Introduction
Nasopharyngeal carcinoma (NPC) is a common type of
malignant tumor in southern China, with the highest incidence in
Guangdong Province (1). Radiotherapy
(RT) is the standard treatment method; however, following RT,
radiation fibrosis or radiation encephalopathy may occur (2,3). The
pathophysiology of radiation encephalopathy is divided into three
periods: The acute reaction period, the early delayed radiation
period and the late delayed radiation period. Radiation-induced
brain injury is irreversible, therefore detection of this in the
early stage is important. Traditionally, the white matter (WM) and
vascular endothelium have been considered to be key structures for
RT-induced brain damage (4). Previous
studies have focused on alterations in the WM of the temporal lobe,
including metabolic changes and abnormalities of water diffusion in
normal-appearing WM in the early phases, using conventional
magnetic resonance imaging (MRI) of patients with NPC (5–7).
Magnetic resonance diffusion tensor imaging (DTI) is
currently the only non-invasive method used to assess the
structure, morphology and function of WM fibers in vivo
(8,9).
DTI technology has been widely used in research of the central
nervous system. Tract-based spatial statistics (TBSS) is a novel
processing method of DTI data, with marked advantages compared with
the manually drawn regions of interest (ROI) method (10). Previous studies have primarily used
the ROI method and have been confined to the temporal lobe
(6–7).
To the best of our knowledge, the present study was the first to
examine the dynamic alterations in the microstructure of whole
brain WM in distinct stages of NPC prior to and following RT, using
the TBSS method.
Although WM is more vulnerable to radiation-induced
injury, compared with gray matter (GM), previous studies have
identified that the GM may be damaged (11,12).
Voxel-based morphometry (VBM) is a suitable method used to assess
GM volume alterations in vivo. A previous study evaluated
radiation-induced GM volume alterations in patients with NPC
(13). The present study aimed at
determining the microstructural alterations in normal-appearing WM
and GM structures in patients with NPC following RT, using
conventional MRI.
Patients and methods
Patients
The present study included 70 subjects (53 males, 17
females; aged between 25 and 59 years; mean age, 44 years) with
pathologically confirmed NPC. Of the 70 subjects, 24 were pre-RT
and the remaining 46 were post-RT. The time between RT and imaging
ranged between 1 week and 4 years. All patients underwent
fractionated RT for the first time with three-dimensional conformal
and intensity-modulated techniques (total dose/fraction
dose/exposures, 66–74 Gy/1.8–2.0 Gy/30-35 times). Prior to MRI
examinations, it was validated that patients exhibited no
occurrences of intracranial tumors or intracranial invasion.
Patients with high blood pressure, diabetes, heart disease, WM
degeneration or vascular lesions were excluded.
Pre-RT subjects constituted the control group.
Post-RT patients were divided into three groups according to the
stage of radiation-induced brain injury which include acute
reaction period, early delayed radiation period and late delayed
radiation period (7): Group 1
(pre-RT, n=24); group 2 (0–6 months post-RT, n=16); group 3
(>6-12 months post-RT, n=16); and group 4 (>12 months
post-RT, n=14). No statistically significant differences were
identified among the groups according to age or sex. The present
study was approved by the Institutional Review Board of Southern
Medical University, Nanfang Hospital (Guangzhou, China) and was
conducted under strict adherence to the Privacy Rules of The Health
Insurance Portability and Accountability Act. All individuals
included were fully informed of the purpose, methods and
precautions of the trial, and written informed consent was obtained
from all participants.
Image acquisition
MRI data were acquired using a 3.0 T clinical
scanner with an eight-channel head coil (SIGNA EXCITE; GE
Healthcare, Chicago, IL, USA). The routine MRI brain protocol
included axial T1-weighted images [repetition time (TR), 600 ms;
echo time (TE), 15 ms], T2-weighted images (TR, 5,200 ms; TE, 140
ms) and T2-weighted fluid attenuated inversion recovery (TR, 9,000
ms; TE, 120 ms; inversion recovery, 2,100 ms). DTI scans were
performed, employing a single-shot echo-planar imaging sequence and
an array spatial sensitivity encoding technique with the following
parameters: TR, 12,000 ms; TE, 75.5 ms; field of view (FOV), 24×24
cm; matrix, 128×128; slice thickness, 3 mm (no inter-slice gap);
number of excitation, 1; and flip angle, 90°. Images were collected
along 25 non-collinear diffusion gradient directions, with a
b-value of 1,000 sec/mm2 and one set of null images with
a b value of 0 sec/mm2. 3D-T1 imaging was performed
using a three-dimensional fast field echo pulse sequence with the
following imaging parameters: TR, 2,500 ms; TE, 1.5 ms; FOV, 24×24
cm; matrix, 256×256; slice thickness, 1 mm; slice gap, 0 mm; NEX,
1.
Post-processing
DTI data were analyzed using the Functional MRI of
the Brain (FMRIB) Software Library (FSL) tools (version 4.19;
www.firirib.ox.ac.uk/fsl). The protocol
used was as follows: i) DTI data were adjusted for head movement
and eddy current distortion, using the Diffusion Toolbox of the FSL
software; ii) mask images of each brain were obtained using each
subject's B0 image with the Brain Extraction Tool; iii) FMRIB
Software Library's Diffusion Toolbox was used to calculate the
diffusion tensor and fractional anisotropy (FA) maps were obtained;
and iv) TBSS processes were monitored (14).
An extension tool of Statistical Parametric Mapping
(SPM), the diffeomorphic anatomical registration through
exponentiated lie algebra tool, was used to process the GM data.
The following procedures were used: i) The original image was
segmented to gray matter, white matter and cerebral spinal fluid
images and subsequently imported into DARTEL for calculation and
obtaining GM images; ii) DARTEL was used to create a GM template;
iii) the GM template was used to normalize each subject's GM image;
iv) the resulting GM images were modulated with a Jacobian
correction; v) a 12 mm full width at half maximum isotropic
Gaussian kernel was used to smooth the resulting images; and vi)
the GM data were transformed to Montreal Neurological Institute
coordinates for SPM analysis. Subsequently, the preprocessed GM
data were analyzed using Statistical parametrical mapping 8
(Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk/spm), with a general linear
model and random field theory.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance with multiple comparisons using the Bonferroni
post hoc test was used to analyze the differences between age among
the groups. Pearson's χ2 test was used to determine sex
differences. Threshold-free cluster enhancement in randomize mode
(15) was used to analyze FA, with
permutation-based correction for multiple comparisons at P<0.05.
The differences between GM volumes among the groups were determined
using a voxel-based comparison. A cluster-level threshold of
P<0.05 were set to produce statistical maps, which were
corrected for multiple comparisons using the false discovery rate
(FDR).
Results
FA
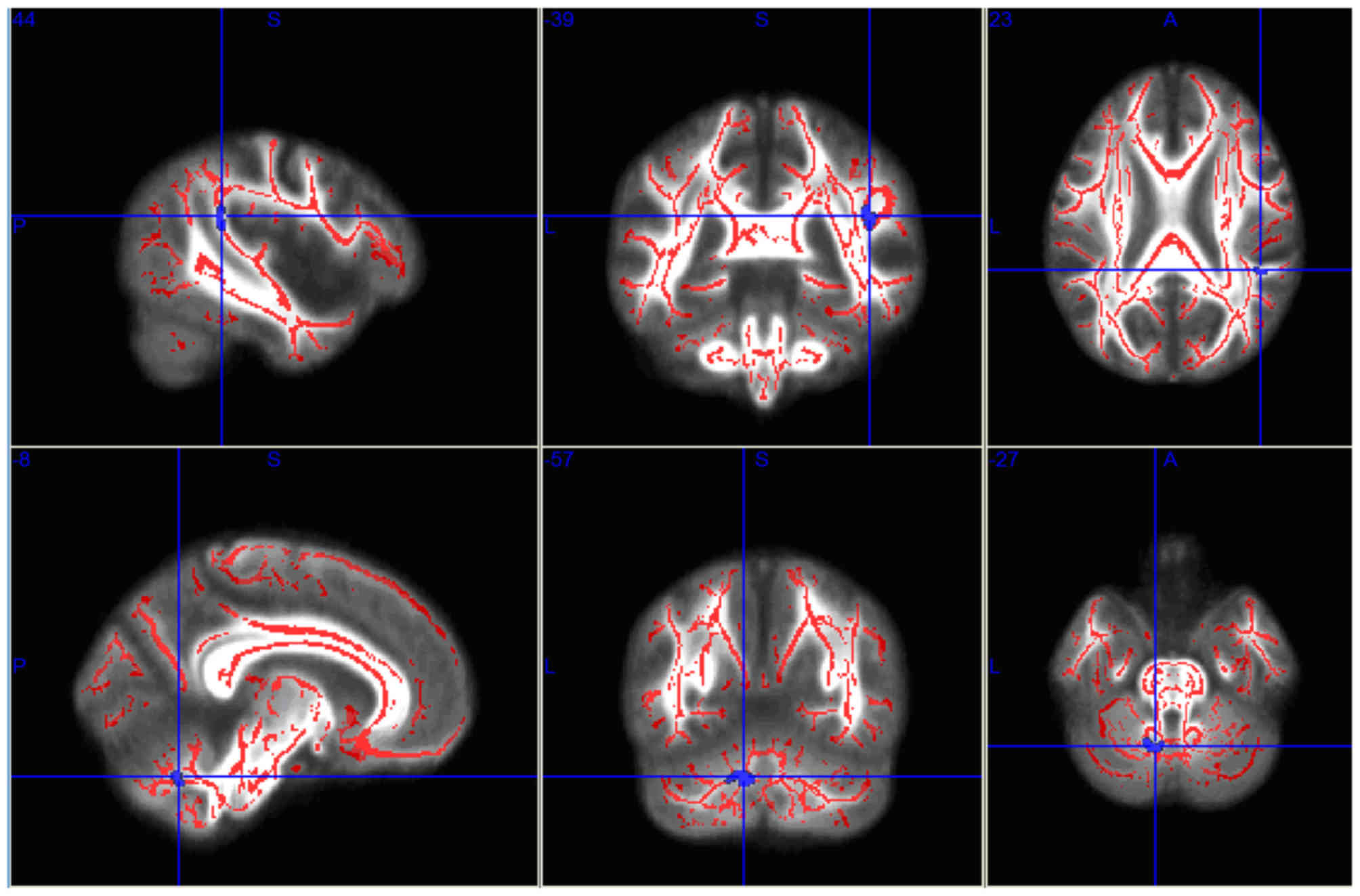
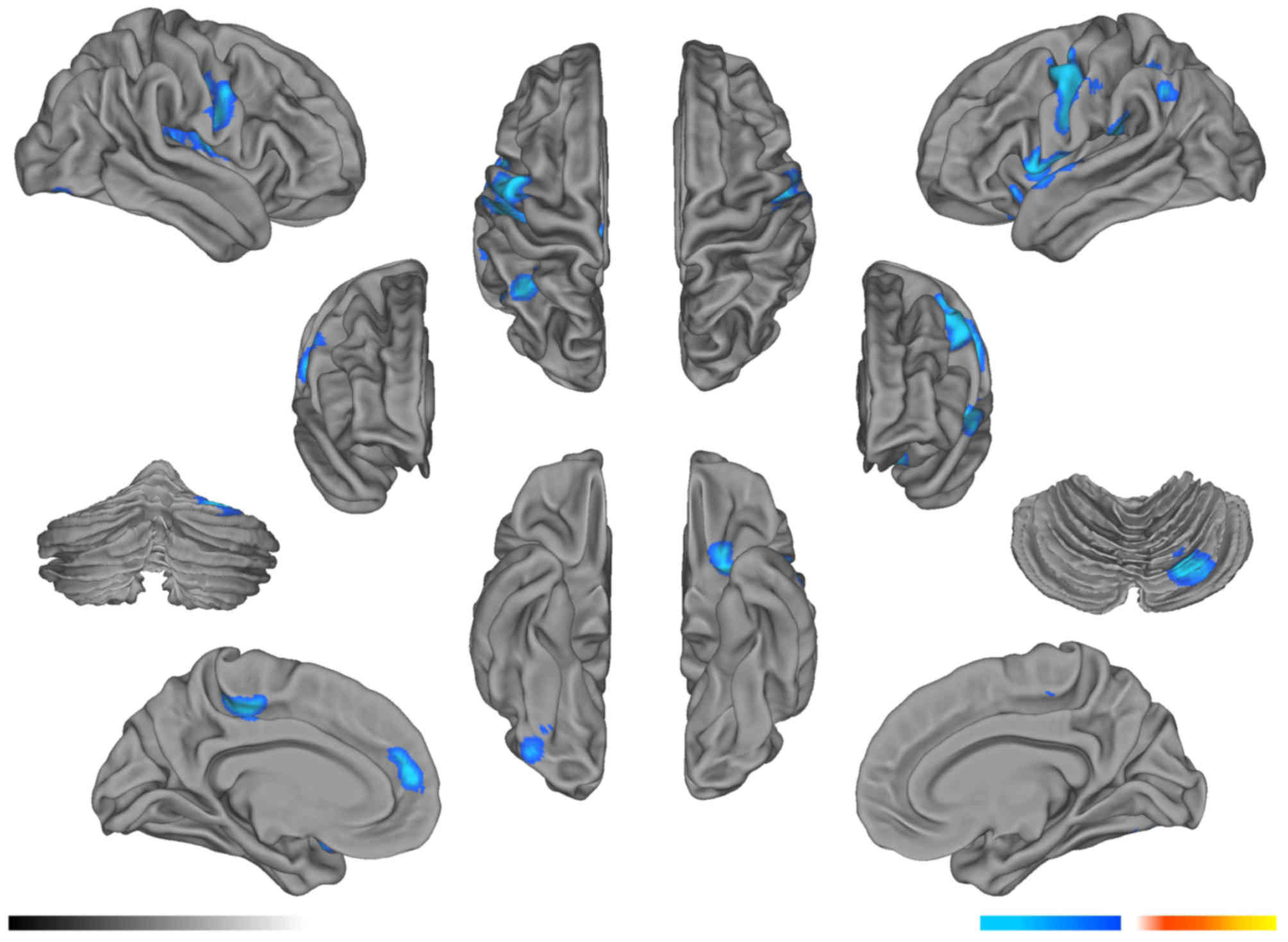
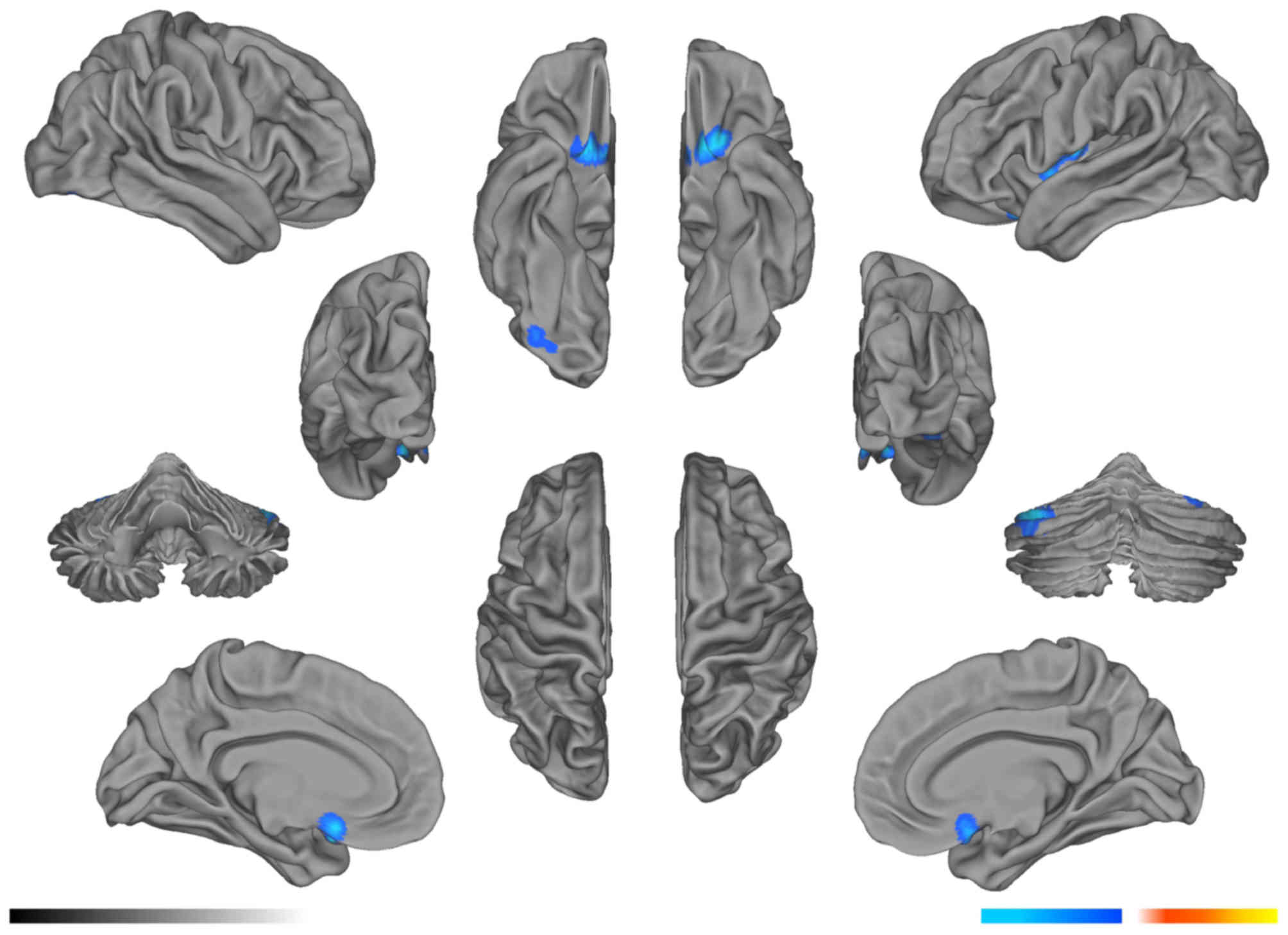
Compared with the pre-RT group, the mean FA values
in the left parietal lobe WM and right cerebellum decreased
significantly in the post-RT 0–6 month group (P<0.05; Fig. 1; Table
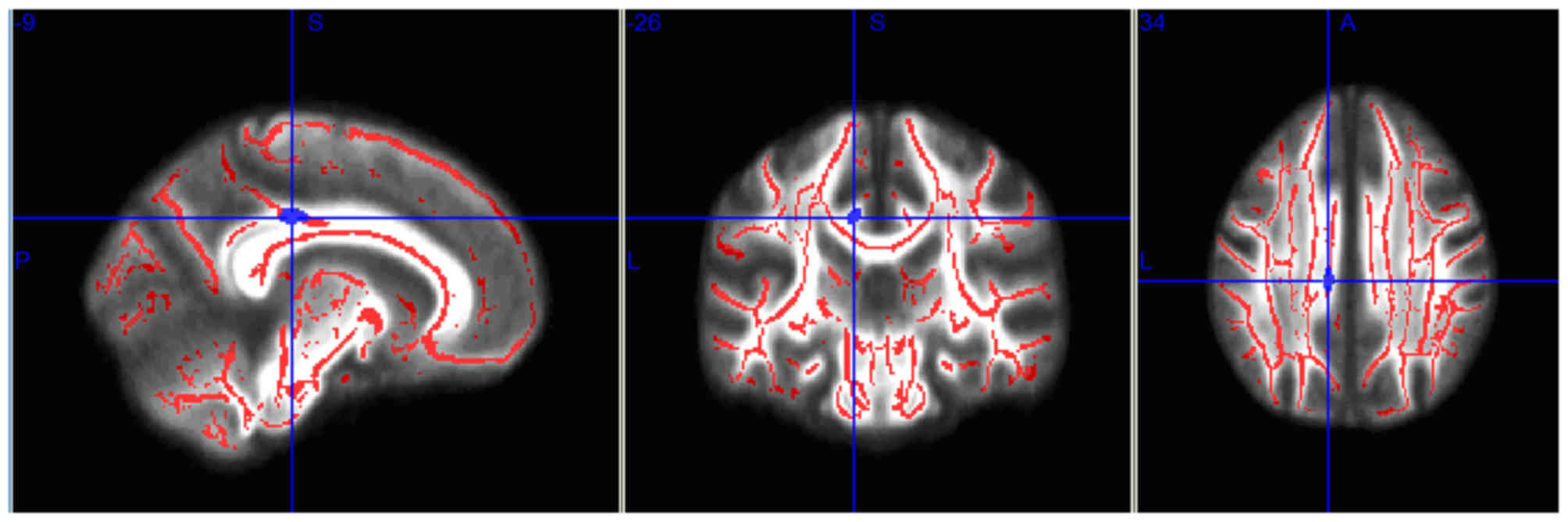
I), and the mean FA values in the right parietal lobe WM
decreased significantly in the post-RT 6–12 month group (P<0.05;
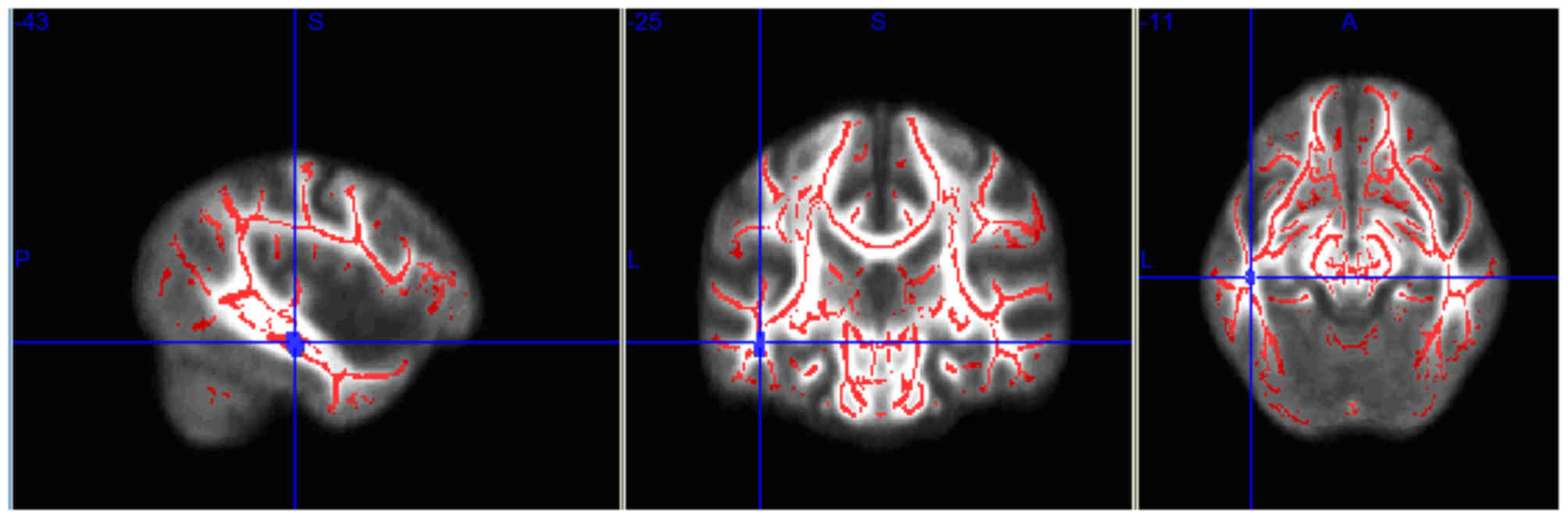
Fig. 2; Table II). In the >12 months post-RT
group, the FA level in the right temporal lobe remained
significantly lower for 1 year after RT, compared with that in the
pre-RT group (P<0.05; Fig. 3;
Table III).
 | Table I.Brain regions with fractional
anisotropy values in the 0–6 month post-radiotherapy group of
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group. |
Table I.
Brain regions with fractional
anisotropy values in the 0–6 month post-radiotherapy group of
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain regions | Voxels | X | Y | Z | P-value |
|---|
| Left parietal
lobe | 30 | −7 | −57 | −30 | <0.0001 |
| Right cerebellum | 24 | 44 | −39 | 18 | <0.0001 |
 | Table II.Brain regions with fractional
anisotropy values in the 6–12 month post-radiotherapy group in
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group. |
Table II.
Brain regions with fractional
anisotropy values in the 6–12 month post-radiotherapy group in
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain region | Voxels | X | Y | Z | P-value |
|---|
| Right parietal
lobe | 28 | −10 | −28 | 33 | 0.019 |
 | Table III.Brain regions with fractional
anisotropy values in the >12 month post-radiotherapy group of
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group. |
Table III.
Brain regions with fractional
anisotropy values in the >12 month post-radiotherapy group of
patients with nasopharyngeal carcinoma, compared with those in the
pre-radiotherapy group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain region | Voxels | X | Y | Z | P-value |
|---|
| Right temporal
lobe | 22 | −43 | −25 | −15 | <0.0001 |
GM volume
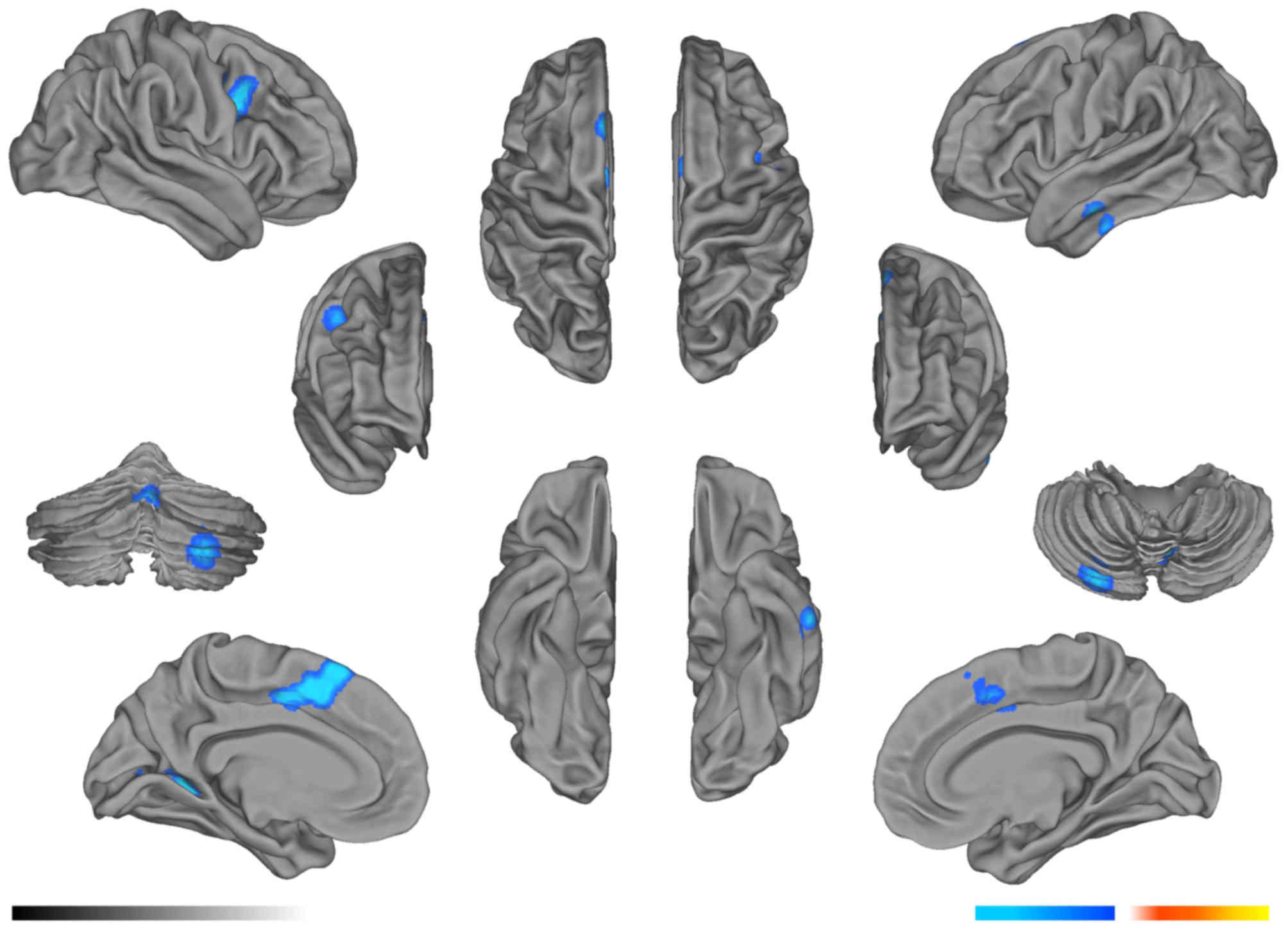
Compared with the pre-RT group, the GM volume in the
bilateral frontal lobe, right occipital lobe, left parietal lobe,
right temporal lobe and left cerebellum decreased significantly in
the post-RT 0–6 month group (P<0.05; FDR corrected; clusters,
100 mm3; Fig. 4; Table IV). In addition, in the bilateral
temporal lobe, parietal lobe, right frontal lobe and left
cerebellum, the GM volume decreased significantly in the post-RT
6–12 month group (P<0.05; Fig. 5;
Table V), compared with that of the
pre-RT group. In the >12 month post-RT group, for 1 year after
RT, the GM volume in the right temporal lobe, bilateral frontal
lobe and bilateral cerebellum remained significantly decreased,
compared with that in the pre-RT group (P<0.05; Fig. 6; Table
VI).
 | Table IV.Brain regions with significantly
reduced gray matter volume in the 0–6 month post-radiotherapy group
of patients with nasopharyngeal carcinoma compared with the
pre-radiation group. |
Table IV.
Brain regions with significantly
reduced gray matter volume in the 0–6 month post-radiotherapy group
of patients with nasopharyngeal carcinoma compared with the
pre-radiation group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain region | Voxels | X | Y | Z | P-value |
|---|
| Right occipital
lobe | 102 | 28 | −76 | −56 | <0.0001 |
| Left occipital
lobe | 110 | −8 | −68 | −38 | <0.0001 |
| Right temporal
lobe | 134 | −60 | −14 | −36 | <0.0001 |
| Left occipital
lobe | 117 | −18 | −50 | −2 | <0.0001 |
| Right frontal
lobe | 187 | 40 | 8 | 22 | <0.0001 |
| Right temporal
lobe | 403 | 6 | 6 | 38 | <0.0001 |
 | Table V.Brain regions with significantly
reduced gray matter volume in the 6–12 month post-radiotherapy
group of patients with nasopharyngeal carcinoma compared with the
pre-radiation group. |
Table V.
Brain regions with significantly
reduced gray matter volume in the 6–12 month post-radiotherapy
group of patients with nasopharyngeal carcinoma compared with the
pre-radiation group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain region | Voxels | X | Y | Z | P-value |
|---|
| Frontal lobe | 230 | 28 | −74 | −26 | <0.0001 |
| Occipital lobe | 110 | −18 | 10 | −26 | <0.0001 |
| Temporal lobe | 139 | 40 | −20 | −6 | <0.0001 |
| Temporal lobe | 455 | −56 | 6 | −6 | <0.0001 |
| Temporal lobe | 222 | 42 | 6 | 4 | <0.0001 |
| Frontal lobe | 114 | −4 | 54 | 6 | <0.0001 |
| Temporal lobe | 141 | −54 | −32 | 14 | <0.0001 |
| Parietal lobe | 218 | 60 | −8 | 18 | <0.0001 |
| Frontal lobe | 743 | −54 | −4 | 20 | <0.0001 |
| Parietal lobe | 176 | −48 | −58 | 34 | 0.001 |
| Temporal lobe | 199 | −10 | −28 | 40 | <0.0001 |
 | Table VI.Brain regions with significantly
reduced gray matter volume in the >12 month post-radiotherapy
group of patients with nasopharyngeal carcinoma compared with the
pre-radiation group. |
Table VI.
Brain regions with significantly
reduced gray matter volume in the >12 month post-radiotherapy
group of patients with nasopharyngeal carcinoma compared with the
pre-radiation group.
|
|
| MNI coordinate
(mm) |
|
|---|
|
|
|
|
|
|---|
| Brain region | Voxels | X | Y | Z | P-value |
|---|
| Left
cerebellum | 225 | −42 | −78 | −38 | <0.0001 |
| Right
cerebellum | 127 | 30 | −70 | −28 | <0.0001 |
| Right temporal
lobe | 282 | 8 | 10 | −26 | <0.0001 |
| Left temporal
lobe | 140 | −44 | 6 | −8 | <0.0001 |
Discussion
To the best of our knowledge, the present study was
the first to analyze the microstructural dynamic alterations in all
brain lobes following RT in patients with NPC at different times,
using DTI-TBSS for WM and VBM for GM volume. The overall brain
alterations were extensive and dynamic, and were not limited to the
temporal lobe, which has not been reported previously.
RT is the preferred and most effective treatment for
NPC; however, the field of RT contains the temporal lobes and the
radiation dose exceeds the brain tissue tolerance.
Radiation-induced injury of brain tissue is one of the most serious
complications, which may influence the prognosis and decrease the
quality of life of patients with NPC. A previous study identified
that the incidence of radiation-induced brain injury was between 4
and 18% (16). Chong et al
(17) reported that radiation-induced
encephalopathy exhibited a long incubation period. Thus, monitoring
the structural alterations in the brain tissue is required.
Alterations in the water diffusion parameters in
normal-appearing WM on conventional MRI scans may be assessed
quantitatively using DTI. DTI has been widely used in studies of
the central nervous system and it has been used to investigate WM
injury following cranial irradiation (18,19). FA
imaging is most widely used in clinical research due to FA
exhibiting the highest Signal to Noise Ratio and the best detail
resolution. To the best of our knowledge, the present study was the
first time that TBSS was used to process the DTI data of patients
with NPC. In addition, previous studies of radiation-induced brain
injury have been confined to the temporal lobes, but, as radiation
may damage WM in other brain regions, the present study
investigated the whole brain.
Previous studies have demonstrated that FA values
decreased markedly in the acute phase and early delayed reaction
period following RT, and subsequently gradually recovered (6,7). Kitahara
et al (19) reported that,
following RT in patients with brain tumors, FA values decreased
markedly between 3 and 5 months, and subsequently recovered after 6
months. The results of the present study identified that FA values
in the left parietal lobe and in the right cerebellar hemisphere
WM, of patients between 0 and 6 months after RT, were statistically
significantly decreased compared with that of the control group,
and this is consistent with previous studies. The aforementioned
two brain regions are located close to the temporal lobe and may
have been included in the radiation field, resulting in WM damage.
In the acute phase and early delayed reaction period, the diffusion
rate of water molecules along the nerve fibers decreased due to
encephaledema, the demyelination of nerve fibers and the
destruction of myelin (19), and the
decreased FA values may reflect these pathological alterations in
the WM. In the present study, the FA values in the late delayed
reaction period remained markedly decreased, compared with that in
the pre-RT group. Additionally, the FA values in the right parietal
lobe WM markedly decreased in the post-RT 6–12 month group and the
values in the right temporal lobe WM markedly decreased after 12
months, compared with that in the pre-RT group. In the late delayed
reaction period, as the brain edema was absorbed, the degree of
demyelination decreased, myelin regeneration occurred and the FA
values in the partial lobe WM recovered gradually (7). In the present study, the FA values in
the left parietal lobe and right cerebellar hemisphere WM did not
markedly decrease after 6 months and the FA values in right
parietal lobe WM did not decrease significantly after 12 months.
The results of the present study indicated that the FA values in a
number of brain regions increased gradually, which was consistent
with previous studies. At the cessation of RT, the pathological
alterations in the WM, due to radiation injury, recovered
partially. The FA value in the right temporal lobe WM of patients
remained markedly decreased after 12 months, indicating that early
WM injury did not return to normal levels following RT and the
temporal lobe may be the most seriously damaged area.
In the present study, the FA values markedly
decreased in the parietal lobe, temporal lobe and cerebellar
hemisphere which indicated that radiation directly or indirectly
damaged the neural structure. There have been a number of
hypotheses to explain this phenomenon including that radiation may
damage the vascular endothelial cells and subsequently cause local
brain tissue ischemia of the whole brain (20). Radiation may induce central nervous
system autoimmune vasculitis, injuring the whole brain WM (21,22). Thus,
the microstructural alterations in the WM are dynamic and extensive
in patients with NPC following RT.
Certain patients with normal conventional MRI scans
exhibited markedly abnormal neurological symptoms, such as memory
loss, following radiotherapy for nasalpharyngeal carcinoma in a
previous study (23). In addition, a
number of neural psychology test studies have revealed that
patients with cranial irradiation experienced a decrease in
cognitive function (24). Previous
studies have been limited to the WM due to radiation sensitivity.
However, serious radiation encephalopathy may involve the GM and
glial cells, located in the GM, are sensitive to radiation.
Additionally, the blood vessels of the GM are richer compared with
that in the WM, which may be damaged by radiation; therefore,
following RT, the GM of the brain may undergo alterations.
Structural imaging using VBM is effective for evaluating GM volume
alterations and has been widely used in the study of diseases where
conventional imaging cannot be used, including drug addiction and
depression (25,26).
The GM volume alterations may be explained by a
number of potential underlying pathophysiological mechanisms,
including increases or decreases in neural or glial cells, cell
size and exchanges between blood and tissue fluid (27). Although a limited number of neurons
died following radiation, the dysfunction in axons and synapses may
induce central nervous system damage (28). In addition, radiation may eliminate
oligodendrocytes (29), resulting in
a decrease in GM volume. The results of the present study
identified that the brain GM volume decreased in the bilateral
temporal lobe, bilateral cerebellar or bilateral frontal lobe and
occipital lobe, which was consistent with a previous animal study,
which demonstrated that irradiation caused early volume deficits in
a number of brain regions in mice (30). The bilateral temporal lobe, bilateral
cerebellum and bilateral occipital lobe may be included in the
radiation field, which may be why the observed decreases in GM
volumes were primarily induced by radiation. Additionally, a
previous study demonstrated that chemotherapy may induce decreases
in the frontal lobe GM volume in patients with breast cancer
(31). Chemotherapy in combination
with RT has previously be used to treat patients with NPC and
therefore, the decrease in frontal lobe GM volume may result from
chemotherapy. The results of the present study demonstrated that
the alterations in the GM were widespread, complex and dynamic, and
did not involve the temporal lobe only. The most drastic
alterations and the most serious injury to brain tissue was
observed between 0 and 6 months; however, after 1 year, patients
recovered to a greater extent.
The present study had a number of limitations.
First, chemotherapy was not considered as a factor, as all the
patients included in the present study received this. Secondly, the
study lacked a healthy control group.
DTI and VBM were more sensitive compared with
conventional MRI in determining radiation-induced brain damage and
these two methods may be used in the long-term follow-up of
patients with NPC following RT, to monitor alterations indicative
of radiation-induced brain damage.
Glossary
Abbreviations
Abbreviations:
|
RT
|
radiotherapy
|
|
NPC
|
nasopharyngeal carcinoma
|
|
DTI
|
diffusion tensor imaging
|
|
TBSS
|
tract-based spatial statistic
|
|
WM
|
white matter
|
|
VBM
|
voxel-based morphometry
|
|
GM
|
gray matter
|
|
FA
|
fractional anisotropy
|
References
|
1
|
Shanmugaratnam K, Chan SH, de-Thé G, Goh
JE, Khor TH, Simons MJ and Tye CY: Histopathology of nasopharyngeal
carcinoma: Correlations with epidemiology, survival rates and other
biological characteristics. Cancer. 44:1029–1044. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Zhou GQ, Qi ZY, Zhang L, Huang SM,
Liu LZ, Li L, Lin AH and Ma J: Radiation-induced temporal lobe
injury after intensity modulated radiotherapy in nasopharyngeal
carcinoma patients: A dose-volume-outcome analysis. BMC Cancer.
13:3972013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Law SC, Ng SH, Chan DK, Poon YF,
Foo W, Tung SY, Cheung FK and Ho JH: Retrospective analysis of
nasopharyngeal carcinoma treated during 1976–1985: Late
complications following megavoltage irradiation. Br J Radiol.
65:918–928. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang Y, Luo D, Rong X, Rong X, Shi X and
Peng Y: Psychological disorders, cognitive dysfunction and quality
of life in nasopharyngeal carcinoma patients with radiation-induced
brain injury. PLoS One. 7:e365292012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glantz MJ, Burger PC, Friedman AH, Radtke
RA, Massey EW and Schold SC Jr: Treatment of radiation-induced
nervous system injury with heparin and warfarin. Neurology.
44:2020–2027. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang HZ, Qiu SJ, Lv XF, Wang YY, Liang Y,
Xiong WF and Ouyang ZB: Diffusion tensor imaging and 1H-MRS study
on radiation-induced brain injury after nasopharyngeal carcinoma
radiotherapy. Clin Radiol. 67:340–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong WF, Qiu SJ, Wang HZ and Lv XF: 1H-MR
spectroscopy and diffusion tensor imaging of normal-appearing
temporal white matter in patients with nasopharyngeal carcinoma
after irradiation: Initial experience. J Magn Reson Imaging.
37:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelles M, Block W, Träber F, Wüllner U,
Schild HH and Urbach H: Combined 3T diffusion tensor tractography
and 1H-MR spectroscopy in motor neuron disease. AJNR Am J
Neuroradiol. 29:1708–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokoyama K, Matsuki M, Shimano H, Sumioka
S, Ikenaga T, Hanabusa K, Yasuda S, Inoue H, Watanabe T, Miyashita
M, et al: Diffusion tensor imaging in chronic subdural hematoma:
Correlation between clinical signs and fractional anisotropy in the
pyramidal tract. AJNR Am J Neuroradiol. 29:1159–1163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang L, Wen W, Zhu W, Trollor J, Kochan
N, Crawford J, Reppermund S, Brodaty H and Sachdev P: White matter
integrity in mild cognitive impairment: A tract-based spatial
statistics study. Neuroimage. 53:16–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan YL, Leung SF, King AD, Choi PH and
Metreweli C: Late radiation injury to the temporal lobes:
Morphologic evaluation at MR imaging. Radiology. 213:800–807. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norris AM, Carrington BM and Slevin NJ:
Late radiation change in the CNS: MR imaging following gadolinium
enhancement. Clin Radiol. 52:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv XF, Zheng XL, Zhang WD, Liu LZ, Zhang
YM, Chen MY and Li L: Radiation-induced changes in normal-appearing
gray matter in patients with nasopharyngeal carcinoma: A magnetic
resonance imaging voxel-based morphometry study. Neuroradiology.
56:423–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith SM, Jenkinson M, Johansen-Berg H,
Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader
MZ, Matthews PM and Behrens TE: Tract-based spatial statistics:
Voxelwise analysis of multi-subject diffusion data. Neuroimage.
31:1487–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith SM and Nichols TE: Threshold-free
cluster enhancement: Addressing problems of smoothing, threshold
dependence and localisation in cluster inference. Neuroimage.
44:83–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valk PE and Dillon WP: Radiation injury of
the brain. AJNR Am J Neuroradiol. 12:45–62. 1991.PubMed/NCBI
|
|
17
|
Chong VF, Fan YF and Mukherji SK:
Radiation-induced temporal lobe changes: CT and MR imaging
characteristics. AJR Am J Roentgenol. 175:431–436. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makki MI, Chugani DC, Janisse J and
Chugani HT: Characteristics of abnormal diffusivity in
normal-appearing white matter investigated with diffusion tensor MR
imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol.
28:1662–1667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitahara S, Nakasu S, Murata K, Sho K and
Ito R: Evaluation of treatment induced cerebral white matter injury
by using diffusion-tensor MR imaging: Initial experience. AJNR Am J
Neuroradiol. 26:2200–2206. 2005.PubMed/NCBI
|
|
20
|
Russo C, Fischbein N, Grant E and Prados
MD: Late radiation injury following hyperfractionated craniospinal
radiotherapy for primitive neuroectodermal tumor. Int J Radiat
Oncol Biol Phys. 44:85–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xavier S, Piek E, Fujii M, Javelaud D,
Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, et
al: Amelioration of radiation-induced fibrosis: Inhibition of
transforming growth factor-beta signaling by halofuginone. J Biol
Chem. 279:15167–15176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SH, Lim DJ, Chung YG, Cho TH, Lim SJ,
Kim WJ and Suh JK: Expression of TNF-alpha and TGF-beta 1 in the
rat brain after a single high-dose irradiation. J Korean Med Sci.
17:242–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parkin AJ and Hunkin NM: Memory loss
following radiotherapy for nasal pharyngeal carcinoma-an unusual
presentation of amnesia. Br J Clin Psychol. 30:349–357. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheung M, Chan AS, Law SC, Chan JH and Tse
VK: Cognitive function of patients with nasopharyngeal carcinoma
with and without temporal lobe radionecrosis. Arch Neurol.
57:1347–1352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Özdemir Hİ, Eker MÇ, Zengin B, Yilmaz DA,
İşman Haznedaroğlu D, Çınar C, Kitiş Ö, Akay A and Gönül AS: Gray
matter changes in patients with deficit schizophrenia and
non-deficit schizophrenia. Turk Psikiyatri Derq. 23:237–246.
2012.
|
|
26
|
Lai CH: Gray matter volume in major
depressive disorder: A meta-analysis of voxel-based morphometry
studies. Psychiatry Res. 211:37–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt-Wilcke T, Leinisch E, Straube A,
Kämpfe N, Draganski B, Diener HC, Bogdahn U and May A: Gray matter
decrease in patients with chronic tension type headache. Neurology.
65:1483–1486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi L, Adams MM, Long A, Carter CC,
Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R and
Brunso-Bechtold JK: Spatial learning and memory deficits after
whole-brain irradiation are associated with changes in NMDA
receptor subunits in the hippocampus. Radiat Res. 166:892–899.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tofilon PJ and Fike JR: The radioresponse
of the central nervous system: A dynamic process. Radiat Res.
153:357–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gazdzinski LM, Cormier K, Lu FG, Lerch JP,
Wong CS and Nieman BJ: Radiation-induced alterations in mouse brain
development characterized by magnetic resonance imaging. Int J
Radiat Oncol Biol Phys. 84:e631–e638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDonald BC, Conroy SK, Ahles TA, West JD
and Saykin AJ: Gray matter reduction associated with systemic
chemotherapy for breast cancer: A prospective MRI study. Breast
Cancer Res Treat. 123:819–828. 2010. View Article : Google Scholar : PubMed/NCBI
|