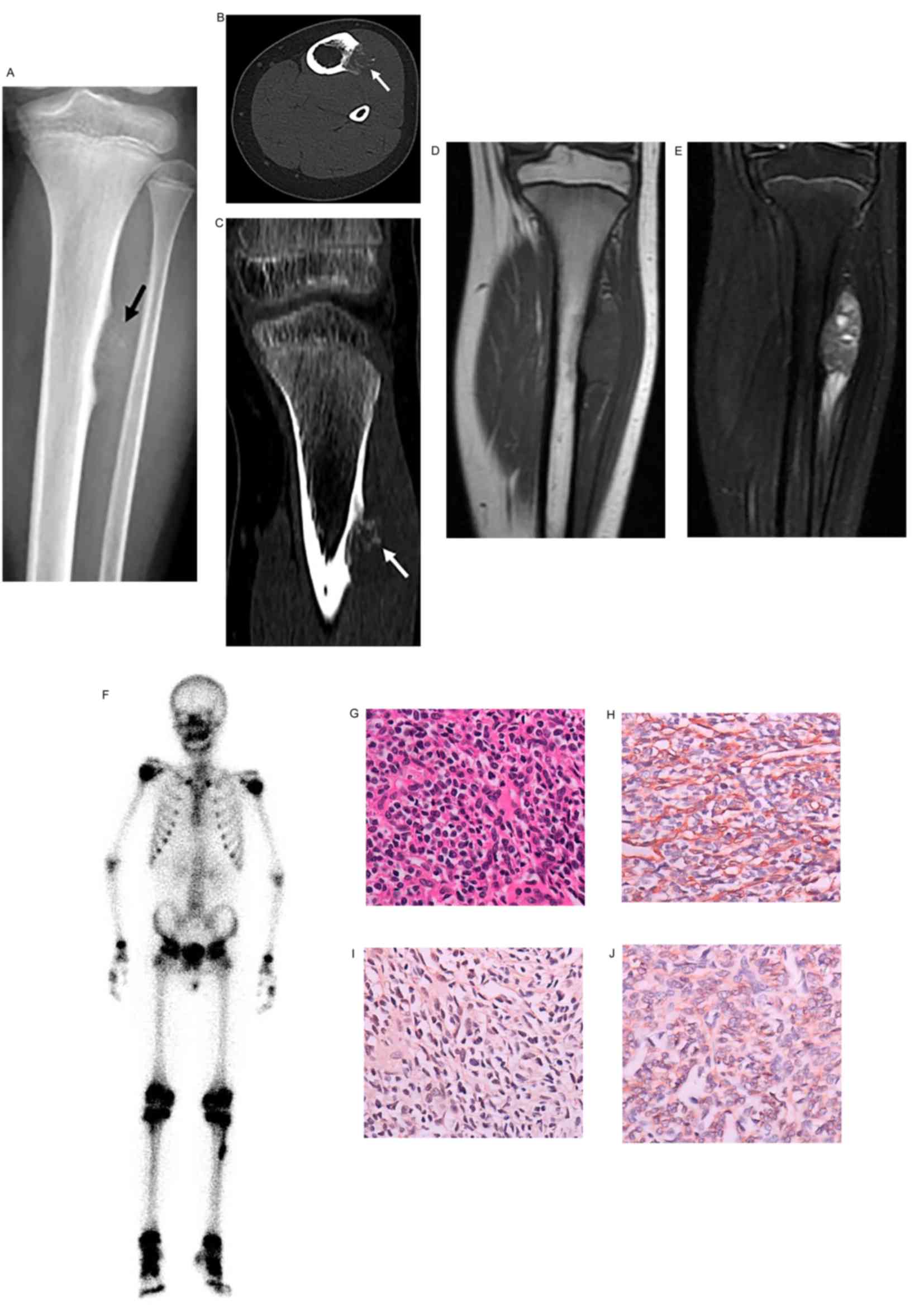
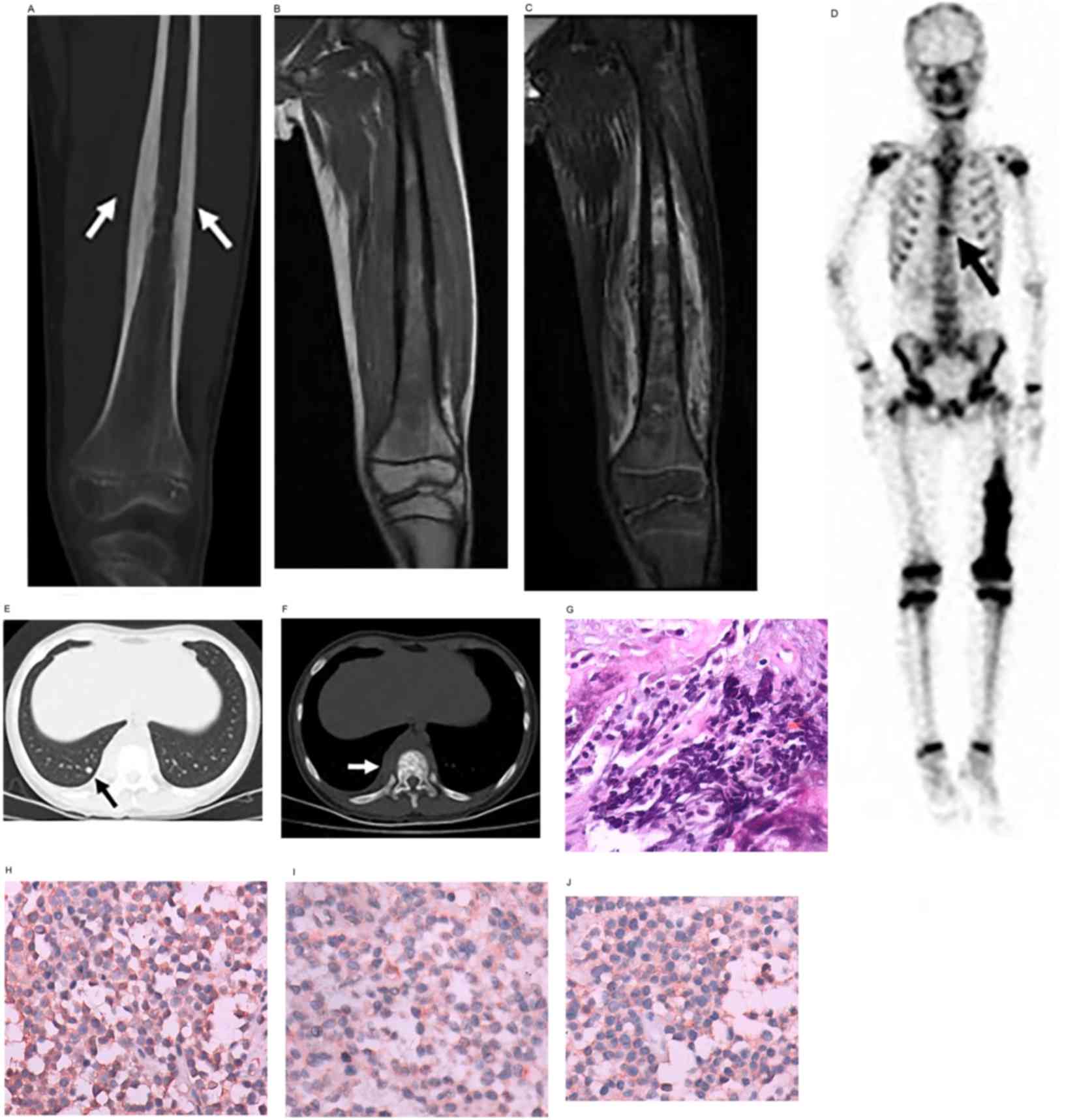 | Figure 3.Ewing sarcoma in a male patient aged
11 years. Ewing sarcoma of the left femur. (A) Coronal
reconstruction of CT showing laminar periosteal thickening of the
femoral diaphysis (arrows), with osteoid matrix in the medullary
cavity. (B) Coronal T1WI and (C) fat suppression T2WI showing
inhomogeneous bone signals in the area of the lesion, with low
signal and high mixed signal, and a soft-tissue mass with mixed
signal, respectively. (D) Whole-body technetium-99 m
methylenediphosphonate bone scan showing increased radionuclide
uptake of the lower and middle sections of the left femur, and
increased radionuclide uptake at the T10 vertebra (arrow), which
indicates bone metastasis. Axial CT of the chest in (E) lung
windows showing metastatic nodules in the lungs (arrow), and in (F)
bone windows showing osteoblastic bone metastasis of the T10
vertebra, with surrounding soft-tissue swelling (arrow). (G)
Photomicrograph showing uniform, small round tumor cells (original
magnification, ×400; hematoxylin and eosin staining). (H)
Vimentin-positive, (I) cluster of differentiation 99-positive and
(J) neuron-specific enolase-positive tissues (original
magnification, ×400; immunohistochemical staining). The diagnosis
was of Ewing sarcoma. CT, computed tomography; WI, weighted
images. |
MRI (4/5) of tumors showed equal or slightly lower
signal compared with adjacent normal bone marrow on T1WI and high
mixed signal on T2WI. Enhanced MRI (1/5) showed markedly
inhomogeneous enhancement. Bone scintigraphy (4/5) showed the
increased radionuclide uptake of the lesions.
Chondrosarcoma
There were 3 cases of chondrosarcoma (2 males and 1
female), with ages ranging from 10 to 16 years. The lesions were
located in the proximal tibia, distal femur and os pubis. The size
of the tumors ranged from 4.9–10.1 cm. Overall, 1 case was a
central/intramedullary chondrosarcoma and 2 cases were
periosteal/juxtacortical chondrosarcomas. The histopathology
included 2 cases of mesenchymal chondrosarcoma and 1 case of grade
2 chondrosarcoma Chondrosarcoma are histologically differentiated
into World Health Organization grade 1, 2 or 3, depending on
cellularity, cellular atypia and mitosis (9). A single case had a pathological
fracture.
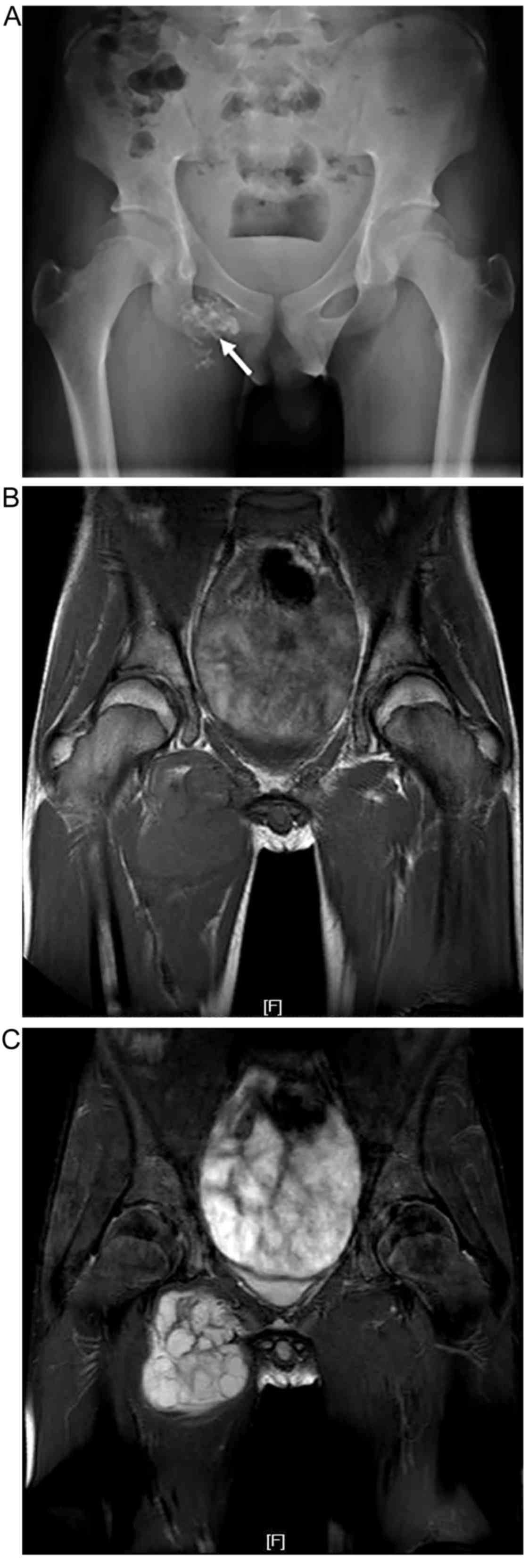
The lesion of 1 case (periosteal/juxtacortical
chondrosarcoma) was adjacent to the cortex of the proximal tibia,
and was shown on radiography and CT as local destruction of the
cortical bone of the lateral tibia, with a soft-tissue mass
containing scattered punctate and amorphous calcifications. MRI
showed long T1 W/long T2 W mixed signals. The pathological
diagnosis was of mesenchymal chondrosarcoma (Fig. 4).
The lesion of another case (central/intramedullary
chondrosarcoma) was located in the medullary cavity of the distal
femur. Radiography showed a local periosteal reaction. MRI showed a
low signal on T1WI and a high mixed signal on T2WI, with a
periosteal reaction and soft-tissue mass. The epiphysis and
epiphyseal plate were involved. The histopathological diagnosis was
of mesenchymal chondrosarcoma.
Radiography and CT of 1 case showed local bone
destruction of the right pubic ramus, with patchy punctate,
cambered calcifications in the surrounding soft-tissue mass. MRI of
the soft-tissue mass showed an equal signal on T1WI and a
heterogeneous high signal on T2WI. The calcifications and septum of
the mass showed low signals on T1WI and T2WI. The histopathological
diagnosis was of grade II chondrosarcoma (Fig. 5).
Of the total cases, 2 underwent bone scintigraphy,
which showed foci of increased radionuclide uptake.
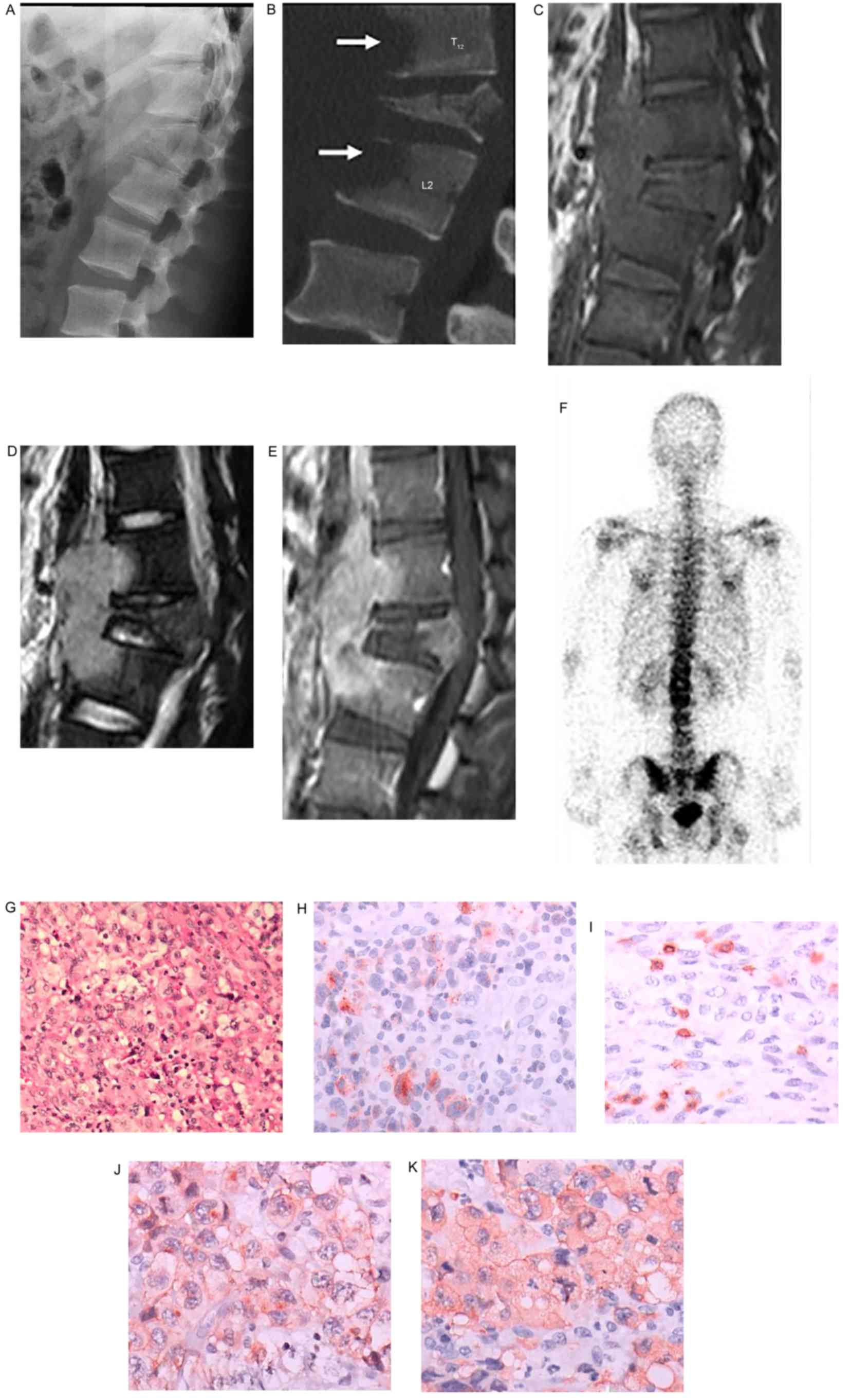
Primary non-Hodgkin lymphoma of the
bone
There was 1 case (male, 17 years old) of primary
non-Hodgkin lymphoma of the bone involving the T12, L1 and L2
vertebrae. The tumor measured 2.7×4.1×6.5 cm. No lymph nodes and
internal organs were involved. CT showed infiltrative bone
destruction of the vertebrae, a soft-tissue mass and a compression
fracture of the L1 vertebra, without an evident periosteal
reaction. MRI showed inhomogeneous shadowing with long-equal
T1WI/long T2WI signals of the vertebrae. The spinal canal was
narrowed, and the spinal cord was compressed due to localized
kyphosis. The surrounding soft-tissue mass was shown as possessing
equal T1WI/long T2WI signals. The areas of the T12, L1 and L2
vertebrae, and the soft-tissue mass showed marked inhomogeneous
enhancement (Fig. 6). Bone
scintigraphy showed slightly increased radionuclide uptake of the
lesions. The pathological diagnosis was of non-Hodgkin lymphoma
(anaplastic large cell lymphoma).
Discussion
In the present study, there were only a few more
male patients than female patients, with an approximate ratio of
1:1. The age of onset of 27 of the 34 cases (79%) was <14 years,
indicating that the majority of the malignant bone tumors in the
cohort occurred in children (<14 years of age). The mean ages at
onset of Ewing sarcoma, osteosarcoma and chondrosarcoma were 8, 11
and 13 years, respectively. Osteosarcoma was the most common
primary malignant bone tumor (74%), followed by Ewing sarcoma
(15%), chondrosarcoma (8%) and lymphoma (3%). The most common site
of the osteosarcomas, Ewing sarcomas and chondrosarcomas was the
long bones. Overall, ~76% originated near the knee joint (distal
femur and proximal tibia), which is consistent with the results of
previous studies. The most common presenting symptom was pain, or
an enlarging soft-tissue mass and pathological fractures. However,
pathological fracture is one of the factors contributing to a poor
prognosis and is indicative of aggressive tumors (10). The most common sites of metastasis
were the lungs and other bones, while the lymph nodes and internal
organs were rarely involved. Skip metastases have been reported in
2–6.5% of osteosarcoma and 4–6% of Ewing sarcoma patients (6,11). Skip
lesions occurred in 8% of the osteosarcoma patients in the present
study, which is a slightly higher rate than that reported in the
literature. None of the patients with Ewing sarcoma developed skip
lesions.
The selection of imaging protocols for bone tumors
in children should follow the ‘As Low As Reasonably Achievable’
principle, which refers to minimizing the dose of ionizing
radiation to the child, while ensuring image quality (6). Therefore, conventional radiography is
the first choice, and in the majority of cases can identify benign
and malignant tumors.
Bone scintigraphy is a method with high sensitivity,
but low specificity. Due to osteogenetic activity or the increase
in blood flow to benign and malignant bone tumors, the uptake of
radionuclide in the lesions is increased. Therefore, in the
majority of cases, Bone scintigraphy cannot differentiate benign
from malignant tumors; however, it can be used to exclude benign
tumors without radionuclide uptake. In addition, Bone scintigraphy
is effective for detecting metastases of the skeletal system
(5,8).
CT is more effective than conventional radiography at revealing
subtle destruction of the bone cortex, the type of tumor matrix and
periosteal reactions. Three-dimensional CT is the best option for
bone tumors in the pelvis, scapula and other complex or overlapping
bones. Additionally, CT is useful for finding pulmonary metastases
(5,8).
MRI is superior for evaluating the extent of
intramedullary and soft-tissue masses. MRI is also markedly more
efficient at detecting skip lesions (5,8).
Functional MRI is able to differentiate not only
benign and malignant bone tumors, but to a certain degree, to also
depict the pathological processes of malignant bone tumors,
including cell apoptosis, cell proliferation and angiogenesis
(2,12–14).
Although functional MRI is in the research stage, the prospect for
clinical applications is promising. At present, functional MRI
techniques for the evaluation of bone tumors include the following:
i) Quantitative dynamic contrast-enhanced (DCE) -MRI can be used to
estimate the extent of necrosis in bone tumors. The percentage of
tumor necrosis is the most important outcome measure for evaluating
whether or not neoadjuvant therapy is clinically effective
(1). Studies have shown that tumor
necrosis of <90% indicates a high risk of local recurrence and a
short overall survival time (6,15). The
flux rate constant and dynamic vector magnitude (DVM) are commonly
used quantitative parameters. A DVM of >1.8 indicates a poor
response to treatment (necrosis <90%), while a DVW of <1.8
indicates a good response to treatment (necrosis >90%) (1). ii) Diffusion-weighted (DW)-MRI is used
to reflect the limitations of the diffusion of water in the body,
in the presence of a pathological process. The apparent diffusion
coefficient (ADC) is a quantitative index that reflects the
apparent freedom of diffusion. Low ADC values indicate areas where
diffusion is restricted by the intact cell membranes in tumor
tissues, while high ADC values indicate acellular regions.
Effective treatment can destroy the membranes of tumor cells,
resulting in the acceleration of water diffusion (12,13). A
marked increase in the ADC value after treatment can be used as a
marker for assessing tumor necrosis (1). Therefore DW-MRI can provide information
about tissue cellularity and cell integrity, and is also effective
at identifying benign and malignant tumors, and assessing response
to treatment. Whole-body DW-MRI can be applied to the detection of
primary tumors and bone metastases. iii) Magnetic resonance
spectroscopy (MRS) is applied to quantify the levels of different
metabolites, and thus depicts the molecular constitution of tumors.
Studies have shown a marked increase in choline levels in malignant
bone tumors. A choline/lipid ratio of >0.2 has been used to
diagnose malignant bone tumors, with a sensitivity of 76% and a
specificity of 88% (16). Therefore,
by quantitative detection of changes in metabolite concentrations,
this technique can identify tumor recurrence and assess the
response to treatment. iv) Blood-oxygen level-dependent imaging
(BOLD) is another functional MRI technique, first developed for
examining brain and now being used in other systems. The source of
BOLD MRI signal indicates the ratio of oxyhaemoglobin and
deoxygenated haemoglobin in the arterioles, capillaries and
post-capillary venules, which is mainly used to assess oxygenation
status in tumors. Oxygen concentration is a prognostic factor in
recurrence of solid tumors, thus a BOLD sequence can be part of
pre-therapy and follow-up MRI protocols in skeletal oncology
(17,18).
18F-FDG PET/CT is most commonly used for
the staging of malignant bone tumors; it plays important roles in
detecting metastasis, assessing recurrence, guiding clinical
surgery and radiotherapy, and assessing therapeutic efficacy
(5,8).
Osteosarcoma is the most common malignant bone tumor
in children (6). In the present
study, the majority of the lesions were located around the knee
joint (distal femur and proximal tibia), and over 33% of cases
involved the epiphysis, while 8% involved the diaphysis. These data
are consistent with the literature (5).
The most common bone tumor on imaging is mixed
osteosarcoma, which refers to the manifestation of osteolytic bone
destruction and osteoblastic bone sclerosis. In the present study,
mixed osteosarcoma accounted for 68% (17/25) of all osteosarcomas,
the osteolytic type accounted for 16% (4/25) and the osteoblastic
type accounted for 16% (4/25). Approximately 96% (24/25) showed the
Codman triangle and radial, spiculate, patchy and other aggressive
periosteal reactions. In total, 92% (23/25) showed a soft-tissue
mass, and 84% (21/25) showed osteoid matrix in the areas of bone
destruction and a soft-tissue mass. The 3 imaging features,
aggressive periosteal reaction, soft-tissue mass and osteoid matrix
are the most typical diagnostic signs for the diagnosis of
malignant bone tumors.
Telangiectatic osteosarcoma is rare, accounting for
1.2–7% of all osteosarcomas. Approximately 90% of the lesions are
located at the metaphysis of the long bones, and the femur (50%)
and tibia (25%) are the most commonly affected bones (19). Dilated blood-containing cavities with
fluid-fluid levels that are in the tumor are characteristic
features, and resemble aneurysmal bone cysts. However, a thickened
cyst wall and septum in a telangiectatic osteosarcoma can appear as
a solid node on enhanced imaging (20).
Ewing sarcoma is the second most common malignant
bone tumor in children (21). The
onset age of Ewing sarcoma is younger than the onset age of
osteosarcoma. In the present study, 80% of cases had an onset age
of <10 years. The most common site of Ewing sarcoma is the
pelvis, followed by the diaphysis of the long bones (femur, tibia,
humerus and rib) (1). However, data
from the present study revealed inconsistent results: The most
common sites of involvement were the diaphysis of the long bones,
then the pelvis. It was likely that this result was obtained
because the number of patients was too small for adequate
statistical analysis.
The imaging manifestations are moth-eaten,
permeative bone destruction, a soft-tissue mass adjacent to the
bone, and onion-skin or spiculate invasive periosteal reactions.
The soft-tissue mass around the tumor is often larger than the area
of bone destruction. The prognosis is poor when the size of the
tumor is >8 cm. Calcification is rare in the areas of bone
destruction and the soft-tissue mass (6,19).
Chondrosarcoma is rare in children. The majority of
these tumors are primary low-grade chondrosarcomas. As little as
0.5% of low-grade chondrosarcomas arise secondarily from benign
chondroid lesions (22).
Chondrosarcomas are most commonly located in the femur, pelvis or
shoulder, and originate from the proximal femur in one-third of
cases. Approximately 50% are located at the metaphysis, 35% at the
diaphysis and 15% at the epiphysis (22).
The size of chondrosarcoma is large, normally >4
cm, or even up to 10–15 cm. In the majority of cases, conventional
radiography reveals osteolytic bone destruction and a punctate,
patchy, circular and cambered cartilaginous matrix. However,
differentiating a low-grade chondrosarcoma from enchondroma is
difficult. The following are the key identifying features (8,22): i) The
depth of endosteal scalloping is more than two-thirds of the
thickness of the bone cortex on radiography or CT. ii) The extent
of edema around the chondrosarcoma is often larger than that of an
enchondroma on MRI. iii) A chondrosarcoma shows peripheral, nodular
or septal enhancement on contrast-enhanced CT or MRI, whereas an
enchondroma only shows peripheral enhancement. DCE-MRI reveals a
fast enhancement rate for chondrosarcoma. iv) 18FDG
PET-CT SUVs of >2.0 have a 91% sensitivity, 100% specificity and
95% accuracy for identifying chondrosarcoma. v) Whole-body
99mTc-MDP bone scans show higher radionuclide uptake in
a chondrosarcoma than the uptake in the normal iliac crest.
Mesenchymal chondrosarcoma is a rare tumor,
accounting for only 3–10% of all chondrosarcomas, and is highly
malignant (6). Approximately 70% of
mesenchymal chondrosarcomas occur in young adults aged 20 to 30
years (23). The 2 cases of
mesenchymal chondrosarcoma in the present study showed bone
destruction and a punctate, circular cartilaginous matrix, with no
characteristic imaging features. Therefore histopathological
examination was required for a definitive diagnosis.
Histologically, this biphasic tumor of the cartilage is composed of
hyaline cartilage and a small round cell component, and is
extremely rare (24).
Primary bone lymphoma accounts for <5% of all
primary bone tumors, and is more common in males, with a
male/female ratio of 1.5:1. The majority of the cases are
non-Hodgkin lymphoma (25). Primary
bone lymphoma in children is likely to occur in the spine or the
diaphyses of the long bones. The following are the diagnostic
criteria of primary bone lymphoma proposed by the World Health
Organization (26): i) Single-bone
invasion, with/without local lymph node involvement; and ii)
multiple-bone invasion, without involvement of the lymph nodes and
internal organs. CT of the chest, abdomen and pelvis must be
performed to exclude lymph node metastasis, which indicates stage
IV disease.
The present study contained 1 patient with primary
bone lymphoma with multiple bone involvement, which had been
misdiagnosed as vertebral tuberculosis preoperatively. According to
the literature, primary bone lymphoma with multiple-bone
involvement is prone to occur in the vertebrae, as opposed to
primary bone lymphoma with single-bone involvement. Primary bone
lymphoma can infiltrate tissues through the vascular channels of
the bone cortex; therefore, the bone cortex may not be damaged
during the formation of a soft-tissue mass (1,8), and there
also may not be a marked periosteal reaction. Calcifications are
rare, and sequestra can be observed in a few cases (19).
In general, children cannot provide a clear medical
history of their complaints; therefore, useful diagnostic
information may be difficult to obtain. In addition, children may
not discover the problem early in the course of the disease, and
parents may not observe the signs of disease. Therefore, children
are examined by a physician when the lesions are large, or when
they have symptoms caused by pressure or metastatic disease, which
negatively affects the chances for early diagnosis and treatment,
and the resultant prognosis. At present, there are few pediatric
oncologists in China (27), and lay
people have limited awareness of pediatric tumors. Therefore,
children with primary malignant bone tumors often do not receive
timely and effective treatment.
In conclusion, in the present study cohort and in
general, osteosarcoma is the most common primary malignant bone
tumor in children, followed by Ewing sarcoma, chondrosarcoma and
lymphoma. Osteosarcoma is more frequently found in children <14
years old, and is most commonly located in the long tubular bones
of the lower extremities. Osteoblastic or osteolytic bone
destruction, an invasive periosteal reaction, a soft-tissue mass,
the tumor matrix and inhomogeneous enhancement are important
imaging features of malignant bone tumors. Conventional radiography
is the preferred method of examination, and CT can be used as a
supplementary method. MRI is used to determine the extent of tumor
invasion, and combined with functional MRI and 18F-FDG
PET/CT, can be used for preoperative evaluation and treatment
assessment.
References
|
1
|
Wootton-Gorges SL: MR Imaging of primary
bone tumors and tumor-like conditions in children. Magn Reson
Imaging Clin N Am. 17:469–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brisse H, Ollivier L, Edeline V,
Pacquement H, Michon J, Glorion C and Neuenschwander S: Imaging of
malignant tumours of the long bones in children: Monitoring
response to neoadjuvant chemotherapy and preoperative assessment.
Pediatr Radiol. 34:595–605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collins M, Wilhelm M, Conyers R, Herschtal
A, Whelan J, Bielack S, Kager L, Kühne T, Sydes M, Gelderblom H, et
al: Benefits and adverse events in younger versus older patients
receiving neoadjuvant chemotherapy for osteosarcoma: Findings from
a meta-analysis. J Clin Oncol. 31:2303–2312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wright EH, Gwilym S, Gibbons CL, Critchley
P and Giele HP: Functional and oncological outcomes after
limb-salvage surgery for primary sarcomas of the upper limb. J
Plast Reconstr Aesthet Surg. 61:382–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eftekhari F: Imaging assessment of
osteosarcoma in childhood and adolescence: Diagnosis, staging and
evaluating response to chemotherapy. Cancer Treat Res. 152:33–62.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaste SC: Imaging pediatric bone sarcomas.
Radiol Clin North Am. 49:749–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costelloe CM and Madewell JE: Radiography
in the initial diagnosis of primary bone tumors. AJR Am J
Roentgenol. 200:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rana KA, Meyer J, Ibrahim S, Ralls M and
Kent PM: The role of imaging of malignant bone tumors in children
and young adults. Curr Probl Cancer. 37:181–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: World Health Organization classification of tumours
of soft tissue and bone [M]. Lyon: IARC Press; pp. 264–81. 2013
|
|
10
|
Lee RK, Chu WC, Leung JH, Cheng FW and Li
CK: Pathological fracture as the presenting feature in pediatric
osteosarcoma. Pediatr Blood Cancer. 60:1118–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphey MD, Senchak LT, Mambalam PK, Logie
CI, Klassen-Fischer MK and Kransdorf MJ: From the radiologic
pathology archives: Ewing sarcoma family of tumors:
Radiologic-pathologic correlation. Radiographics. 33:803–831. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fayad LM, Jacobs MA, Wang X, Carrino JA
and Bluemke DA: Musculoskeletal tumors: How to use anatomic,
functional and metabolic MR techniques. Radiology. 265:340–356.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benassi MS, Rimondi E, Balladelli A,
Ghinelli C, Magagnoli G and Vanel D: The role of imaging for
translational research in bone tumors. Eur J Radiol. 82:2115–2123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang S and Panicek DM: The evolution of
musculoskeletal tumor imaging. Radiol Clin North Am. 47:435–453.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meyers PA, Gorlick R, Heller G, Casper E,
Lane J, Huvos AG and Healey JH: Intensification of preoperative
chemotherapy for osteogenic sarcoma: Results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol. 16:2452–2458. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Cheng K, Ding Y, Liang W, Ding Y,
Vanel D and Cheng X: Study of single voxel 1H MR spectroscopy of
bone tumors: Differentiation of benign from malignant tumors. Eur J
Radiol. 82:2124–2128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dallaudiere B, Hummel V, Hess A, Lincot J,
Preux PM, Maubon A and Monteil J: Tumoral hypoxia in osteosarcoma
in rats: Preliminary study of blood oxygenation level-dependent
functional MRI and 18F-misonidazole PET/CT with diffusion-weighted
MRI correlation. AJR Am J Roentgenol. 200:187–192. 2013. View Article : Google Scholar
|
|
18
|
Padhani AR, Krohn KA, Lewis JS and Alber
M: Imaging oxygenation of human tumours. Eur Radiol. 17:861–872.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yarmish G, Klein MJ, Landa J, Lefkowitz RA
and Hwang S: Imaging characteristics of primary osteosarcoma:
Nonconventional subtypes. Radiographics. 30:1653–1672. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphey MD, wan Jaovisidha S, Temple HT,
Gannon FH, Jelinek JS and Malawer MM: Telangiectatic osteosarcoma:
Radiologic-pathologic comparison. Radiology. 229:545–553. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balamuth NJ and Womer RB: Ewing's sarcoma.
Lancet Oncol. 11:184–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mosier SM, Patel T, Strenge K and Mosier
AD: Chondrosarcoma in childhood: The radiologic and clinical
conundrum. J Radiol Case Rep. 6:32–42. 2012.PubMed/NCBI
|
|
23
|
Küpeli S, Varan A, Gedikoğlu G and
Büyükpamukçu M: Sacral mesenchymal chondrosarcoma in childhood: A
case report and review of the literature. Pediatr Hematol Oncol.
27:564–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qasem SA and DeYoung BR: Cartilage-forming
tumors. Semin Diagn Pathol. 31:10–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carroll G, Breidahl W and Robbins P:
Musculoskeletal lymphoma: MRI of bone or soft tissue presentations.
J Med Imaging Radiat Oncol. 57:663–673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demircay E, Hornicek FJ Jr, Mankin HJ and
Degroot H III: Malignant lymphoma of bone: A review of 119
patients. Clin Orthop Relat Res. 471:2684–2690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu KJ, Sun ZZ, Rui YJ, Mi JY and Ren MX:
Shortage of paediatricians in China. Lancet. 383:9542014.
View Article : Google Scholar : PubMed/NCBI
|