Introduction
Breast cancer (BC) represents the leading cause of
cancer-associated mortality in females worldwide (1). As a heterogeneous disease, a series of
genetic markers have been evaluated and associated with clinical
prognostic parameters in patients with BC (2–4). However,
these markers are not yet effective enough to be used in clinical
practice, and additional studies are required to produce effective
targets that can be used to predict prognosis and drug resistance
(5).
The high mobility group A1 (HMGA1) proteins are
architectural non-histone chromatin factors, which form
stereospecific multiprotein complexes termed enhanceosomes on the
promoter/enhancer regions of genes that regulate gene
transcription. Each HMGA1 protein has three AT-hook domains that
bind to the minor groove of AT-rich DNA sequences and interact with
various transcription factors to enhance or inhibit gene
transcription (6,7). HMGA1 is involved in a variety of
cellular processes, including embryogenesis, cell cycle regulation,
senescence, differentiation and DNA repair (8–11). HMGA1
protein overexpression is a feature of malignant tumors, including
pancreas, breast and colorectal cancers (12–18). A
previous in vitro study provided evidence that HMGA1 exerts
an important role in the pathogenesis of breast cancer; exogenous
expression of HMGA1 in normal human breast cells may lead to
malignant phenotype transformation (19). HMGA1 promoted metastatic processes in
breast cancer cells through enhancing cell proliferation, the Hippo
signaling pathway and epithelial-to-mesenchymal transition
(20–25). In addition, HMGA1 expression in breast
cancer cells diminished cellular DNA repair activity by inducing
enhanced apoptosis and sensitizing cells to cisplatin-induced death
(26). Knockdown of HMGA1 expression
altered breast cancer cells to a more differentiated phenotype and
reduced breast tumorigenesis (21,27).
A high body mass index (BMI) is an independent risk
factor for cardiovascular disease (28,29) and
cancer (30,31). Several studies have demonstrated that
BMI influences the outcomes of patients with BC and is considered a
prognosis factor (32–35). Furthermore, HMGA protein expression in
tumors may also be associated with BMI (36).
To elucidate the role of HMGA1 and BMI in the
prognosis of BC, HMGA1 protein expression was evaluated by
immunohistochemical staining in two large cohorts of BC samples. It
was identified that HMGA1 expression indicated an advanced BC
malignancy, while its expression did not show significant
prognostic value. However, the combined evaluation of HMGA1
expression and high BMI may serve as a biomarker of poor prognosis
in patients with BC.
Materials and methods
Patients
The eligible BCs were collected based on inclusion
and exclusion criteria. Inclusion criteria: BCs with pathological
diagnosis; informed consent obtained or waiver of consent; and
follow-up information available. Exclusion criteria: Failed to get
informed consent; multiple cancers; lack of histological diagnosis;
and no follow-up information. A total of 273 BCs who received
surgical operation in the Second Affiliated Hospital of Zhejiang
University (Zhejiang, China) were entered as the training set. The
validation set, which consisted of 310 patients with BC who
received surgical operation were collected from the National
Engineering Center for Biochip (Shanghai, China). In the training
set, all patients who received surgical operation between January
2004 and September 2010 were followed up until August 2015. The 310
BCs in the validation set received operations between January 2001
and December 2008, and the last follow-up time was July 2014.
Construction of tissue microarray
(TMA)
Formalin-fixed and paraffin-embedded tumor specimens
were prepared for TMA using the Beecher Manual Tissue Arrayer
(Beecher Instruments, Inc., Sun Prairie, WI, USA). Briefly, one
core tissue biopsy with a diameter of 1 mm was taken from a
representative region of an individual paraffin-embedded BC sample
and placed into a new recipient paraffin block. Every sample
included 2–3 tissue cores for biomarker analysis. Consecutive
sections of 4–5 mm were cut from TMA blocks and placed on glass
slides for subsequent immunohistochemical analysis. The tumor
blocks also contained tumor and normal breast tissue samples as
positive and negative controls for each IHC staining.
HMGA1 immunohistochemistry
Paraffin sections of 5–6 µm were deparaffinized and
antigen was retrieved by boiling for 15 min in 0.1 M citrate
buffer. The endogenous peroxidase activity was blocked with 3%
hydrogen peroxide for 15 min. Array slides were then incubated with
normal goat serum (catalog no. ZLI-9021; ZSGB-Bio, Beijing China).
for 15 min. The primary antibody HMGA1 (catalog no. ab129153;
dilution, 1:250; Abcam, Cambridge, UK) was incubated overnight at
4°C in a humidified chamber. The rabbit antibody against HMGA1
(catalog. no. A380388; dilution, 1:5,000) used in the present study
was purchased from ALEXIS Biochemicals (San Diego, CA, USA). PBS
was used as a negative control. The array slides were incubated
with horseradish peroxidase-labeled polymer conjugated with
corresponding antibodies for 30 min. Diaminobenzidine (catalog no.
D8230; Solarbio, Beijing, China) was then applied for 5 and 10 min,
respectively. Each slide was counterstained with hematoxylin (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA).
Scoring of HMGA1 expression
HMGA1 staining was assessed for the percentage of
nuclear immunoreactivity in tumor cells by two independent
observations. Results were grouped into the following categories:
No nuclear staining (−); with nuclear staining <20% (+); 20–50%
of nuclear positive cells (++); and >50% of nuclear positive
cells (+++). All clinicopathological data (pathological diagnosis,
grade and tumor node metastasis stage) and immunohistochemical data
[estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor 2 (HER2)] were reevaluated by pathologists
from the Department of Pathology (Second Affiliated Hospital,
Zhejiang University School of Medicine, Zhejiang, China). BMI
scores were divided by 24 according to the Chinese standard which
determines that >24 kg/m2 is categorized as
overweight or obese (37).
Statistical analysis
SPSS 21.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analysis. The association between HMGA1
expression/BMI and clinicopathological factors was estimated using
the Pearson's χ2 test. Overall survival (OS) curves were
constructed using the Kaplan-Meier method by the log-rank test.
Univariate analysis was performed with the log-rank test, and Cox's
regression test was applied for multivariable analysis. The factors
of ER status, PR status, HER2 status, tumor size and lymph node
involvement were excluded when performed multivariable analysis, as
these factors have the collinear relation with TNBC and TNM stage.
Hazard ratios (HRs) were reported with 95% confidence intervals
(CIs). P<0.05 was considered to indicate a statistically
significant difference.
Results
HMGA1 expression and
clinicopathological characteristics in BC
Associations between HMGA1 expression and
clinicopathological characteristics of BC patients are shown in
Table I. HMGA1 expression was
observed in 105/273 (38.5%) patients with BC in the training set
and 191/310 (61.6%) in the validation set. HMGA1 staining was
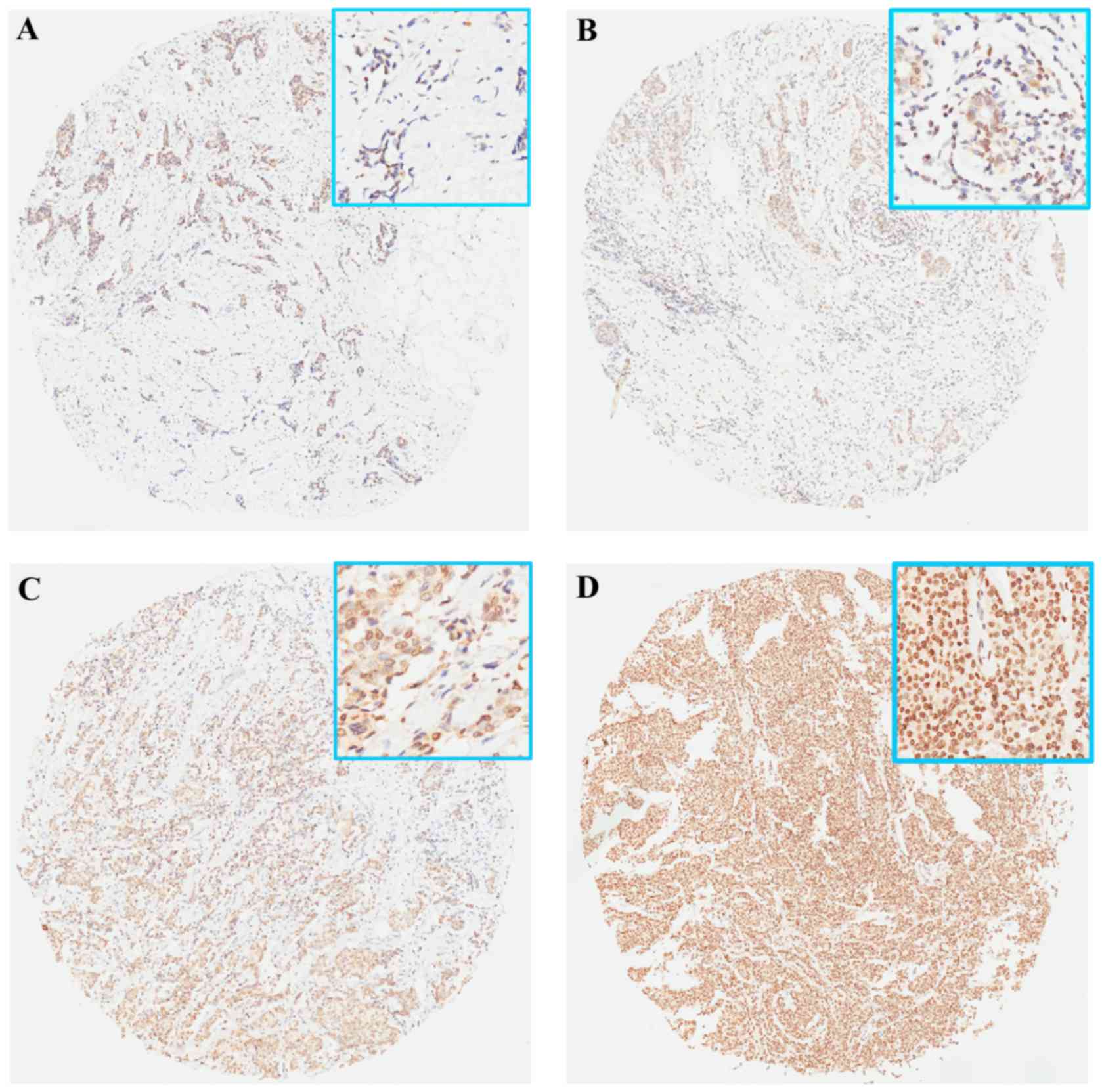
negative in all the normal samples (Fig.
1A). The association between positive HMGA1 expression and
clinicopathological parameters was then analyzed. It was identified
that HMGA1 overexpression was significantly associated with
histological grade in the training set (P=0.031) and the validation
set (P<0.001). However, no significant difference in HMGA1
expression, according to age, tumor location, stage of disease,
triple-negative BC (TNBC) or other parameters, was observed
(Table I).
 | Table I.HMGA1 protein expression and
clinicopathological characteristics in breast cancer. |
Table I.
HMGA1 protein expression and
clinicopathological characteristics in breast cancer.
|
| Training set (ZJU,
n=273) |
| Validation set
(SBC, n=310) |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Patients, n | HMGA1+,
n (%) | P-value | Patients, n | HMGA+, n
(%) | P-value |
|---|
| Age |
|
| 0.863 |
|
| 0.983 |
| ≤50
years | 136 | 53 (39.0) |
| 117 | 72 (61.5) |
|
| >50
years | 137 | 52 (38.0) |
| 193 | 119 (61.7) |
|
| Tumor
locationa |
|
| 0.377 |
|
| 0.604 |
|
Left | 147 | 53 (36.1) |
| 136 | 86 (63.2) |
|
| Right
bilateral | 126 | 52 (41.3) |
| 174 | 105 (60.3) |
|
| Histological
gradeb |
|
| 0.031 |
|
| <0.001 |
| I | 45 | 13 (28.9) |
| 51 | 18 (35.3) |
|
| II | 119 | 45 (37.8) |
| 195 | 121 (62.1) |
|
|
III | 24 | 14 (58.3) |
| 64 | 52 (81.3) |
|
| Tumor size |
|
| 0.188 |
|
| 0.614 |
| T1 | 125 | 45 (36.0) |
| 78 | 41 (52.6) |
|
| T2 | 129 | 50 (38.8) |
| 199 | 131 (65.8) |
|
| T3 and
T4 | 19 | 10 (52.6) |
| 33 | 19 (57.6) |
|
| Lymph node
involvement |
|
| 0.631 |
|
| 0.073 |
| N
(−) | 148 | 55 (37.2) |
| 145 | 97 (66.9) |
|
| N
(+) | 125 | 50 (40.0) |
| 165 | 94 (57.0) |
|
| AJCC stage |
|
| 0.133 |
|
| 0.664 |
| I | 82 | 26 (31.7) |
| 41 | 24 (58.5) |
|
| II | 128 | 52 (40.6) |
| 181 | 117 (64.6) |
|
|
III | 63 | 27 (42.9) |
| 88 | 50 (56.8) |
|
| ER status |
|
| 0.798 |
|
| 0.192 |
|
Negative | 104 | 39 (37.5) |
| 113 | 75 (66.4) |
|
|
Positive | 169 | 66 (39.1) |
| 197 | 116 (58.9) |
|
| PR status |
|
| 0.510 |
|
| 0.501 |
|
Negative | 116 | 42 (36.2) |
| 156 | 99 (63.5) |
|
|
Positive | 157 | 63 (40.1) |
| 154 | 92 (59.7) |
|
| HER2 status |
|
| 0.609 |
|
| 0.075 |
|
Negative | 214 | 84 (39.3) |
| 208 | 121 (58.2) |
|
|
Positive | 59 | 21 (35.6) |
| 102 | 70 (67.6) |
|
|
Triple-negative |
|
| 0.740 |
|
| 0.442 |
|
TNBC | 68 | 25 (36.8) |
| 46 | 26 (56.5) |
|
|
Others | 205 | 80 (39.0) |
| 264 | 165 (62.5) |
|
BMI and clinicopathological
parameters
A total of 158/273 patients with BC in the training
set were recorded with BMI, the association between BMI and
associated clinicopathological parameters was analyzed. BMI did not
show any association with age, location, tumor stage or other
parameters (Table II), however, high
BMI (>24 kg/m2) was significantly associated with
HMGA1 expression (P=0.033).
 | Table II.Association between body mass index
and clinicopathological characteristics of patients with breast
cancer. |
Table II.
Association between body mass index
and clinicopathological characteristics of patients with breast
cancer.
|
| BMI,
kg/m2 |
|---|
|
|
|
|---|
| Characteristic | <24 | ≥24 | P-value |
|---|
| Age (years) |
|
| 0.249 |
|
≤50 | 51 | 22 |
|
|
>50 | 57 | 28 |
|
| Tumor
locationa |
|
| 0.249 |
|
Left | 52 | 29 |
|
| Right
bilateral | 56 | 21 |
|
| Histological
gradeb |
|
| 0.080 |
| I | 25 | 8 |
|
| II | 53 | 18 |
|
|
III | 8 | 7 |
|
| Tumor size |
|
| 0.437 |
| T1 | 53 | 25 |
|
| T2 | 48 | 20 |
|
| T3 and
T4 | 7 | 5 |
|
| Lymph node
involvement |
|
| 0.455 |
| N
(−) | 63 | 26 |
|
| N
(+) | 45 | 24 |
|
| AJCC stage |
|
| 0.851 |
| I | 34 | 15 |
|
| II | 55 | 21 |
|
|
III | 19 | 14 |
|
| ER status |
|
| 0.410 |
|
Negative | 38 | 21 |
|
|
Positive | 70 | 29 |
|
| PR status |
|
| 0.384 |
|
Negative | 46 | 25 |
|
|
Positive | 62 | 25 |
|
| HER2 status |
|
| 0.169 |
|
Negative | 97 | 41 |
|
|
Positive | 11 | 9 |
|
|
Triple-negative |
|
| 0.893 |
|
TNBC | 81 | 37 |
|
|
Others | 27 | 13 |
|
| HMGA1 status |
|
| 0.033 |
|
Negative | 65 | 21 |
|
|
Positive | 43 | 29 |
|
Survival analysis
In order to clarify whether HMGA1 affects the
prognosis of patients with BC, Kaplan-Meier analysis was performed,
and it was revealed that HMGA1 level did not predict survival
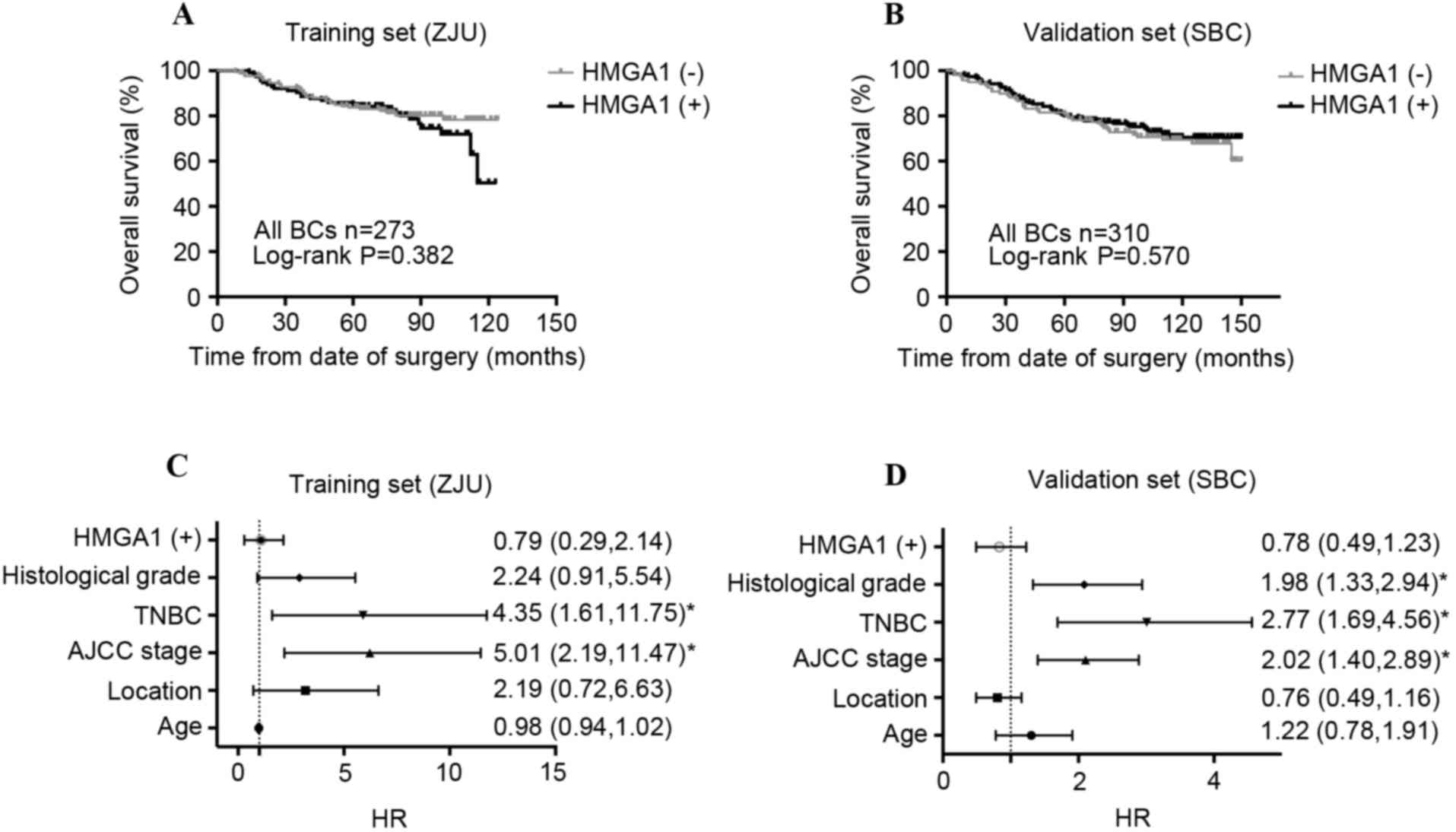
significance in patients with BC. As shown in Fig. 2A and B, HMGA1 did not affect the OS of
patients with BC in the training set (P=0.382) or the validation
set (P=0.570). The results of Cox's regression test are presented
in Table III. As expected, the
univariate analysis revealed that tumor stages 3 and 4, lymph node
involvement, American Joint Committee on Cancer (AJCC) stages II
and III, histological grade III and TNBC subtype were associated
with unfavorable prognosis; while ER (+) and PR (+) were associated
with favorable prognosis in the training cohort. These results were
confirmed in the validation cohort. Multivariate analysis indicated
that the AJCC stage in the training set (HR, 5.01; CI, 2.19–11.47)
and the validation set (HR, 2.02; CI, 1.20–2.89), and TNBC status
in the training set (HR, 4.35; CI, 1.61–11.75) and the validation
set (HR, 2.77; CI, 1.69–4.56) were the independent prognostic risk
of patients with BC. HMGA1 expression was not associated with OS in
the training set (HR, 0.79; CI, 0.29–2.14; Fig. 2C) or in the validation set (HR, 0.78;
CI, 0.49–1.23; Fig. 2D).
 | Table III.Univariate and multivariate Cox
analysis for high mobility group A1 and survival of breast
cancer. |
Table III.
Univariate and multivariate Cox
analysis for high mobility group A1 and survival of breast
cancer.
|
| Training set (ZJU,
n=273) | Validation set
(SBC, n=310) |
|---|
|
|
|
|
|---|
| Characteristic | Univariate, HR (95%
CI) | Multivariate, HR
(95% CI) | Univariate, HR (95%
CI) | Multivariate, HR
(95% CI) |
|---|
| Age (>50 vs. ≤50
years) | 1.02
(0.99–1.04) | 0.98
(0.94–1.02) | 1.10
(0.71–1.70) | 1.22
(0.78–1.91) |
| Location (right vs.
left) | 1.19
(0.69–2.03) | 2.19
(0.72–6.63) | 0.86
(0.57–1.29) | 0.76
(0.49–1.16) |
| ER status (+ vs.
-) | 0.33
(0.19–0.58)a |
| 0.60
(0.39–0.91)a |
|
| PR status (+ vs.
-) | 0.28
(0.16–0.50)a |
| 0.54
(0.35–0.83)a |
|
| HER2 status (+ vs.
-) | 1.53
(0.84–2.79) |
| 1.50
(0.98–2.31) |
|
| Tumor size (T3/4
vs. T1/2) | 2.01
(1.35–2.99)a |
| 1.73
(1.23–2.44)a |
|
| Lymph node
involvement (+ vs. -) | 1.98
(1.58–2.48)a |
| 1.46
(1.19–1.80)a |
|
| AJCC stage (II/III
vs. I) | 3.62
(2.35–5.58)a | 5.01
(2.19–11.47)a | 2.10
(1.47–2.99)a | 2.02
(1.40–2.89)a |
| TNBC (TNBC vs.
non-TNBC) | 3.32
(1.94–5.70)a | 4.35
(1.61–11.75)a | 2.45
(1.52–3.94)a | 2.77
(1.69–4.56)a |
| Histological grade
(III vs. I/II) | 3.65
(1.61–8.31)a | 2.24
(0.91–5.54) | 1.70
(1.19–2.42)a | 1.98
(1.33–2.94)a |
| HMGA1 (+ vs.
-) | 1.05
(0.61–1.82) | 0.79
(0.29–2.14) | 0.88
(0.58–1.35) | 0.78
(0.49–1.23) |
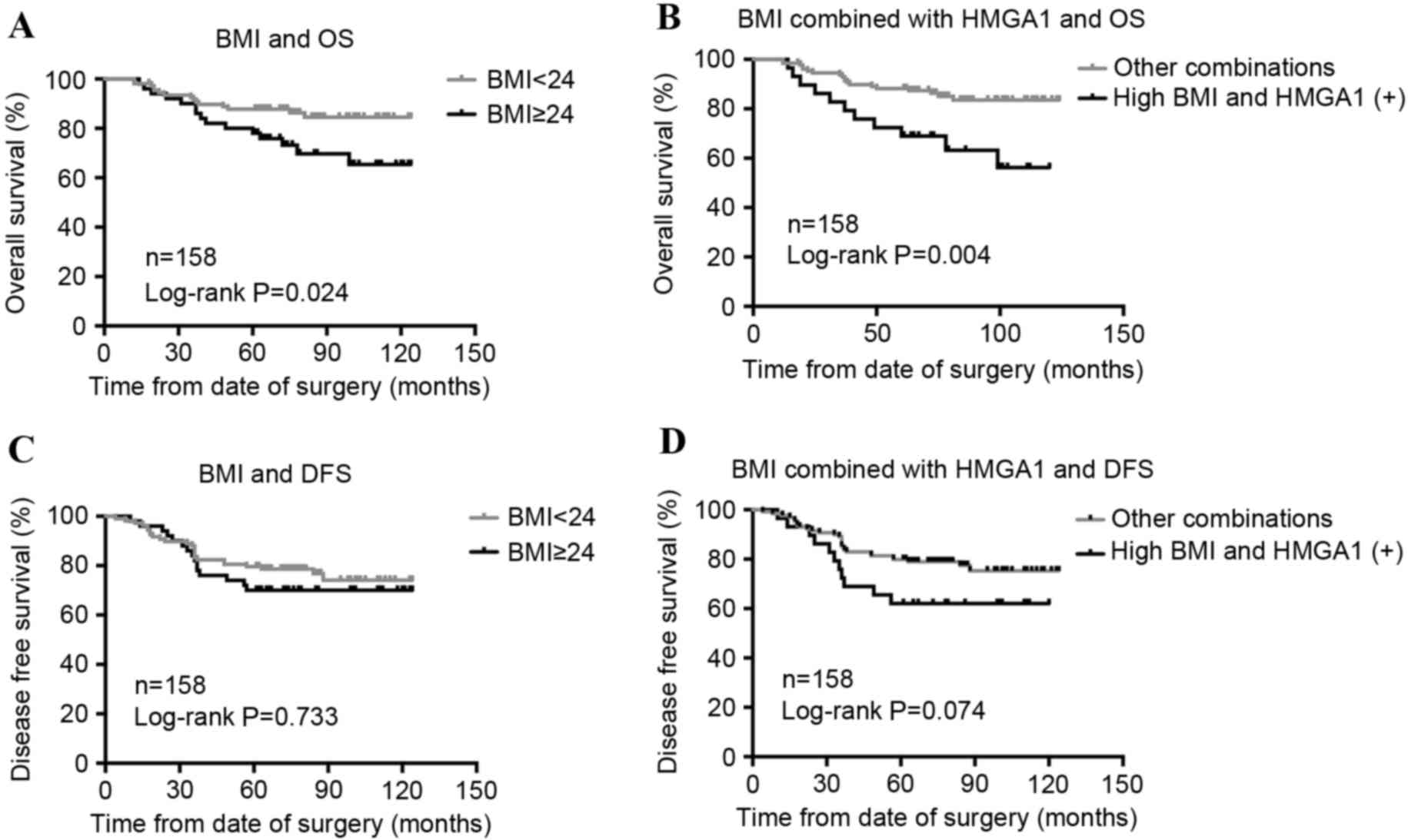
The association between BMI and prognosis in the
group of 158 patients with BC was then analyzed, and it was
identified that high BMI was associated with poor OS (P=0.024;
Fig. 3A), but not associated with
disease-free survival (DFS; P=0.733; Fig.
3B). In addition, BMI and HMGA1 combined (HMGA1 positive and
BMI >24 kg/m2) evaluation had a stronger association
with OS (P=0.004; Fig. 3C) and DFS
(P=0.074; Fig. 3D). Cox's regression
test was then performed (Table IV).
BMI (HR, 2.23; CI, 1.09–4.56) and BMI/HMGA2 combined score (HR,
2.83; CI, 1.25–5.95) had a significant adverse prognosis value for
OS (HR, 1.32; CI, 0.70–2.50), but not with DFS (HR, 1.86; CI,
0.93–3.73) in univariate analysis. However, the prognostic value of
the combined BMI and HMGA1 score was dampened in multivariate
analysis; the HRs of the high BMI-HMGA1 combined score with DFS and
OS were 1.82 (CI, 0.58–5.62) and 4.21 (CI, 0.61–29.00),
respectively.
 | Table IV.Univariate and multivariate Cox
analysis of prognostic factors for disease-free survival and
overall survival in 158 patients with breast cancer. |
Table IV.
Univariate and multivariate Cox
analysis of prognostic factors for disease-free survival and
overall survival in 158 patients with breast cancer.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Factors | Univariate, HR (95%
CI) | Multivariate, HR
(95% CI) | Univariate, HR (95%
CI) | Multivariate, HR
(95% CI) |
|---|
| Location (right vs.
left) | 1.15
(0.62–2.14) |
| 1.31
(0.64–2.70) |
|
| HMGA1 (+ vs.
-) | 1.55
(0.83–2.90) |
| 1.90
(0.92–3.95) |
|
| BMI (≥24 vs.
<24) | 1.32
(0.70–2.50) |
| 2.23
(1.09–4.56)a |
|
| Age (>50 vs. ≤50
years) | 1.03
(1.01–1.05)a | 1.01
(0.98–1.05) | 1.03
(1.00–1.06)a | 0.96
(0.88–1.04) |
| AJCC stage (II/III
vs. I) | 2.94
(1.83–4.71)a | 1.66
(0.82–3.37) | 4.95
(2.69–9.11)a | 2.76
(0.68–11.25) |
| TNBC (TNBC vs.
non-TNBC) | 3.58
(1.93–6.67)a | 3.12
(1.25–7.83)a | 4.63
(2.24–9.55)a | 5.07
(0.95–27.12) |
| Histological grade
(III vs. I/II) | 1.69
(0.79–3.61) | 1.06
(0.47–2.41) | 14.63
(3.43–62.52)a | 8.70
(1.21–62.28)a |
| BMI-HMGA1 combined
score | 1.86
(0.93–3.73) | 1.82
(0.58–5.64) | 2.83
(1.35–5.95)a | 4.21
(0.61–29.00) |
| (high vs. low) |
|
|
|
|
Discussion
In the present study, HMGA1 expression in 583
patients with BC was retrospectively analyzed from two medical
centers, to clarify the expression patterns of HMGA1 in BC samples.
In total, 38.5% of patients with BC in the training set and 61.6%
of patients in the validation set showed HMGA1 expression. The
discrepancy of HMGA1 expression ratio may be due to the difference
of baseline characteristics of patients from these two sets. In the
training set, 93.0% of patients were stage I and II, and 24.9% of
patients had TNBC, while in the validation set, 83.2% of patients
were identified as stage I and II, and only 14.8% of patients were
classified as TNBC. The oncogenic protein HMGA1 has been
established as the prognostic and predictive marker of survival in
various types of cancers (16,38,39).
Its expression preceded the appearance of the malignant phenotype,
as only 40% of hyperplastic lesions with cellular atypia were
stained for HMGA1, while 62% of breast carcinomas were HMGA1
expression (12). In the present
study, HMGA1 expression determined by immunohistochemistry did not
show any association with OS in patients with BC, while HMGA1
expression was significantly associated with histological grade of
patients with BC, which is consistent with previous studies
(12,40,41).
BMI is a simple measurement based on individual
weight and height, it is widely used to define overweight and
obesity. High BMI has been identified as a major risk of type 2
diabetes mellitus (T2DM) (42). The
presence of a functional variant of the HMGA1 gene was also
associated with T2DM (43), and this
HMGA1 variant positively associated with BMI (44). From these results, it was inferred
that an association may exist between HMGA1 and BMI. Notably, HMGA1
expression was found to be significantly associated with BMI in
patients with BC; 62.5% of HMGA1 positive patients were overweight
or obese (BMI ≥24 kg/m2), while only 17.8% of HMGA1
negative patients were overweight. Survival analysis resulted in
poor OS for BC patients with high BMI (≥24 kg/m2).
Although HMGA1 expression did not indicate any prognostic value in
patients with BC, the HMGA1 expression and BMI combined score had a
stronger prognostic value for OS (Fig.
3B, P=0.004). Similarly, BMI did not have prognostic value for
DFS (P=0.733), but the HMGA1 expression and BMI combined score
showed a trend in association with DFS (P=0.074). It was
hypothesized that HMGA1 and BMI may perform a synergistic role in
the process of tumorigenesis. HMGA1 protein directly binds to an
adipose-specific promoter CCAAT-enhancer-binding protein-β to exert
a critical role in adipocyte hemostasis, and suppression of HMGA1
expression impaired adipocytic differentiation and decreased fat
tissue development (45). Adipocytes
promoted the secretion of peptide hormone cholecystokinin of cancer
cells and enhanced the proliferation of prostate cancer stem cells
(46). In addition, the leptin, which
was produced by adipocytes, was suggested to contribute to tumor
development and progression through activating the Janus
kinase/signal transducers and activators of transcription,
phosphatidylinositol 3-kinase/AKT and extracellular signal-related
kinase signaling pathways (47,48). It
was hypothesized that HMGA1 may enhance the proliferation of
adipocytes, particularly adipocytes around the cancer cells, which
may interact with cancer cells by secreting specific cytokines to
promote the malignant biological properties of cancer cells.
However, additional studies are required to elucidate the
particular molecular mechanisms underlying this connection among
HMGA1, obesity and BC.
The BC tissues included in the present study were
collected from two medical centers; however, complete pathological
BMI and DFS data could not be obtained for all samples. Therefore,
the potential selection bias and confounding bias was inevitable.
BMI and HMGA2 combined score had a significant adverse prognosis
value for OS in univariate analysis. However, it did not indicate
an independent risk in multivariate analysis. A lack of enough
samples of patients with BMI, the presence of collinearity between
BMI-HMGA1 combined score and other clinicopathological parameters,
or other confounding factors, may affect the reliability of the
results.
In conclusion, the present study demonstrated that
HMGA1 expression in BC is positively associated with pathological
differentiation. However, HMGA1 expression is not prognostic of
survival in patients with BC. The combined evaluation of HMGA1
expression and high BMI can be a more effective marker in
predicting poor prognosis of patients with BC. HMGA1 and BMI may
play a synergistic role in the development and progression of
BC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues Oliveira FF, Dos Santos RE, de
Oliveira AL, de Lima Rozenowicz R, de Melo MB and Scheffer DK:
Prognostic assessment of polymorphisms of the MDR-1 and GSTP1 genes
in patients with stage II and III breast cancer submitted to
neoadjuvant chemotherapy. Breast J. 18:185–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horak CE, Pusztai L, Xing G, Trifan OC,
Saura C, Tseng LM, Chan S, Welcher R and Liu D: Biomarker analysis
of neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone
or paclitaxel in early-stage breast cancer. Clin Cancer Res.
19:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silva JM, Dominguez G, Silva J, Garcia JM,
Sanchez A, Rodriguez O, Provencio M, España P and Bonilla F:
Detection of epithelial messenger RNA in the plasma of breast
cancer patients is associated with poor prognosis tumor
characteristics. Clin Cancer Res. 7:2821–2825. 2001.PubMed/NCBI
|
|
5
|
Cianfrocca M and Gradishar W: New
molecular classifications of breast cancer. CA Cancer J Clin.
59:303–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thanos D and Maniatis T: Virus induction
of human IFN beta gene expression requires the assembly of an
enhanceosome. Cell. 83:1091–1100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reeves R and Nissen MS: The
A.T-DNA-binding domain of mammalian high mobility group I
chromosomal proteins. A novel peptide motif for recognizing DNA
structure. J Biol Chem. 265:8573–8582. 1990.PubMed/NCBI
|
|
8
|
Chiappetta G, Avantaggiato V, Visconti R,
Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti
V, Santoro M, et al: High level expression of the HMGI (Y) gene
during embryonic development. Oncogene. 13:2439–2446.
1996.PubMed/NCBI
|
|
9
|
Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla
LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau
C, et al: Prediction of central nervous system embryonal tumour
outcome based on gene expression. Nature. 415:436–442. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Narita M, Narita M, Krizhanovsky V, Nuñez
S, Chicas A, Hearn SA, Myers MP and Lowe SW: A novel role for
high-mobility group a proteins in cellular senescence and
heterochromatin formation. Cell. 126:503–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adair JE, Maloney SC, Dement GA, Wertzler
KJ, Smerdon MJ and Reeves R: High-mobility group A1 proteins
inhibit expression of nucleotide excision repair factor xeroderma
pigmentosum group A. Cancer Res. 67:6044–6052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiappetta G, Botti G, Monaco M,
Pasquinelli R, Pentimalli F, Di Bonito M, D'Aiuto G, Fedele M,
Iuliano R, Palmieri EA, et al: HMGA1 protein overexpression in
human breast carcinomas: Correlation with ErbB2 expression. Clin
Cancer Res. 10:7637–7644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liau SS, Jazag A and Whang EE: HMGA1 is a
determinant of cellular invasiveness and in vivo metastatic
potential in pancreatic adenocarcinoma. Cancer Res. 66:11613–11622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piscuoglio S, Zlobec I, Pallante P, Sepe
R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A
and Karamitopoulou E: HMGA1 and HMGA2 protein expression correlates
with advanced tumour grade and lymph node metastasis in pancreatic
adenocarcinoma. Histopathology. 60:397–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fedele M, Fidanza V, Battista S,
Pentimalli F, Klein-Szanto AJ, Visone R, De Martino I, Curcio A,
Morisco C, Del Vecchio L, et al: Haploinsufficiency of the Hmga1
gene causes cardiac hypertrophy and myelo-lymphoproliferative
disorders in mice. Cancer Res. 66:2536–2543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liau SS, Rocha F, Matros E, Redston M and
Whang E: High mobility group AT-hook 1 (HMGA1) is an independent
prognostic factor and novel therapeutic target in pancreatic
adenocarcinoma. Cancer. 113:302–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liau SS and Whang E: HMGA1 is a molecular
determinant of chemoresistance to gemcitabine in pancreatic
adenocarcinoma. Clin Cancer Res. 14:1470–1477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams MD, Zhang X, Belton AS, Xian L,
Huso T, Park JJ, Siems WF, Gang DR, Resar LM, Reeves R and Hill HH
Jr: HMGA1 drives metabolic reprogramming of intestinal epithelium
during hyperproliferation, polyposis, and colorectal
carcinogenesis. J Proteome Res. 14:1420–1431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dolde CE, Mukherjee M, Cho C and Resar LM:
HMG-I/Y in human breast cancer cell lines. Breast Cancer Res Treat.
71:181–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pegoraro S, Ros G, Ciani Y, Sgarra R,
Piazza S and Manfioletti G: A novel HMGA1-CCNE2-YAP axis regulates
breast cancer aggressiveness. Oncotarget. 6:19087–19101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pegoraro S, Ros G, Piazza S, Sommaggio R,
Ciani Y, Rosato A, Sgarra R, Del Sal G and Manfioletti G: HMGA1
promotes metastatic processes in basal-like breast cancer
regulating EMT and stemness. Oncotarget. 4:1293–1308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Treff NR, Dement GA, Adair JE, Britt RL,
Nie R, Shima JE, Taylor WE and Reeves R: Human KIT ligand promoter
is positively regulated by HMGA1 in breast and ovarian cancer
cells. Oncogene. 23:8557–8562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WM, Guerra-Vladusic FK, Kurakata S,
Lupu R and Kohwi-Shigematsu T: HMG-I(Y) recognizes base-unpairing
regions of matrix attachment sequences and its increased expression
is directly linked to metastatic breast cancer phenotype. Cancer
Res. 59:5695–5703. 1999.PubMed/NCBI
|
|
24
|
Reeves R, Edberg DD and Li Y:
Architectural transcription factor HMGI(Y) promotes tumor
progression and mesenchymal transition of human epithelial cells.
Mol Cell Biol. 21:575–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Resar LM: The high mobility group A1 gene:
Transforming inflammatory signals into cancer? Cancer Res.
70:436–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldassarre G, Belletti B, Battista S,
Nicoloso MS, Pentimalli F, Fedele M, Croce CM and Fusco A: HMGA1
protein expression sensitizes cells to cisplatin-induced cell
death. Oncogene. 24:6809–6819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Cello F, Shin J, Harbom K and Brayton
C: Knockdown of HMGA1 inhibits human breast cancer cell growth and
metastasis in immunodeficient mice. Biochem Biophys Res Commun.
434:70–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manson JE, Colditz GA, Stampfer MJ,
Willett WC, Rosner B, Monson RR, Speizer FE and Hennekens CH: A
prospective study of obesity and risk of coronary heart disease in
women. N Engl J Med. 322:882–889. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song YM, Sung J, Smith Davey G and Ebrahim
S: Body mass index and ischemic and hemorrhagic stroke: A
prospective study in Korean men. Stroke. 35:831–836. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reeves GK, Pirie K, Beral V, Green J,
Spencer E and Bull D: Million Women Study Collaboration: Cancer
incidence and mortality in relation to body mass index in the
Million Women Study: Cohort study. BMJ. 335:11342007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sparano JA, Wang M, Zhao F, Stearns V,
Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW Jr,
et al: Obesity at diagnosis is associated with inferior outcomes in
hormone receptor-positive operable breast cancer. Cancer.
118:5937–5946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berclaz G, Li S, Price KN, Coates AS,
Castiglione-Gertsch M, Rudenstam CM, Holmberg SB, Lindtner J, Erien
D, Collins J, et al: Body mass index as a prognostic feature in
operable breast cancer: The international breast cancer study group
experience. Ann Oncol. 15:875–884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan DS, Vieira AR, Aune D, Bandera EV,
Greenwood DC, McTiernan A, Rosenblatt Navarro D, Thune I, Vieira R
and Norat T: Body mass index and survival in women with breast
cancer-systematic literature review and meta-analysis of 82
follow-up studies. Ann Oncol. 25:1901–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Widschwendter P, Friedl TW, Schwentner L,
DeGregorio N, Jaeger B, Schramm A, Bekes I, Deniz M, Lato K,
Weissenbacher T, et al: The influence of obesity on survival in
early, high-risk breast cancer: Results from the randomized SUCCESS
A trial. Breast Cancer Res. 17:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Califano D, Pignata S, Losito NS, Ottaiano
A, Greggi S, De Simone V, Cecere S, Aiello C, Esposito F, Fusco A
and Chiappetta G: High HMGA2 expression and high body mass index
negatively affect the prognosis of patients with ovarian cancer. J
Cell Physiol. 229:53–59. 2014.PubMed/NCBI
|
|
37
|
Adult weight determination. The national
health and family planning commission of the People's Republic of
China. 2013 04 18;
|
|
38
|
Huang R, Huang D, Dai W and Yang F:
Overexpression of HMGA1 correlates with the malignant status and
prognosis of breast cancer. Mol Cell Biochem. 404:251–257. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fedele M and Fusco A: HMGA and cancer.
Biochim Biophys Acta. 1799:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ram TG, Reeves R and Hosick HL: Elevated
high mobility group-I(Y) gene expression is associated with
progressive transformation of mouse mammary epithelial cells.
Cancer Res. 53:2655–2660. 1993.PubMed/NCBI
|
|
41
|
Flohr AM, Rogalla P, Bonk U, Puettmann B,
Buerger H, Gohla G, Packeisen J, Wosniok W, Loeschke S and
Bullerdiek J: High mobility group protein HMGA1 expression in
breast cancer reveals a positive correlation with tumour grade.
Histol Histopathol. 18:999–1004. 2003.PubMed/NCBI
|
|
42
|
Ganz ML, Wintfeld N, Li Q, Alas V, Langer
J and Hammer M: The association of body mass index with the risk of
type 2 diabetes: A case-control study nested in an electronic
health records system in the United States. Diabetol Metab Syndr.
6:502014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiefari E, Tanyolac S, Paonessa F,
Pullinger CR, Capula C, Iiritano S, Mazza T, Forlin M, Fusco A,
Durlach V, et al: Functional variants of the HMGA1 gene and type 2
diabetes mellitus. JAMA. 305:903–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chiefari E, Tanyolaç S, Iiritano S,
Sciacqua A, Capula C, Arcidiacono B, Nocera A, Possidente K, Baudi
F, Ventura V, et al: A polymorphism of HMGA1 is associated with
increased risk of metabolic syndrome and related components. Sci
Rep. 3:14912013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Melillo RM, Pierantoni GM, Scala S,
Battista S, Fedele M, Stella A, De Biasio MC, Chiappetta G, Fidanza
V, Condorelli G, et al: Critical role of the HMGI(Y) proteins in
adipocytic cell growth and differentiation. Mol Cell Biol.
21:2485–2495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang KD, Liu J, Jovanovic L, An J, Hill
MM, Vela I, Lee TK, Ma S, Nelson C, Russell PJ, et al: Adipocytes
promote prostate cancer stem cell self-renewal through
amplification of the cholecystokinin autocrine loop. Oncotarget.
7:4939–4948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Uddin S, Bu R, Ahmed M, Abubaker J,
Al-Dayel F, Bavi P and Al-Kuraya KS: Overexpression of leptin
receptor predicts an unfavorable outcome in Middle Eastern ovarian
cancer. Mol Cancer. 8:742009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT and ERK signaling is involved in leptin-mediated promotion
of invasion and migration of hepatocellular carcinoma cells. Cancer
Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|