Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide (1). Over the last
20 years, and the last decade in particular, the clinical outcome
for metastatic CRC patients has improved greatly due to patients
undergoing advanced surgical resection of localized metastasis and
advanced systemic chemotherapy (2,3). The
leucovorin (FOL) and fluorouracil (5-FU) plus oxaliplatin (l-OHP;
FOLFOX) or FOL and 5-FU plus irinotecan (SN-38; FOLFIRI) with
molecularly-targeted drugs are used as first-line chemotherapy
regimens worldwide in the treatment of advanced CRC (4,5). Recently
studies have revealed that the median survival time (MST) of
advanced CRC with the chemotherapy was >30 months with the
integration of multiple cytotoxic agents and molecularly-targeted
therapies (6–8). It is common knowledge that the treatment
period of the first-line chemotherapy is the longest, and that the
response rate of the first-line chemotherapy is the highest
(9). However, second-line
chemotherapy must be abandoned in certain cases due to disease
progression and adverse effects. In addition, high medical costs
have been reported to be a significant problem (10–14).
Therefore, a more effective regimen should be selected as
first-line chemotherapy treatment in a clinical setting.
A previous report demonstrated that
individualization of first-line chemotherapy was possible using the
collagen gel droplet-embedded culture drug sensitivity test
(CD-DST) and individualized first-line chemotherapy using CD-DST
may improve the prognosis of patients with unresectable CRC
(15,16).
The aim of this prospective cohort study was to
evaluate the individualization of first-line chemotherapy using
CD-DST, focusing on prognosis.
Materials and methods
Patients
During the period between March 2008 and December
2015, tumor specimens were obtained from 120 patients with CRC.
Lymph node metastasis and/or distant metastasis was reported in
these patients. No patient was treated with preoperative
chemotherapy or chemoradiotherapy. Written informed consent for
measurement of individual chemosensitivity was obtained from all
patients. Approval for the present study was obtained from the Tobu
Chiiki Hospital Institutional Review Board (No. 02.03.29. #1).
Methods
The CD-DST was performed using a Human Cancer
Primary Culture System Kit; Primastarä (Kurabo Industries, Ltd.,
Chuo-ku, Osaka, Japan). Tumor tissue was excised from primary
surgical specimens and subjected to the CD-DST. The CD-DST allows
for the evaluation of drug sensitivity using isolated
3-dimensionally cultured tumor cells in a small collagen gel
droplet, and was used to evaluate the sensitivity of the tumors to
5-FU, which was performed according to a previous description by
Kobayashi et al (17,18). Each specimen was washed 5 times with
50 ml saline, followed by additional washing 5 times with 50 ml
antibiotic fluid containing 1.0 mg/ml piperacillin and 0.5 mg/ml
kanamycin. The transport bottle contained 1.0 mg/ml piperacillin,
0.5 mg/ml kanamycin and 2.5 µg/ml amphotericin B. Tissue (1 g) was
treated for 2 h at 37°C with a cocktail containing 1.0% dispersion
enzyme EZ™ (Kurabo Industries, Ltd.). Dispersed cell suspensions
were inoculated into pre-culture media on collagen-coated flasks
(CG-flusk™, Kurabo Industries, Ltd.) overnight. Viable tumor cells
were subsequently recovered by 0.05% collagenase treatment.
Recovered cells were embedded in 30 µl collagen gel droplets.
The embedded cells were cultivated in culture media
containing 5-FU and l-OHP at 6.0 and 3.0 µg/ml (FOLFOX regimen), or
5-FU and SN-38 at 6.0 and 0.2 µg/ml (FOLFIRI regimen),
respectively, for 24 h at 37°C. Following the removal of the
anticancer agent-containing media, cells were additionally cultured
for 7 days in serum-free culture media (PCM-2™, Kurabo Japan) to
prevent the growth of fibroblasts. Viable cells were stained with
neutral red solution and counted using the imaging colorimetric
quantification method (Primage™, Kurabo Japan). The surviving cell
number ratio between the drug-treated and control group, which
received no drug treatment, was calculated. A growth rate <0.8
was regarded a successful culture.
The histograms and the cumulative distributions of
the growth inhibition rates (IRs) under the two conditions were
evaluated based on the evidence that the clinical response rates to
FOLFOX and FOLFIRI were ~50% (9,19–21). Therefore, taking the median of the
histogram as the cut off value in each regimen, the patients were
divided into responder and poor responder.
All patients were divided into 4 cohorts: FOLFOX and
FOLFIRI responder (dual responder), FOLFOX responder, FOLFIRI
responder and poor responder.
All patients were divided into 3 cohorts: FOLFOX
recommended, FOLFIRI recommended and the two regimens
recommended.
The patients with the chemotherapy were divided into
2 cohorts: Treated with appropriate first-line regimen and treated
with inappropriate first-line regimen.
First-line regimens were selected by the attending
physician. Frequencies of chemotherapy and prognosis were
prospectively evaluated and compared among the cohorts.
Statistical analysis
Histograms were analyzed with the D'Agostino-Pearson
omnibus normality test. Frequencies of chemotherapy were compared
between the two cohorts using the t-test. The MST was calculated by
the Kaplan-Meier method. The overall survival (OS) curves of the
two cohorts were compared by the log-rank test. Data are presented
as the means ± standard deviation (SD) and were analyzed using
GraphPad Prism (version 5.04; GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics are demonstrated in Table I. The median follow-up period was 1124
days. The individual growth IRs under each of the two conditions
are presented in Table II.
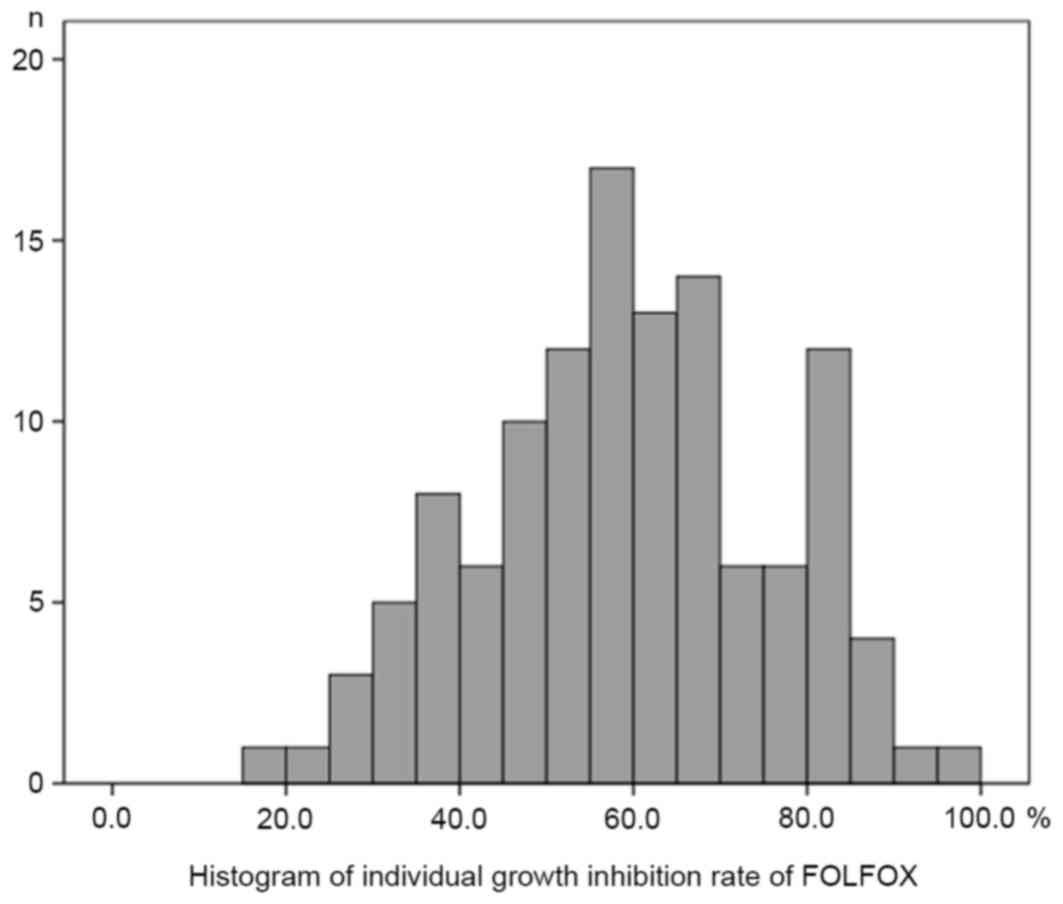
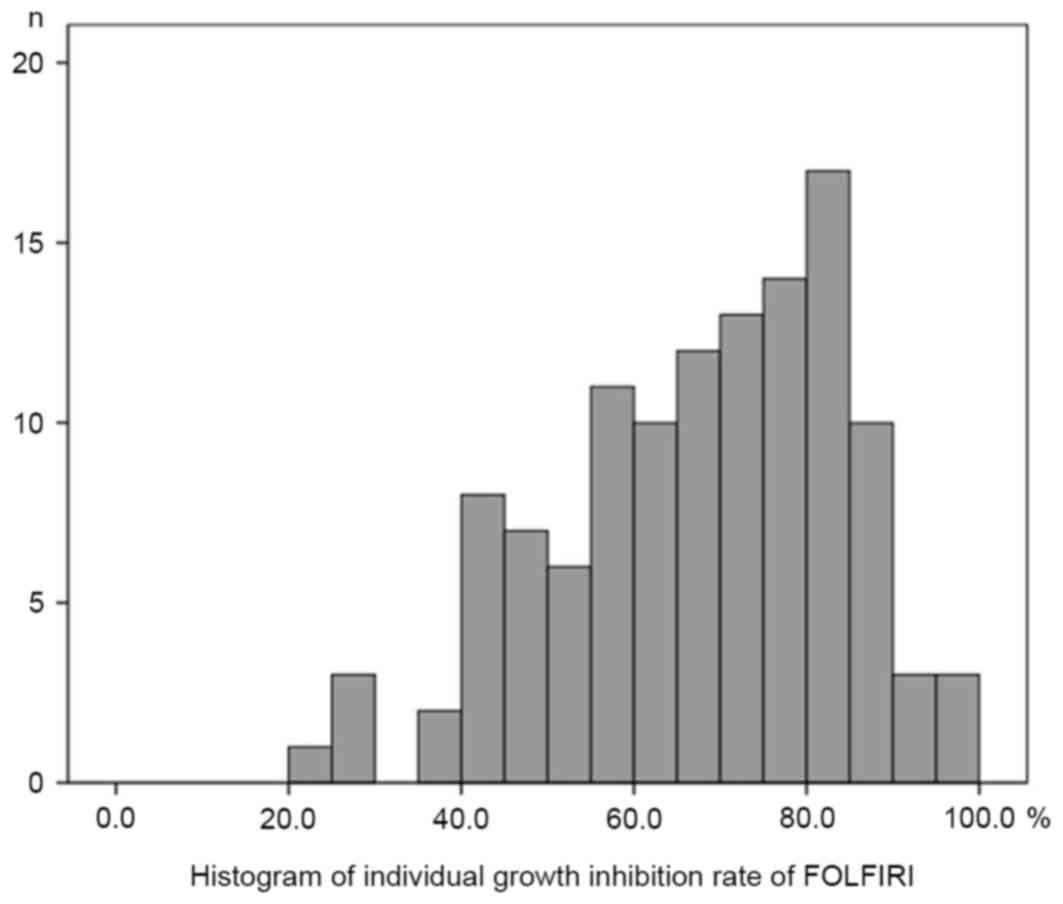
Histograms of the individual growth IRs (%) under the conditions of
the FOLFOX regimen and FOLFIRI regimen are presented in Figs. 1 and 2,
respectively. The median, mean, SD and standard error (SE) of the
mean with the FOLFOX regimen were 59.6, 59.5, 16.8 and 1.54,
respectively. The median, mean, SD and SE of the mean with the
FOLFIRI regimen were 70.0, 67.7, 16.8 and 1.53, respectively. The
histograms passed the normality test (α=0.05; FOLFOX regimen,
P=0.52; FOLFIRI regimen, P=0.07).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Value |
|---|
| Age, years, mean
(range) | 66.1 (36–83) |
| Gender |
|
|
Male/female | 79/41 |
| Histological
type |
|
|
Papillary adenocarcinoma | 2 |
| Well
differentiated adenocarcinoma | 22 |
|
Moderately differentiated
adenocarcinoma | 80 |
| Poorly
differentiated adenocarcinoma | 6 |
|
Mucinous adenocarcinoma | 9 |
|
Squamous cell carcinoma | 1 |
| Primary tumor
site |
|
|
Colon/rectum | 76/44 |
 | Table II.Growth inhibition rates (%) of FOLFOX
and FOLFIRI. |
Table II.
Growth inhibition rates (%) of FOLFOX
and FOLFIRI.
| Patient no. | FOLFOX | FOLFIRI |
|---|
| 1 | 80.1 | 82.9 |
| 2 | 71.3 | 79.2 |
| 3 | 81.2 | 83.4 |
| 4 | 60.0 | 68.7 |
| 5 | 29.9 | 66.5 |
| 6 | 69.7 | 89.6 |
| 7 | 58.7 | 63.2 |
| 8 | 73.0 | 85.2 |
| 9 | 63.2 | 75.9 |
| 10 | 77.9 | 85.5 |
| 11 | 76.3 | 85.6 |
| 12 | 53.6 | 62.6 |
| 13 | 41.9 | 60.7 |
| 14 | 81.3 | 80.9 |
| 15 | 42.3 | 70.2 |
| 16 | 84.8 | 86.8 |
| 17 | 75.9 | 83.9 |
| 18 | 59.2 | 76.4 |
| 19 | 69.9 | 85.5 |
| 20 | 57.0 | 49.7 |
| 21 | 79.2 | 83.0 |
| 22 | 86.1 | 89.1 |
| 23 | 67.3 | 74.4 |
| 24 | 81.3 | 85.2 |
| 25 | 60.4 | 71.9 |
| 26 | 93.4 | 98.6 |
| 27 | 62.6 | 84.9 |
| 28 | 58.5 | 54.8 |
| 29 | 81.2 | 84.0 |
| 30 | 66.5 | 73.3 |
| 31 | 81.3 | 78.1 |
| 32 | 59.9 | 74.1 |
| 33 | 53.3 | 65.0 |
| 34 | 49.3 | 48.3 |
| 35 | 44.7 | 49.3 |
| 36 | 68.8 | 72.1 |
| 37 | 59.7 | 69.4 |
| 38 | 50.8 | 59.3 |
| 39 | 51.6 | 56.5 |
| 40 | 57.9 | 70.2 |
| 41 | 58.5 | 63.7 |
| 42 | 62.4 | 72.5 |
| 43 | 15.2 | 21.5 |
| 44 | 82.9 | 84.3 |
| 45 | 66.3 | 68.1 |
| 46 | 46.7 | 57.2 |
| 47 | 71.3 | 70.0 |
| 48 | 32.6 | 43.2 |
| 49 | 56.4 | 59.2 |
| 50 | 63.4 | 64.6 |
| 51 | 59.7 | 55.3 |
| 52 | 46.2 | 45.4 |
| 53 | 68.7 | 77.7 |
| 54 | 49.2 | 42.7 |
| 55 | 31.5 | 43.8 |
| 56 | 35.5 | 41.5 |
| 57 | 69.5 | 76.6 |
| 58 | 57.0 | 53.7 |
| 59 | 53.7 | 55.8 |
| 60 | 72.4 | 83.4 |
| 61 | 29.4 | 43.8 |
| 62 | 37.7 | 65.1 |
| 63 | 68.4 | 77.2 |
| 64 | 47.9 | 73.7 |
| 65 | 58.8 | 69.1 |
| 66 | 50.5 | 76.5 |
| 67 | 74.3 | 81.9 |
| 68 | 36.4 | 46.3 |
| 69 | 81.4 | 82.4 |
| 70 | 68.0 | 80.9 |
| 71 | 64.3 | 78.2 |
| 72 | 69.9 | 83.7 |
| 73 | 40.7 | 58.0 |
| 74 | 61.4 | 55.3 |
| 75 | 60.0 | 79.5 |
| 76 | 44.8 | 60.2 |
| 77 | 73.3 | 93.9 |
| 78 | 66.6 | 79.8 |
| 79 | 27.8 | 25.5 |
| 80 | 47.7 | 66.2 |
| 81 | 51.5 | 62.6 |
| 82 | 59.4 | 69.9 |
| 83 | 30.6 | 26.6 |
| 84 | 68.6 | 75.6 |
| 85 | 66.5 | 81.8 |
| 86 | 75.0 | 73.5 |
| 87 | 56.3 | 57.1 |
| 88 | 58.0 | 69.0 |
| 89 | 31.0 | 47.3 |
| 90 | 84.5 | 89.5 |
| 91 | 42.1 | 26.9 |
| 92 | 51.1 | 54.2 |
| 93 | 86.8 | 92.0 |
| 94 | 54.8 | 56.9 |
| 95 | 52.0 | 70.1 |
| 96 | 49.4 | 71.7 |
| 97 | 23.7 | 53.5 |
| 98 | 45.4 | 63.0 |
| 99 | 47.9 | 52.6 |
| 100 | 64.6 | 63.5 |
| 101 | 64.9 | 78.5 |
| 102 | 53.9 | 61.4 |
| 103 | 45.4 | 59.9 |
| 104 | 60.1 | 79.2 |
| 105 | 36.0 | 37.1 |
| 106 | 59.1 | 81.3 |
| 107 | 60.1 | 69.4 |
| 108 | 34.5 | 39.9 |
| 109 | 39.8 | 47.3 |
| 110 | 39.0 | 44.9 |
| 111 | 38.3 | 43.3 |
| 112 | 39.9 | 40.3 |
| 113 | 54.6 | 66.8 |
| 114 | 79.0 | 86.8 |
| 115 | 88.1 | 95.0 |
| 116 | 58.6 | 52.0 |
| 117 | 88.5 | 94.1 |
| 118 | 84.9 | 84.6 |
| 119 | 83.3 | 83.7 |
| 120 | 99.7 | 99.9 |
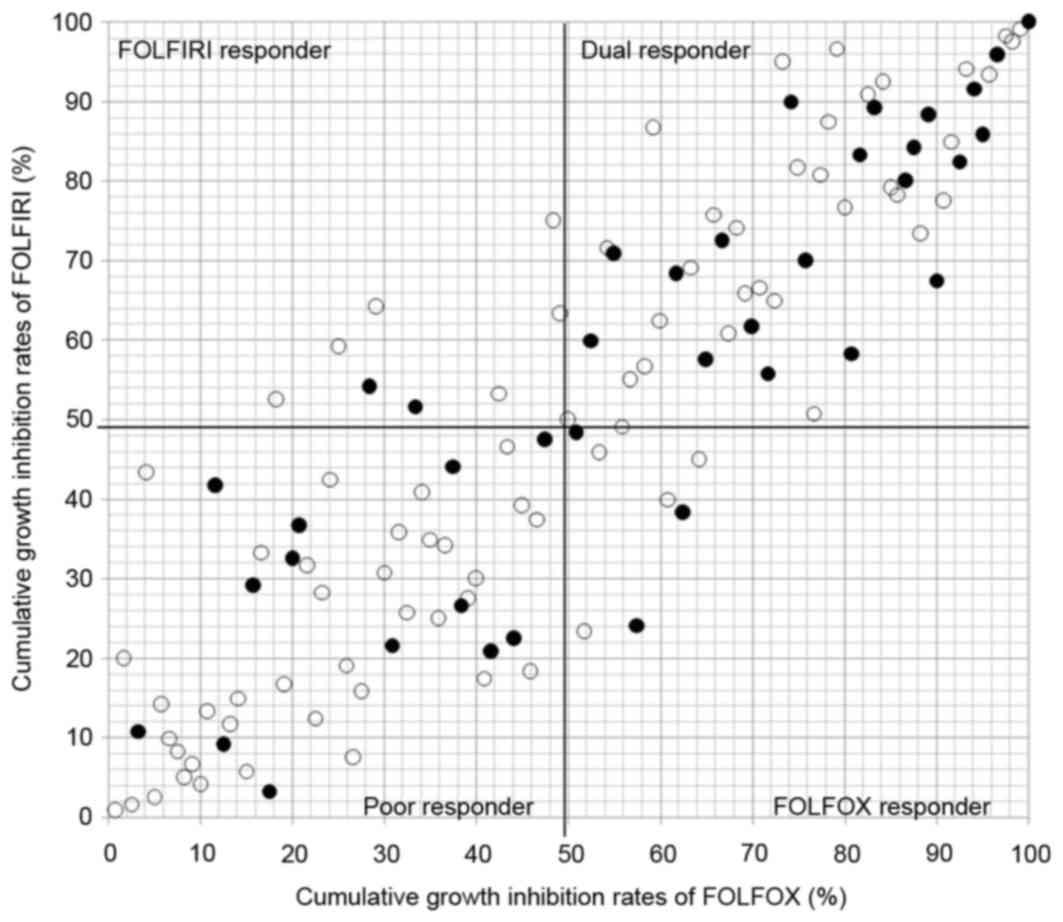
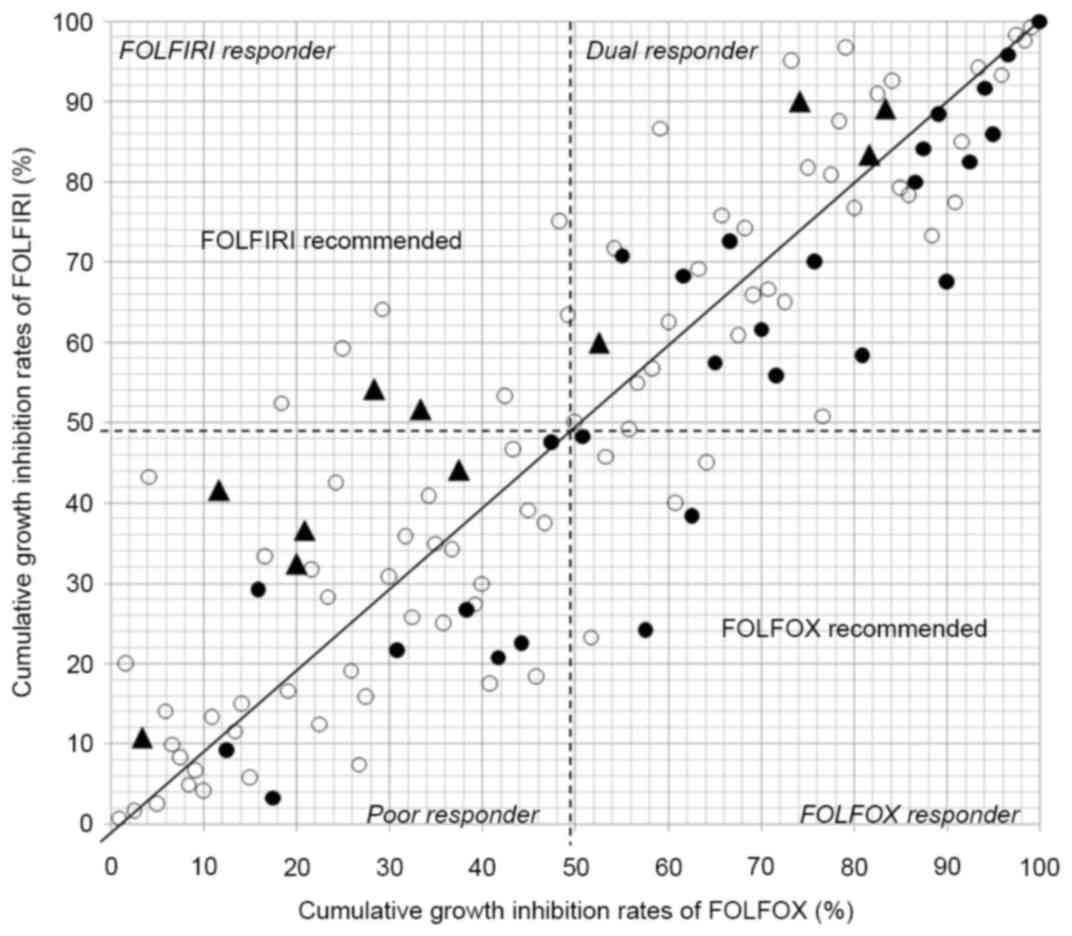
Four cohorts (dual, FOLFOX, FOLFIRI,
and poor responder)
The 4 cohorts based on the cumulative distribution
of the individual growth IRs between the two conditions is shown in
Fig. 3. Individualization of
first-line chemotherapy was possible in all 120 patients, with dual
responder, FOLFOX responder, FOLFIRI responder and poor responder
in 53, 8, 8, and 51 patients, respectively. Thirty-nine of the
patients eventually received the chemotherapy in dual responder
(n=21), FOLFOX responder (n=3), FOLFIRI responder (n=2) and poor
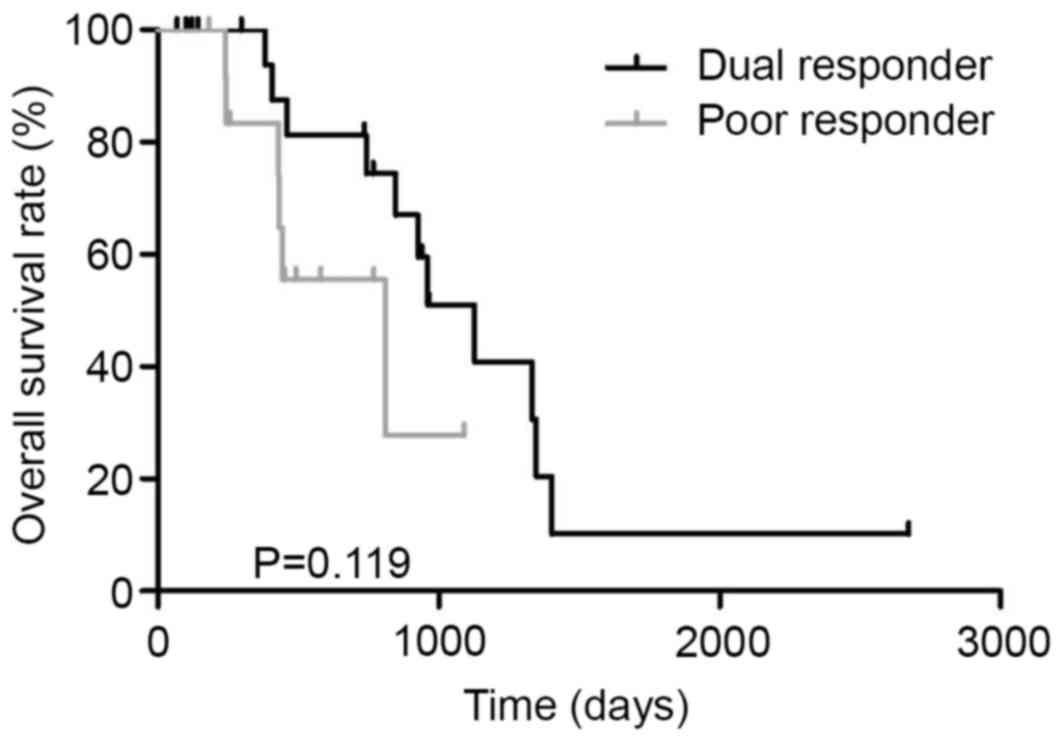
responder (n=13). The MST in dual responder and poor responder was
1128 and 810 days, respectively (P=0.119, Fig. 4).
Three cohorts (FOLFOX, FOLFIRI and the
two regimens recommended)
Individualization of first-line chemotherapy was
possible in all 120 patients, with FOLFOX and FOLFIRI showing
higher efficacy in 63 and 51 patients, respectively, and equal
efficacy in 6 cases (Fig. 5).
Thirty-nine of the patients eventually received the chemotherapy in
FOLFOX recommended (n=22), FOLFIRI recommended (n=15), and the two
regimens recommended (n=2).
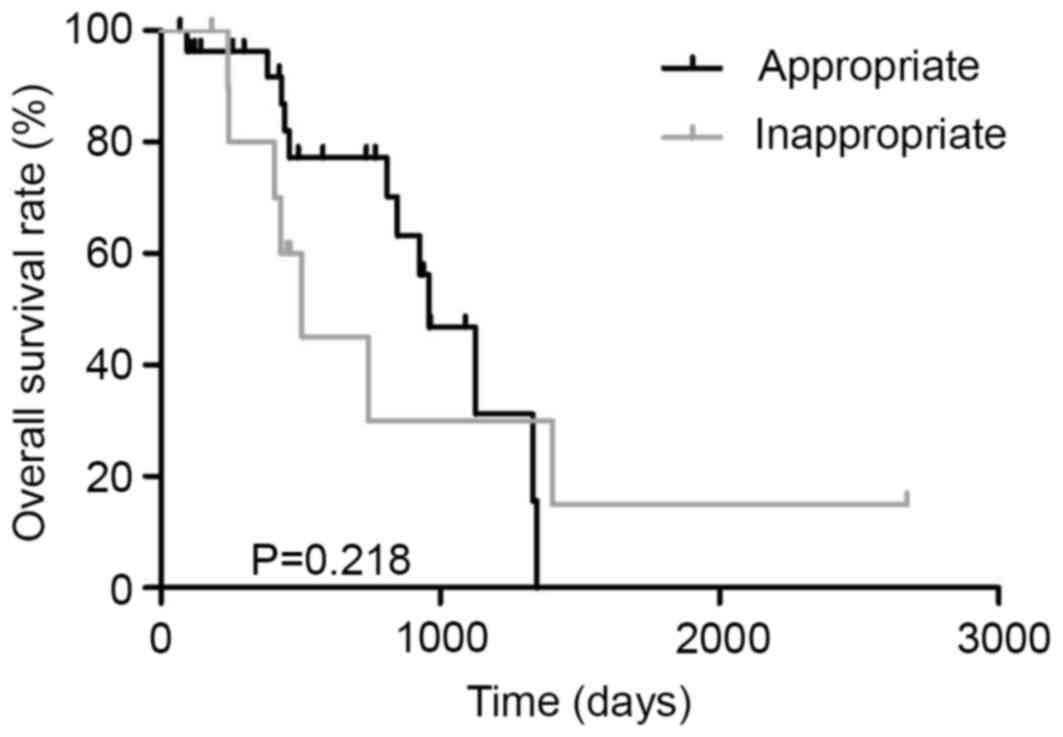
Two cohorts (appropriate and
inappropriate first-line chemotherapy)
Thirty-nine patients with unresectable CRC were
treated with chemotherapy. Patients treated with appropriate
first-line regimen and those treated with inappropriate first-line
regimen were 28 and 11, respectively. All patients treated with
inappropriate first-line regimen received FOLFOX therapy (Fig. 5). The MST in patients treated with the
appropriate first-line regimen and those treated with an
inappropriate first-line regimen was 960 and 506 days, respectively
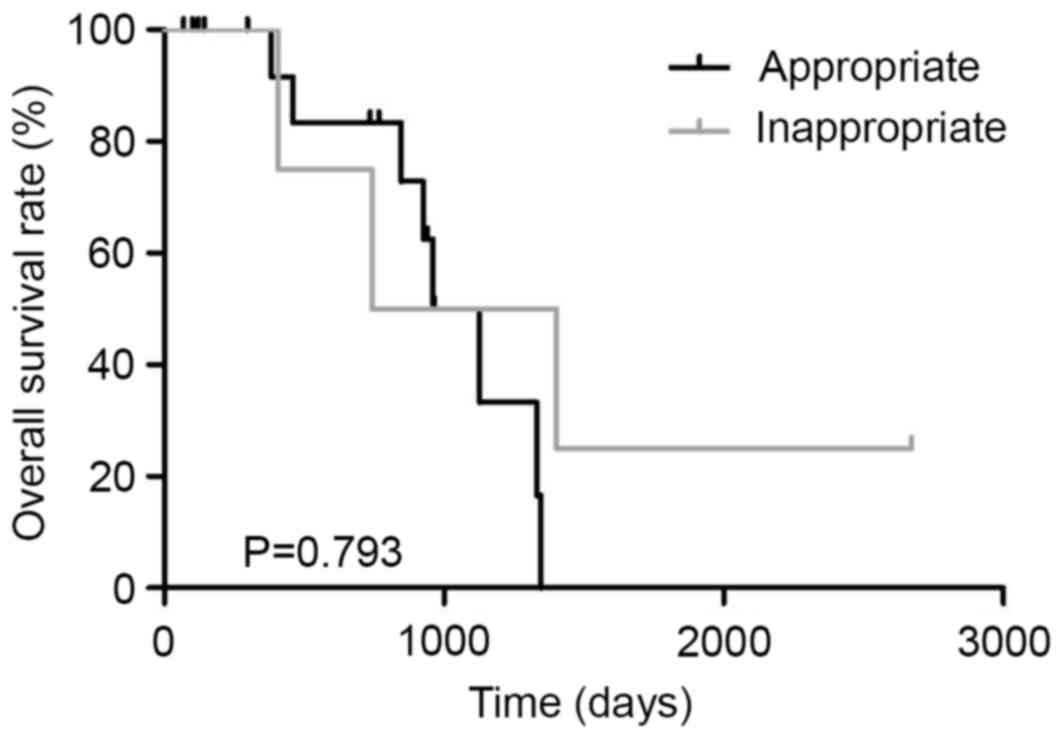
(P=0.218, Fig. 6). In dual
responders, the MST in patients treated with the appropriate
first-line regimen (n=17) and those treated with the inappropriate
first-line regimen (n=4) was 1044 and 1073 days, respectively
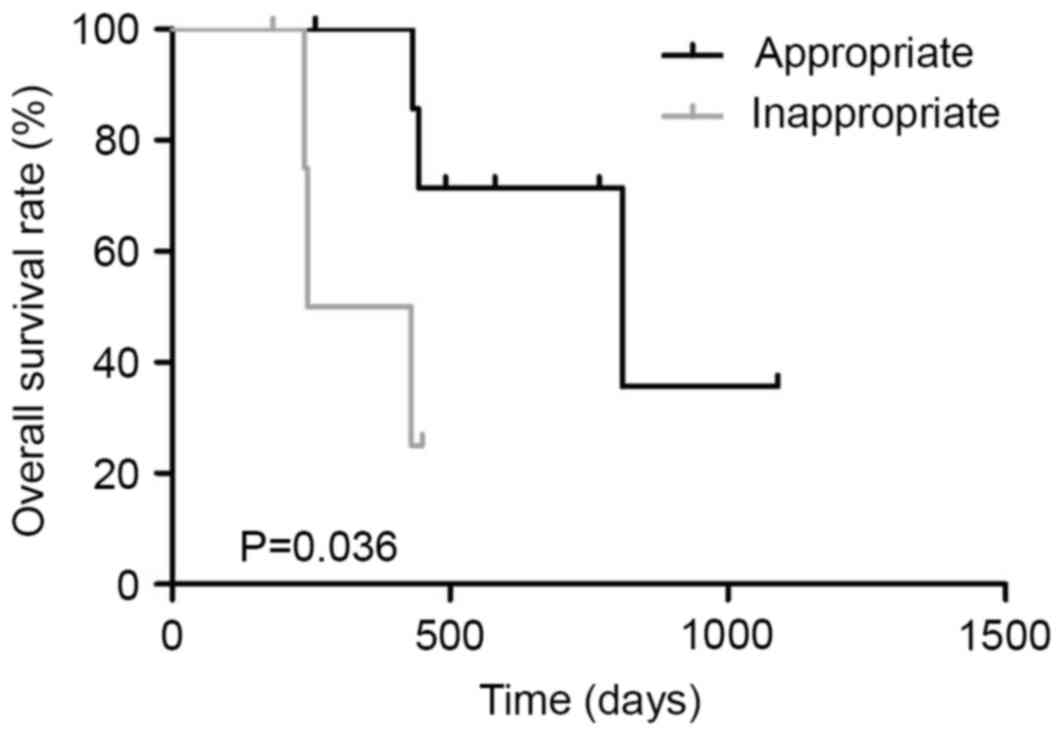
(P=0.793, Fig. 7). In the poor
responder group, the MST in patients treated with appropriate
first-line regimen (n=8) and those treated with inappropriate
first-line regimen (n=5) was 810 and 337 days, respectively
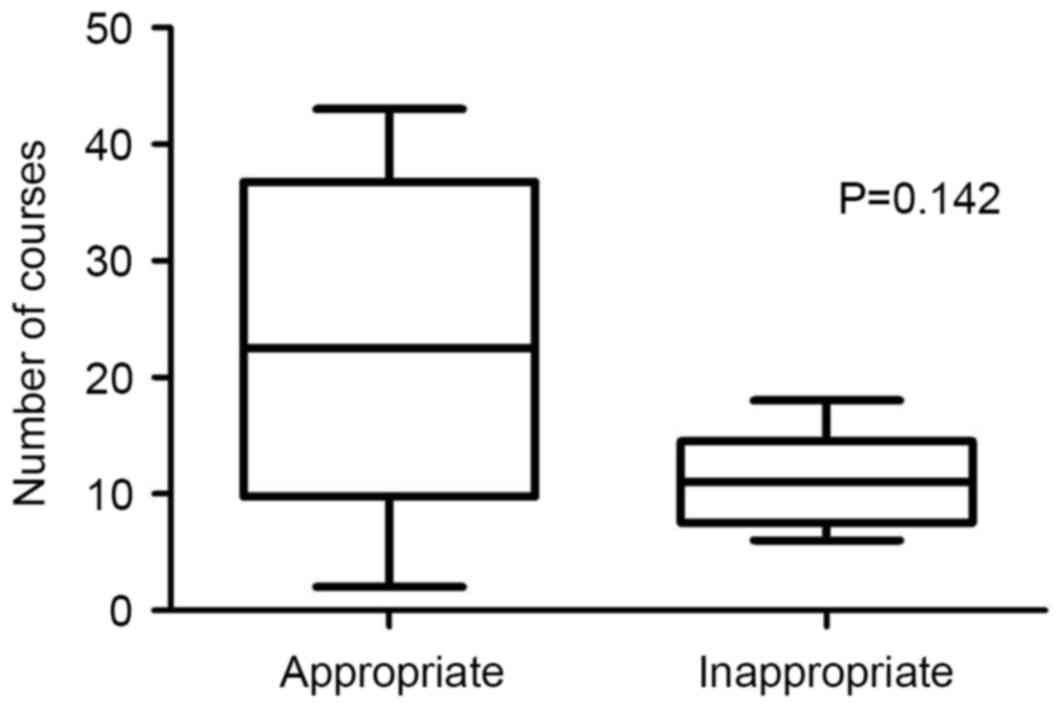
(P=0.036, Fig. 8). The mean frequency
of appropriate and inappropriate regimen in the two cohorts was
22.8±5.17 and 11.0±1.98 courses, respectively (P=0.142, Fig. 9).
Discussion
The present study demonstrated three things.
Firstly, the prognosis of a dual responder was improved compared
with that of poor responders. Secondly, there was no different
prognosis between patients treated with the appropriate first-line
regimen and patients treated with an inappropriate first-line
regimen in dual responders. Thirdly, in poor responders, there were
significant differences in the prognosis between patients treated
with an appropriate first-line regimen and patients treated with an
inappropriate first-line regimen.
The prognosis of dual responders was improved
compared with that of a poor responder. However, there no
significant difference was identified between the two cohorts. The
reason for this may be that the periods of observation of 4
patients in the dual responder group were <150 days. In the dual
responder group, the longest-term survivor (>2700 days) was
treated with an inappropriate first-line regimen. However, the
patient's growth IRs of FOLFOX and FOLFIRI were 77.9 and 85.5%,
respectively. These growth IRs were high level. For certain
patients, whose growth IRs of FOLFOX and FOLFIRI were high-level,
it was not significant whether FOLFOX or FOLFIRI was administered
first. This result may support Grothey's report in dual responders.
Several studies have investigated individualization in 5-FU-based
chemotherapy based on the 5-FU metabolism-associated enzymatic and
genetic characteristics of the individual patient (22–29). In
addition, the individualization of 5-FU-based chemotherapy based on
the serum 5-FU concentration has been reported lately (30–34).
However, individualization in 5-FU-based chemotherapy remains to be
implemented in a clinical setting. Therefore, CD-DST may be useful
to detect poor responder in 5-FU-based chemotherapy. On the other
hand, the individualized chemotherapy with molecularly-targeted
anticancer agents may be implemented based on the genetic
characteristics of the individual patient. Recently, the importance
of the biomarker for molecularly-targeted anticancer agents has
become increasingly evident in a clinical setting (35–37). In
poor responders, the biomarker for molecularly-targeted anticancer
agents is required in order to improve the prognosis. Therefore,
advanced studies into biomarkers are important.
There was no different prognosis between patients
treated with the appropriate first-line regimen and patients
treated with the inappropriate first-line regimen in dual
responders. Grothey et al (38) reported that while it was not
significant whether FOLFOX or FOLFIRI was administered first, it
was crucial that full administration of the targeted dosages of all
3 drugs (5-FU, oxaliplatin and irinotecan) was achieved (38). However, second-line chemotherapy must
be abandoned in certain patients due to disease progression,
adverse effects or high medical cost in a clinical setting. The
cost of molecularly-targeted anticancer agents in particular is
expensive. Therefore, the number of reports on cost effectiveness
analysis and cost utility analysis are rapidly increasing (10–14). In
randomized controlled trials (RCTs), the rate of second-line
chemotherapy enforcement in PRIME study, OPUS study, NO16966 study
and FIRE-3 study was 62, <50, 53 and 69.9%, respectively
(39–42). Even in those recent RCTs with strict
eligible standard, second-line chemotherapy could not be carried
out in >30% of the patients. First-line chemotherapy is usually
administered over a long period of time (9). In addition, the response rate of the
first-line chemotherapy is typically higher compared with
second-line chemotherapy (43,44).
Therefore, selection of a more effective regimen as the first-line
chemotherapy using CD-DST is extremely important even for dual
responder patients in a clinical setting. The present study has
already reported the following: When the clinical response rates of
FOLFOX and FOLFIRI were 50%, responders were identified using the
median based on the histograms of the individual growth IRs. The
efficacies of FOLFOX and FOLFIRI were not exactly equivalent in all
the individuals. By using CD-DST, it was possible to individualize
the first line chemotherapy and may also improve the prognosis of
patients with unresectable CRC.
In poor responders, there were significant
differences of prognosis between patients treated with appropriate
first-line regimen and patients treated with an inappropriate
first-line regimen. Moreover, more chemotherapy in patients treated
with the appropriate first-line regimen was performed. It is
crucial to administer the appropriate first-line regimen.
Administration of a more effective first-line regimen leads to
prolonging the period of first-line chemotherapy and increases the
total number of chemotherapy cycles. This indicates the importance
for the detection of poor responders and the selection of
first-line chemotherapy using CD-DST.
There were certain limitations to the present cohort
study. Firstly, the sample size was small; a larger sample size
would have improved the quality of the data. Secondly, in the
present study, the periods of observation of 4 patients in dual
responder were shorter (<150 days) compared with other patients.
The short periods of observation may influence statistical
analysis. Moreover, the present study was not randomized. The
individualization of first-line chemotherapy using CD-DST requires
additional prospective randomized studies.
In conclusion, the results from the present study
suggest that the administration of the recommended first-line
regimen using CD-DST for patients with unresectable CRC is
important for improvement in prognosis. It is important to
administrate appropriate first-line regimen, particularly in poor
responders.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adam R, De Gramont A, Figueras J, Guthrie
A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P,
Rubbia-Brandt L, et al: The oncosurgery approach to managing liver
metastases from colorectal cancer: A multidisciplinary
international consensus. Oncologist. 17:1225–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones RP, Stättner S, Sutton P, Dunne DF,
McWhirter D, Fenwick SW, Malik HZ and Poston GJ: Controversies in
the oncosurgical management of liver limited stage IV colorectal
cancer. Surg Oncol. 23:53–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN: NCCN Clinical Practice Guidelines in
Oncology. Colon Cancer. Version 1 2017. http://www.nccn.orgFebruary 24–2017
|
|
5
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar E Aranda, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venook A, Niedzwiecki D, Lenz H, Mahoney
M, Innocenti F, O'Neil B, Shaw J, Polite B, Hochster H, Goldberg R,
et al: CALGB/SWOG 80405: Analysis of patients undergoing surgery as
part of treatment strategy. Ann Oncol. 25:1–41. 2014. View Article : Google Scholar
|
|
8
|
Recondo G Jr, Díaz-Cantón E, de la Vega M,
Greco M, Recondo G Sr and Valsecchi ME: Advances and new
perspectives in the treatment of metastatic colon cancer. World J
Gastrointest Oncol. 6:211–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung HW, Chan AL, Leung MS and Lu CL:
Systematic review and quality assessment of cost-effectiveness
analysis of pharmaceutical therapies for advanced colorectal
cancer. Ann Pharmacother. 47:506–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drummond MF, Sculpher MJ, Torrance GW,
O'Brien BJ and Stoddart GL: Methods for the economic evaluation of
health care programmes. Oxford University Press; New York, NY:
2005
|
|
13
|
Manca A, Asseburg C, Vergel Y Bravo,
Seymour MT, Meade A, Stephens R, Parmar M and Sculpher MJ: The
cost-effectiveness of different chemotherapy strategies for
patients with poor prognosis advanced colorectal cancer (MRC
FOCUS). Value Health. 15:22–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lange A, Prenzler A, Frank M, Kirstein M
and der Schulenburg JM Vogel Aandvon: A systematic review of
cost-effectiveness of monoclonal antibodies for metastatic
colorectal cancer. Eur J Cancer. 50:40–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Hashiguchi T, Nakatani A, Marusasa T, Muraki A, Nagaoka
I and Futagawa S: Leucovorin and fluorouracil plus oxaliplatin or
leucovorin and fluorouracil plus irinotecan as individualized
first-line therapy based on a drug sensitivity test. Exp Ther Med.
1:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ochiai T, Nishimura K, Watanabe T,
Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T,
Kishine K, et al: Individualized chemotherapy for colorectal cancer
based on the collagen gel droplet-embedded drug sensitivity test.
Oncol Lett. 4:621–624. 2012.PubMed/NCBI
|
|
17
|
Kobayashi H, Tanisaka K, Doi O, Kodama K,
Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, Yasutomi M, et
al: An in vitro chemosensitivity test for solid tumors using
collagen gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.PubMed/NCBI
|
|
18
|
Kobayashi H, Higashiyama M, Minamigawa K,
Tanisaka K, Takano T, Yokouchi H, Kodama K and Hata T: Examination
of in vitro chemosensitivity test using collagen droplet culture
method with colorimetric endpoint quantification. Jpn J Cancer Res.
92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomized trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ochiai T, Nishimura K, Noguchi H, Kitajima
M, Tsukada A, Watanabe E, Nagaoka I and Futagawa S: Prognostic
impact of orotate phosphoribosyl transferase among 5-fluorouracil
metabolic enzymes in resectable colorectal cancers treated by oral
5-fluorouracil-based adjuvant chemotherapy. Int J Cancer.
118:3084–3088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aki F, Bando Y, Takahashi T, Uehara H,
Numoto S, Ito S, Sasa M and Izumi K: A retrospective study on TS
mRNA expression and prediction of the effects of adjuvant oral
5-fluorouracil in breast cancer. Oncol Lett. 1:981–987.
2010.PubMed/NCBI
|
|
24
|
Komori S, Osada S, Tomita H, Nishio K,
Kumazawa I, Tachibana S, Tsuchiya J and Yoshida K: Predictive value
of orotate phosphoribosyltransferase in colorectal cancer patients
receiving 5-FU-based chemotherapy. Mol Clin Oncol. 1:453–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochiai T, Umeki M, Miyake H, Iida T,
Okumura M, Ohno K, Sakamoto M, Miyoshi N, Takahashi M, Tsumura H,
et al: Impact of 5-fluorouracil metabolizing enzymes on
chemotherapy in patients with resectable colorectal cancer. Oncol
Rep. 32:887–892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasako M, Terashima M, Ichikawa W, Ochiai
A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T and Imamura
H: Impact of the expression of thymidylate synthase and
dihydropyrimidine dehydrogenase genes on survival in stage II/III
gastric cancer. Gastric Cancer. 18:538–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawakami H, Zaanan A and Sinicrope FA:
Implications of mismatch repair-deficient status on management of
early stage colorectal cancer. J Gastrointest Oncol. 6:676–684.
2015.PubMed/NCBI
|
|
28
|
Jung SH, Kim SH and Kim JH: Prognostic
impact of microsatellite instability in colorectal cancer
presenting with mucinous, signet-ring, and poorly differentiated
cells. Ann Coloproctol. 32:58–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashktorab H, Ahuja S, Kannan L, Llor X,
Ellis NA, Xicola RM, Laiyemo AO, Carethers JM, Brim H and Nouraie
M: A meta-analysis of MSI frequency and race in colorectal cancer.
Oncotarget. 23:34546–34557. 2016. View Article : Google Scholar
|
|
30
|
van Kuilenburg AB and Maring JG:
Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic
drug monitoring in cancer patients. Pharmacogenomics. 14:799–811.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paci A, Veal G, Bardin C, Levêque D,
Widmer N, Beijnen J, Astier A and Chatelut E: Review of therapeutic
drug monitoring ofanticancer drugs part 1-Cytotoxics. Eur J Cancer.
50:2010–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Derissen EJ, Jacobs BA, Huitema AD, Rosing
H, Schellens JH and Beijnen JH: Exploring the intracellular
pharmacokinetics of the 5-fluorouracil nucleotides during
capecitabine treatment. Br J Clin Pharmacol. 81:949–957. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang R, Zhang Y, Zhou H, Zhang P, Yang P,
Tong Q, Lyu Y and Han Y: Individual 5-fluorouracil dose adjustment
via pharmacokinetic monitoring versus conventional
body-area-surface method: A meta-analysis. Ther Drug Monit.
38:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JJ, Beumer JH and Chu E: Therapeutic
drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol.
78:447–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bencsikova B, Bortlicek Z, Halamkova J,
Ostrizkova L, Kiss I, Melichar B, Pavlik T, Dusek L, Valik D,
Vyzula R and Zdrazilova-Dubska L: Efficacy of bevacizumab and
chemotherapy in the first-line treatment of metastatic colorectal
cancer: Broadening KRAS-focused clinical view. BMC Gastroenterol.
15:372015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hecht JR, Cohn A, Dakhil S, Saleh M,
Piperdi B, Cline-Burkhardt M, Tian Y and Go WY: SPIRITT: A
randomized, multicenter, phase II study of panitumumab with FOLFIRI
and bevacizumab with FOLFIRI as second-line treatment in patients
with unresectable wild type KRAS metastatic colorectal cancer. Clin
Colorectal Cancer. 14:72–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sartore-Bianchi A, Trusolino L, Martino C,
Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I,
Martinelli E, et al: Dual-targeted therapy with trastuzumab and
lapatinib in treatment-refractory, KRAS codon 12/13 wild-type,
HER2-positive metastatic colorectal cancer (HERACLES): A
proof-of-concept, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:738–746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan andoxaliplatin in the course of treatment. J Clin Oncol.
22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bokemeyer C, Bondarenko I, Hartmann, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. An Oncol. 22:1535–1546. 2011. View Article : Google Scholar
|
|
42
|
Modest DP, Stintzing S, von Weikersthal
LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T,
Lerchenmüller C, Kahl C, et al: Impact of subsequent therapies on
outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with
FOLFIRI plus cetuximab or bevacizumab in patients with KRAS
wild-type tumors in metastatic colorectal cancer. J Clin Oncol.
33:3718–3726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haller DG, Rothenberg ML, Wong AO,
Koralewski PM, Miller WH Jr, Bodoky G, Habboubi N, Garay C and
Olivatto LO: Oxaliplatin plus irinotecan compared with irinotecan
alone as second-line treatment after single-agent fluoropyrimidine
therapy for metastatic colorectal carcinoma. J Clin Oncol.
26:4544–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koopman M, Antonini NF, Douma J, Wals J,
Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G,
Loosveld OJ, et al: Sequential versus combination chemotherapy with
capecitabine, irinotecan, and oxaliplatin in advanced colorectal
cancer (CAIRO): A phase III randomised controlled trial. Lancet.
370:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|