Introduction
Although there have been various improvements in
detection, diagnosis and treatment, cancer remains the number two
cause of mortality in the world, and accounts for a higher number
of mortalities than heart disease in those under 85 years of age
(1). One of the biggest challenges
currently in cancer treatment is to provide effective anticancer
therapy without substantial adverse effects, while simultaneously
minimizing toxicity. Considering the severe side effects of
chemotherapy, studies have been increasingly focusing on the
anticancer potential of various vegetables, including potatoes
(2,3).
Potatoes are one of the most commonly consumed
vegetables worldwide and are a good source of antioxidants,
including phenolic compounds, vitamin C and carotenoids (4). Previous studies have demonstrated that
potato extract (PE) exhibits anticancer, antiviral and
anti-parasite activities in vitro and in vivo
(5–7).
Cheng et al (8) observed that
rhamnogalacturonan I domain-rich pectin from potato inhibits the
proliferation of human colon cancer HT-29 cells and induces
significant G2/M cell cycle arrest. Additionally, Yan et al
(9) and Gundala et al
(10) have reported that chlorogenic
acid, the predominant phenolic compound in potato, inhibits
carcinogenesis in liver and prostate cancer cells in vitro
and in vivo.
Studies on the anticancer properties of potato have
utilized PEs derived from various methods (6,11,12). In a comparative analysis of eight
phytoplankton chlorophyll-extraction methods (13), it has been shown that the freeze-thaw
method produces high quality and stable phytoplankton chlorophyll,
and is convenient to use. The authors of the present study
successfully patented the ‘potato freeze-thaw solution (PFTS)’ in
the national patented invention (grant no. CN105211794A) (14). Our previous study demonstrated that
PFTS exhibits an anti-inflammatory effect on the lung tissue of
rats with chronic obstructive pulmonary disease induced by
cigarette smoke (15). Considering
the bioactivity of PFTS, it is unknown whether the bioactive
constituents of PFTS possess anticancer properties. In the present
study, the effect of PEs from PFTS on the immune function was
investigated, including white blood cell (WBC) counts, macrophage
phagocytosis and lymphocyte transformation in tumor-bearing mice.
It was also examined whether PFTS has an antitumor property by
measuring the survival time of tumor-bearing mice.
Materials and methods
Ethics
All experimental animal procedures were conducted
according to the Institutional Animal Care and Use Committee at
Inner Mongolia Medical University (Inner Mongolia Medical
University, Jinshan Economic and Technological Development Zone,
Hohhot, China). The protocol of the present study was approved by
the Ethics Committee of Inner Mongolia Medical University prior to
the initiation of the study, and permission was obtained to perform
the study.
Preparation of PFTS and amino acid
analysis
Fresh potato Kexing IV was developed by the Potato
Research Institute of Heilongjiang Academy of Agricultural Sciences
(Heilongjiang, China), and cultivated by Sheng Feng Potato Industry
Planting Base in the Wuchua area of the Inner Mongolia Autonomous
Region in China. The PE was isolated by PFTS as described in our
previous study (15). Briefly,
potatoes were first frozen at −30°C for 12 h, and then thawed at
35°C and disrupted. Following centrifugation at 1,000 × g for 30
min at 4°C, the supernatant was collected. The extracted liquid was
then purified by macroporous adsorptive resins (0.45 µm; Nantong
FilterBio Technology, Jiangsu, China) at room temperature, and
stored at 4°C until used for experiments. Amino acid compositions
of PFTS were described in our previous study (15).
Amino acid composition of PE was detected by an
amino acid analyzer (L-8900; Hitachi, Ltd., Tokyo, Japan).
Different amino acids were eluted sequentially based on their ionic
strength (acidic amino acids were firstly obtained, neutral amino
acids were then obtained, and finally, basic amino acids were
obtained). Subsequently, these amino acids were reacted with
ninhydrin at 135°C. Finally, the concentrations of amino acids were
quantified by an ultraviolet detector (VIS-7220N; Beijing
Beifen-Ruili Analytical Instrument (Group) Co. Ltd., Beijing,
China) at 570 and 440 nm.
Mice treatment
A total of 80 6–8-week-old Kunming mice (40 males
and 40 females; weight, 18–22 g) were purchased from Beijing
Weitong Lihua Experimental Animal Technology Co., Ltd. (Beijing,
China). To suppress the immune function of mice, all mice were
injected intramuscularly with cyclophosphamide (4 mg) 1 and 3 days
prior to further treatment (16). In
total, 20 mice were then each administered either 1 ml of PFTS or
1.8 mg of Ganoderma lucidum (G. lucidum; Research Center of
Bioresource & Bioenergy, School of Biotechnology, Jiangnan
University, Jiangsu, China) twice a day by gavage. Another 20 mice
were treated with 1 ml of PBS. An additional 20 mice receiving no
treatment served as the negative control group. On days 3 and 10,
peripheral blood was collected from the tails and WBC count
(neutrophils, eosinophils, basophils, monocytes and lymphocytes)
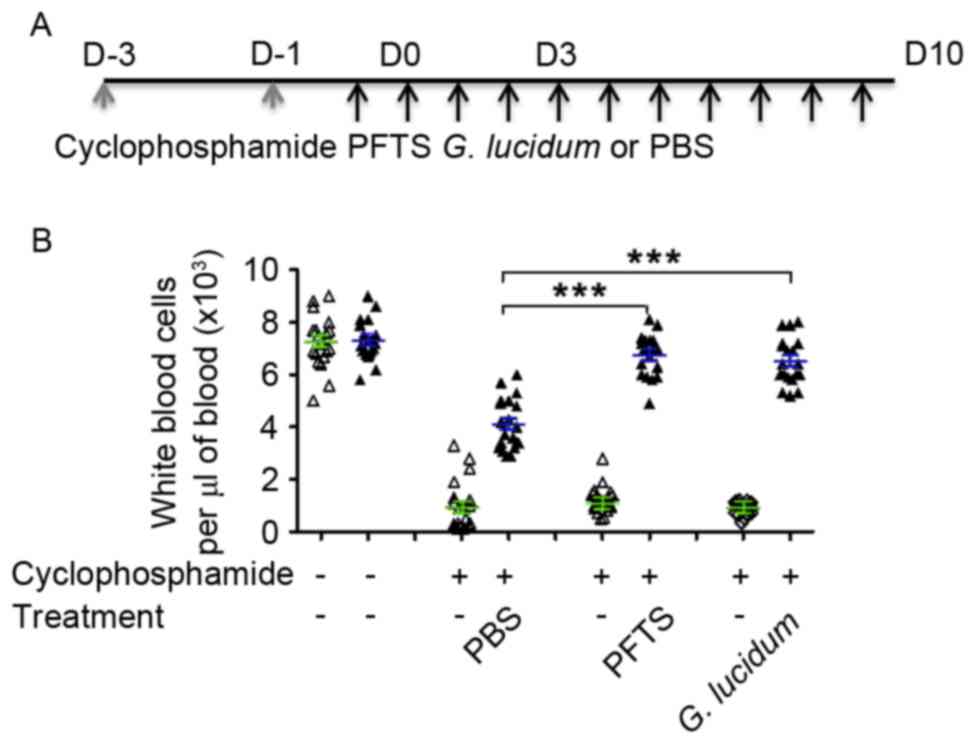
was measured. The schema of mouse treatment is shown in Fig. 1A. All mice were housed at 25°C with a
12-h light/12-h dark cycle with free access to pellet chow and
water.
Peritoneal macrophage phagocytic
index
To investigate whether PFTS could enhance innate
immune functions, 20 mice were each treated with either 1 ml of
PFTS or 1.8 mg of G. lucidum twice a day for 20 days by
gavage. On day 20 these mice were injected intraperitoneally with
0.5 ml of 1% thioglycollate broth (Sigma-Aldrich; Merck KGaA) to
induce the infiltration of macrophages. On the following day, these
mice were injected intraperitoneally with 0.5 ml of 1% red blood
cells from chicken (Bersee Biotechnology, Beijing, China). At 40
min post-injection of red blood cells, the peritoneal fluid was
collected. Subsequent to centrifugation (1,500 × g), peritoneal
cells were smeared and stained using the Giemsa stain
(Sigma-Aldrich; Merck KGaA). In brief, cell films on slides were
incubated in May-Grünwald stain at room temperature for 5 min.
Subsequent to washing in phosphate buffer, cell films were then
incubated in dilute Giemsa solution for 20 min at room temperature,
followed by rinsing in deionized water. Morphological and
morphometrical analyses were performed using a microscopy system at
×20 magnification (BX 50; Olympus Corporation, Tokyo, Japan)
connected to a digital camera (DP70; Olympus Corporation).
Measurements of areas of interest were conducted using MetaMorph
software (version NX2.5, Molecular Devices, LLC, Sunnyvale, CA,
USA). The macrophage phagocytic index was calculated as a
percentage of macrophages engulfing chicken red blood cells.
Lymphocyte transformation rate
To investigate whether PFTS enhances the rate of
lymphocyte transformation, 20 mice were each treated with either
PFTS or G. lucidum twice a day for 20 days by gavage. On day
20, these mice were injected intramuscularly with 0.4 mg of
phytohaemagglutinin (Ziqi Biotechology, Shanghai, China). Blood was
collected from tails, and smeared and stained using the
aforementioned Giemsa stain method. Morphological and
morphometrical analyses were performed using a microscopy system
(BX 50; Olympus Corporation) connected to a digital camera (DP70;
Olympus Corporation). Measurements of areas of interest were
conducted using MetaMorph software (version NX2.5, Molecular
Devices, LLC). The percentage of lymphocyte transformation was
calculated by dividing the number of lymphoblasts by the total
number of lymphocytes and lymphoblasts.
Ascites tumor model
To investigate whether PFTS extends the survival
time of tumor-bearing mice, 20 mice were inoculated
intraperitoneally with 1 ml of 2.3×109 S-180 ascites
tumor cells (purchased from X-Y Biotechnology, Shanghai, China). A
total of 10 mice were treated with 1 ml of PFTS three times per day
by gavage and another 10 mice were treated with PBS (serving as the
control). The growth of ascites tumor was monitored by body weight
and the appearance of the abdomen. The survival time of
tumor-bearing mice was measured.
Statistical analysis
All data are expressed as the mean ± standard error
of mean. Statistical analysis was performed by SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). For the analysis of WBC counts,
macrophage phagocytic index and lymphocyte transformation rate, the
Mann-Whitney test was used. The log-rank test was performed to
analyze difference of survival between tumor-bearing and control
mice. P<0.05 was considered to indicate a statistically
significant difference.
Results
Amino acid compositions of PFTS
Total content of amino acids was 648.3 mg/ml in PE.
It was identified to be composed by various amino acids, including
aspartic acid (227.348 mg/100 ml), glutamic acid (127.686 mg/100
ml), valine (44.407 mg/100 ml), alanine (25.295 mg/100 ml),
threonine (23.350 mg/100 ml), leucine (22.354 mg/100 ml),
isoleucine (21.290 mg/100 ml), phenylalanine (21.909 mg/100 ml),
glycine (15.796 mg/100 ml), serine (15.620 mg/100 ml), cystine
(9.005 mg/100 ml), methionine (12.986 mg/100 ml), lysine (24.309
mg/100 ml), arginine (20.140 mg/100 ml), proline (21.162 mg/100 ml)
and histidine (8.583 mg/100 ml).
PFTS increases peripheral WBCs
suppressed by cyclophosphamide comparably to G. lucidum
To test the effect of PFTS on peripheral WBC counts,
pre-treatment with cyclophosphamide, a well-established
immunosuppressant was used to suppress immune function of mice.
Following two doses of cyclophosphamide treatment, WBC counts
dropped 10-fold from the value detected in the untreated mice group
(7.2×103 cells/µl) to that observed in the
cyclophosphamide-treated mice groups (0.7×103 cells/µl)
(Fig. 1B). Cyclophosphamide-treated
mice were then fed with PFTS or G. lucidum for 3 or 10 days
and WBC counts were measured in these mice. As shown in Fig. 1B, WBC counts were significantly higher
in mice treated with either PFTS or G. lucidum for 10 days
compared with those exhibited by PBS-treated mice. WBC counts in
the PFTS-treated mice reached a similar level to those in the
cyclophosphamide-untreated mice (6.76±0.77 vs. 7.28±0.76,
respectively; P=0.0978 by the Mann-Whitney test). Notably, the
PFTS-mediated effect was comparable to that caused by G.
lucidum, a well-known agent that increases WBC counts (17).
In addition, it was observed that the proportion of
subsets of WBCs (neutrophils, eosinophils, basophils, monocytes and
lymphocytes) was unchanged (data not shown) in PFTS-treated mice,
which was comparable to the results obtained in
cyclophosphamide-untreated mice. Thus, as food nutrition, PFTS may
serve as a dietary supplement to increase the WBC count of patients
receiving chemotherapy.
PFTS enhances peritoneal macrophage
phagocytosis
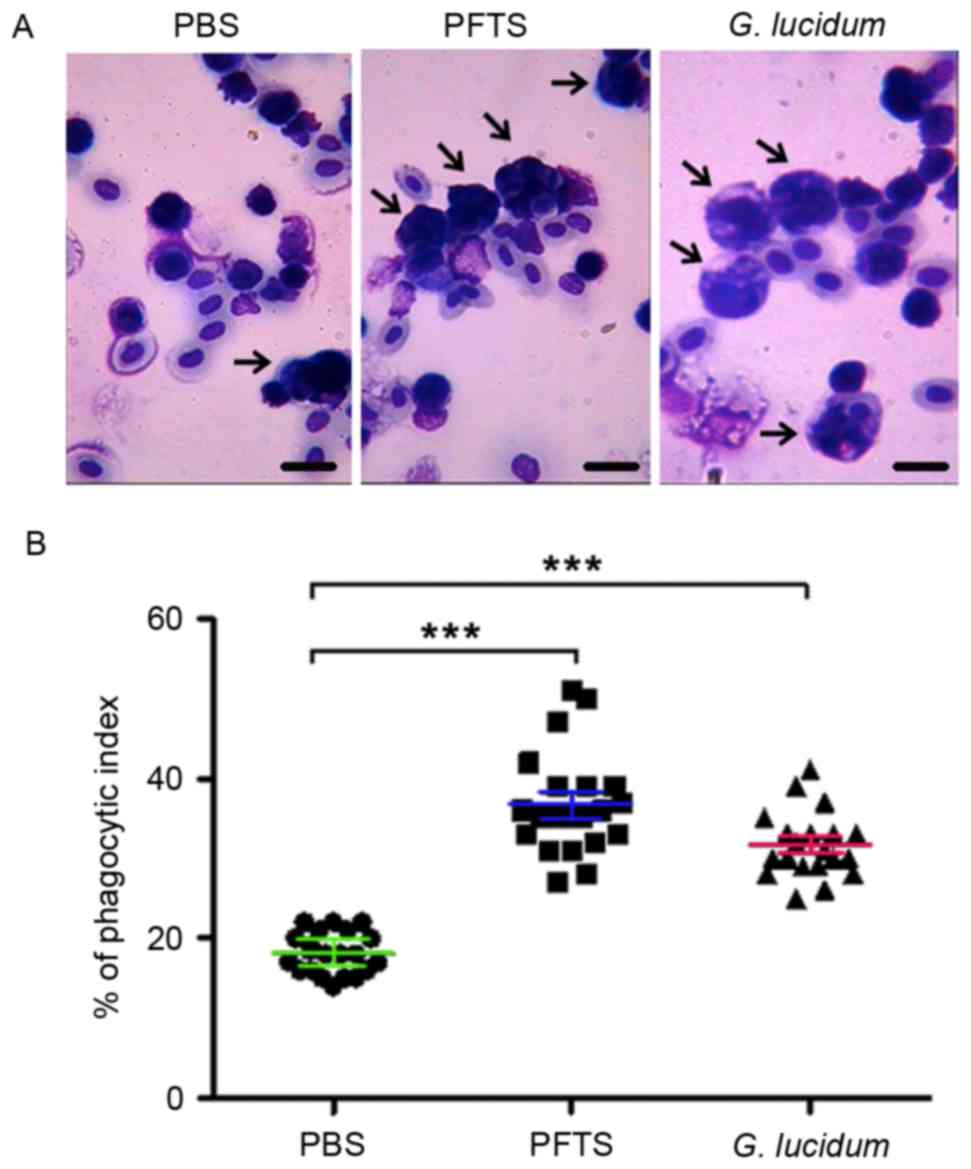
To investigate whether PFTS enhances innate immune
function, the capacity of macrophages engulfing chicken red blood
cells in mice treated with PFTS was determined. By Giemsa staining,
it was revealed that macrophages engulfed more chicken red blood
cells in the PFTS and G. lucidum groups than in the
PBS-treated group (Fig. 2A). The
phagocytic index was significantly higher in PFTS-treated mice
(36.8±4.1%) compared with that in PBS-treated mice (18.1±2.8%), and
was comparable to that in G. lucidum-treated mice
(31.6±4.7%) (Fig. 2B). These results
indicated that PFTS promotes innate immune function by enhancing
peritoneal macrophage phagocytosis.
PFTS improves the lymphocyte
transformation rate
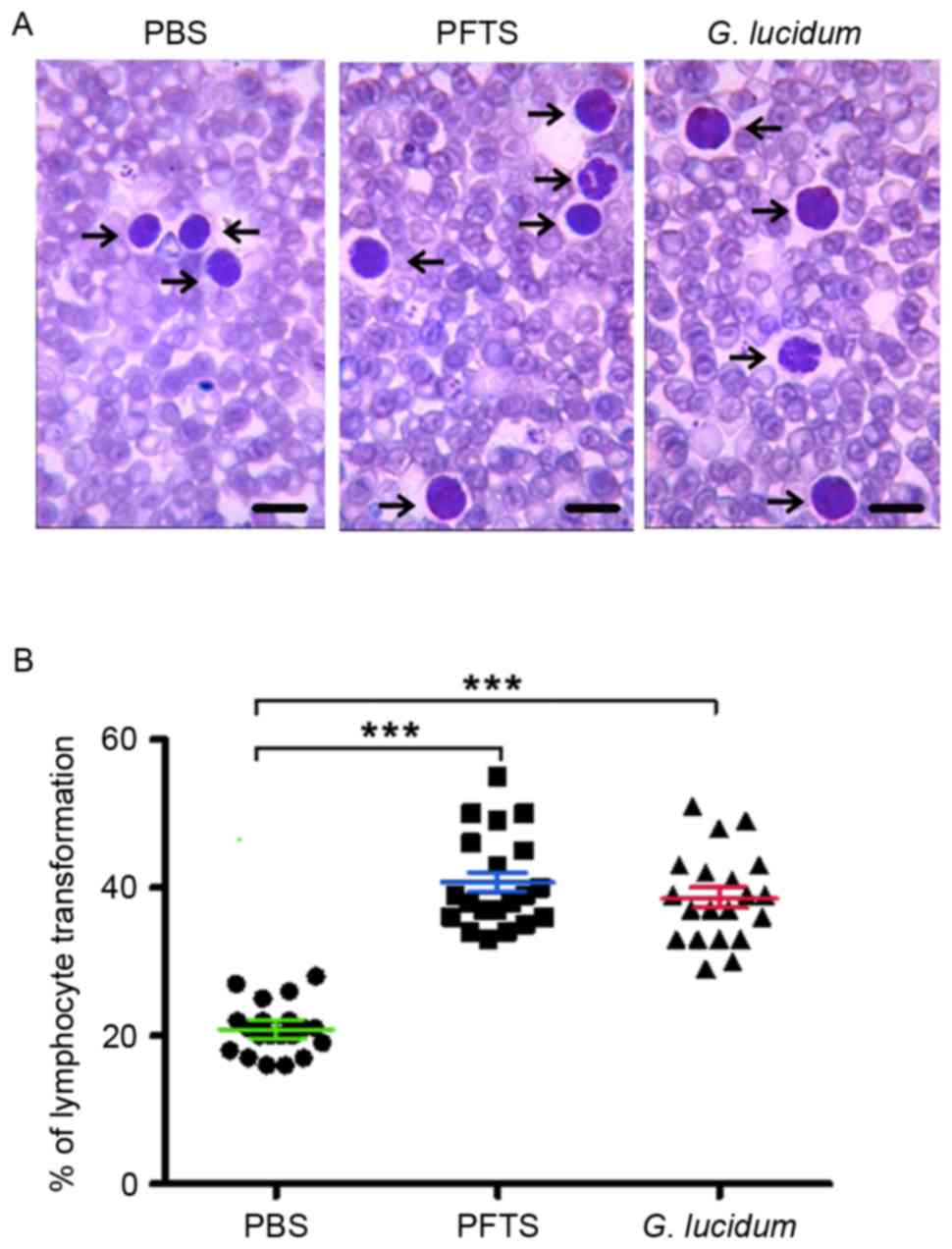
It was then investigated whether PFTS improves the
lymphocyte transformation rate. By Giemsa staining, it was revealed
that treatment with phytohaemagglutinin transformed more
lymphocytes in PFTS- or G. lucidum-treated mice than in
PBS-treated mice (Fig. 3A). The
lymphocyte transformation rate was 40.7±5.1 and 38.6±5.7% in the
PFTS and G. lucidum groups, respectively, which was
significantly higher than that in the PBS-treated group (20.9±3.9%)
(Fig. 3B).
PFTS prolongs the survival of
tumor-bearing mice
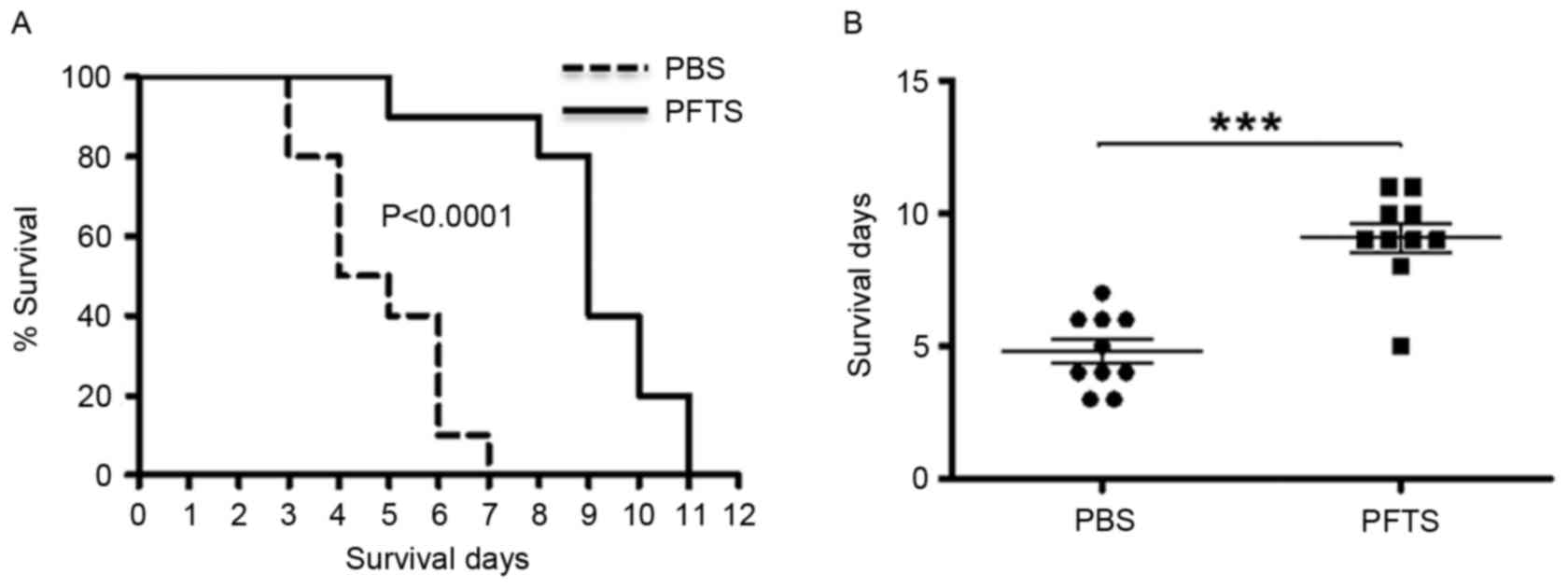
The present study demonstrated that PFTS enhances
the immune response in mice, and then aimed to test whether PFTS
possesses antitumor properties. A tumor-bearing mouse model was
established by inoculating mice intraperitoneally with S-180 tumor
cells, and the survival of these mice with either PFTS or PBS
treatment was then measured. As shown in Fig. 4A, the median survival time was 9.1 and
4.8 days in PFTS- and PBS-treated mice, respectively. Using the
log-rank test analysis, PFTS-treated mice had a significantly
longer survival time compared with that exhibited by PBS-treated
mice (P<0.0001; Fig. 4B).
Discussion
In the present study, PFTS was demonstrated to
enhance peripheral WBCs suppressed by cyclophosphamide, improve
peritoneal macrophage phagocytosis and increase the rate of
lymphocyte transformation in mice. Furthermore, PFTS extended the
survival time of tumor-bearing mice. To the best of our knowledge,
the present study is the first to clarify the effects of the
freeze-thaw-extracted PFTS on immune function and antitumor
activity in vivo.
Leukocytes or WBCs are a major component of the
immune system and perform a crucial role in defending the body
against infectious organisms and carcinogenesis (18). Abrogation of leukocytes with
chemotherapy drugs is life-threatening for patients with cancer
(19). Although granulocyte
macrophage-colony-stimulating factor is usually effective in
raising leukocyte count following chemotherapy, its cost and side
effects pose a challenge for patients (20). In a previous study, PFTS significantly
enhanced peripheral leukocytes and almost completely reversed the
leukopenia in mice induced by cyclophosphamide, to a similar level
to that mediated by G. lucidum, a well-known immune
activator (21). These findings are
compatible with a notion that dietary supplementation of purple
sweet potato extract attenuates the suppression of T cell and B
cell proliferation and T helper 1/T helper 2 cytokine imbalance in
immunodeficient mice, partly contributing to ameliorate immune
dysfunction (22). Collectively, it
was speculated that stimulation of leukocyte proliferation or
counteraction of bone marrow suppression may contribute to
PFTS-mediated reversal of leukopenia by cyclophosphamide (23).
It is of interest to know whether PFTS has the
potential to increase immune functions. This question was addressed
by measuring the peritoneal macrophage phagocytosis and lymphocyte
transformation in mice treated with PFTS. It has been well
established that macrophage phagocytosis characterizes the
activation of macrophages and reflects innate immune responses, and
that lymphocyte transformation reflects the ability of lymphocytes
to respond to antigen stimulation and represents adaptive immunity
(24). Innate and adaptive immunity
serve important roles in preventing infections and surveying
tumorigenesis (25,26). The present data demonstrated that, as
with G. lucidum, PFTS increased peritoneal macrophage
phagocytosis and the rate of lymphocyte transformation. In this
line, a dose-dependent manner of purified sweet potato
polysaccharide has been demonstrated to affect the phagocytic
function (27).
The finding that PFTS is able to promote immune
functions led us to test directly whether PFTS has antitumor
properties. Using a mouse tumor model, it was observed that mice
fed with PFTS had significantly longer survival times compared with
those of mice fed with PBS, demonstrating its antitumor property.
These results were supported by a previous study showing that
polyphenol-rich sweet potato greens extract extends survival time
by inhibiting proliferation and inducing apoptosis in prostate
cancer cells in vitro and in vivo (28). The mechanisms underlying the antitumor
function of PFTS remain to be elucidated.
There are four caveats to the current study that
require brief mention. First, only potato cultivated in the Wuchuan
area of Inner Mongolia, mid-west region of China was tested, where
the climate is dry and sunshine is abundant. It is unknown whether
potatoes cultivated in other geographical regions are similar to
those used in the current study. Second, the freeze-thaw method
causes cells to swell and shrink and break up ultimately due to the
ice crystals formed in the freezing process. Several freeze-thaw
cycles may be required to facilitate cell membrane breakage and the
release of cell components (29).
Methodological modification is ongoing for maximizing the PFTS
bioactivity. Third, following the routine drug discovery approach,
PFTS bioactivity was tested but not observed in vitro. This
may be a result of physical or chemical cytotoxicity. Lastly, the
effect of PFTS on macrophage phagocytosis and lymphocyte
transformation was assessed at only one time point (day 20), but
its long-term effects were not evaluated. These assessments from
multiple doses and time points are required in future studies.
In conclusion, the present study demonstrated for
the first time that PFTS improves immune functions and extends the
survival time of mice with ascite tumors. With its ease of
production and dietary administration path, PFTS may possess the
potential to become a clinical option for the prevention and
treatment of cancer. While the present study is only a first step
towards uncovering the anticancer properties of PFTS, further
investigation is warranted to assess the bioactivity and clinical
potential of PFTS comprehensively.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560013) and the
Hospital Fund from Inner Mongolia Autonomous Region People's
Hospital (grant no. 201529), Inner Mongolia Autonomous Region,
China. The authors thank Dr Xingyu Yao (Hulun Buir City Hospital of
Chinese and Mongolian Medicine, Hulun Buir, Inner Mongolia, China)
for technical assistance during the experiments and English
correction of the manuscript.
References
|
1
|
Jemal A, Siege R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charepalli V, Reddivari L, Radhakrishnan
S, Vadde R, Agarwal R and Vanamala JK: Anthocyanin-containing
purple-fleshed potatoes suppress colon tumorigenesis via
elimination of colon cancer stem cells. J Nutr Biochem.
26:1641–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman M: Chemistry and anticarcinogenic
mechanisms of glycoalkaloids produced by eggplants, potatoes, and
tomatoes. J Agric Food Chem. 63:3323–3337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Deng L, Chen J, Zhou S, Liu S, Fu Y,
Yang C, Liao Z and Chen M: An analytical pipeline to compare and
characterise the anthocyanin antioxidant activities of purple sweet
potato cultivars. Food Chem. 194:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugata M, Lin CY and Shih YC:
Anti-Inflammatory and anticancer activities of Taiwanese
purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts.
Biomed Res Int. 2015:7680932015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Ashaal HA: Regeneration, in vitro
glycoalkaloids production and evaluation of bioactivity of callus
methanolic extract of Solanum tuberosum L. Fitoterapia. 81:600–606.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuber T, Holm D, Byrne P, Ducreux L,
Taylor M, Kaiser M and Stushnoff C: Optimization of in vitro
inhibition of HT-29 colon cancer cell cultures by Solanum tuberosum
L. extracts. Food Funct. 6:72–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng H, Zhang Z, Leng J, Liu D, Hao M,
Gao X, Tai G and Zhou Y: The inhibitory effects and mechanisms of
rhamnogalacturonan I pectin from potato on HT-29 colon cancer cell
proliferation and cell cycle progression. Int J Food Sci Nutr.
64:36–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan Y, Li J, Han J, Hou N, Song Y and Dong
L: Chlorogenic acid enhances the effects of 5-fluorouracil in human
hepatocellular carcinoma cells through the inhibition of
extracellular signal-regulated kinases. Anticancer Drugs.
26:540–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gundala SR, Yang C, Lakshminarayana N,
Asif G, Gupta MV, Shamsi S and Aneja R: Polar biophenolics in sweet
potato greens extract synergize to inhibit prostate cancer cell
proliferation and in vivo tumor growth. Carcinogenesis.
34:2039–2049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Truong VD, Hu Z, Thompson RL, Yencho GC
and Pecota KV: Pressurized liquid extraction and quantification of
anthocyanins in purple-fleshed sweet potato genotypes. J Food
Compost Anal. 26:96–103. 2012. View Article : Google Scholar
|
|
12
|
Madiwale GP, Reddivari L, Stone M, Holm DG
and Vanamala J: Combined effects of storage and processing on the
bioactive compounds and pro-apoptotic properties of color-fleshed
potatoes in human colon cancer cells. J Agric Food Chem.
60:11088–11096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng J, Li Yb and Zhu Q: Comparison of
methods for phytoplankton chlorophyII-a concentration measurement.
Ecol & Envir. 2:524–528. 2008.(In Chinese).
|
|
14
|
Limin Yang: Method for extracting full
active nutrient solution of Potato by ultra low temperature (grant
no. CN105211794A). State Intellectual Property Office of China.
2016.
|
|
15
|
Xu GH, Shen J, Sun P, Yang ML, Zhao PW,
Niu Y, Lu JK, Wang ZQ, Gao C, Han X, et al: Anti-inflammatory
effects of potato extract on a rat model of cigarette smoke-induced
chronic obstructive pulmonary disease. Food Nutr Res. 59:288792015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saida Y, Watanabe S, Tanaka T, Baba J,
Sato K, Shoji S, Igarashi N, Kondo R, Okajima M, Koshio J, et al:
Critical roles of chemoresistant effector and regulatory T cells in
antitumor immunity after lymphodepleting chemotherapy. J Immunol.
195:726–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng S and Sliva D: Ganoderma lucidum for
cancer treatment: We are close but still not there. Integr Cancer
Ther. 14:249–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller WA: Getting leukocytes to the site
of inflammation. Vet Pathol. 50:7–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta RG, Murillo G, Naithani R and Peng
X: Cancer chemoprevention by natural products: How far have we
come? Pharm Res. 27:950–961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong IS: Stimulatory versus suppressive
effects of GM-CSF on tumor progression in multiple cancer types.
Exp Mol Med. 48:e2422016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferreira IC, Heleno SA, Reis FS, Stojkovic
D, Queiroz MJ, Vasconcelos MH and Sokovic M: Chemical features of
Ganoderma polysaccharides with antioxidant, antitumor and
antimicrobial activities. Phytochemistry. 114:38–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim OK, Nam DE, Yoon HG, Baek SJ, Jun W
and Lee J: Immunomodulatory and antioxidant effects of purple sweet
potato extract in LP-BM5 murine leukemia virus-induced murine
acquired immune deficiency syndrome. J Med Food. 18:882–889. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neboh EE and Ufelle SA: Myeloprotective
activity of crude methanolic leaf extract of Cassia occidentalis
incyclophosphamide-induced bone marrow suppression in Wistar rats.
Adv Biomed Res. 4:52015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ley K, Pramod AB, Croft M, Ravichandran KS
and Ting JP: How mouse macrophages sense what is going on. Front
Immunol. 7:2042016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu G, Bi Y, Wang R and Wang X:
Self-eating and self-defense: Autophagy controls innate immunity
and adaptive immunity. J Leukoc Biol. 93:511–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Djaldetti M and Bessler H: Modulators
affecting the immune dialogue between human immune and colon cancer
cells. World J Gastrointest Oncol. 6:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Kan J, Li Z and Chen Z:
Characterization and immunostimulatory activity of an
(1→6)-a-D-glucan from the root of Ipomoea batatas. Int
Immunopharmacol. 5:1436–1445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karna P, Gundala SR, Gupta MV, Shamsi SA,
Pace RD, Yates C, Narayan S and Aneja R: Polyphenol-rich sweet
potato greens extract inhibits proliferation and induces apoptosis
in prostate cancer cells in vitro and in vivo. Carcinogenesis.
32:1872–1880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barbas JP and Mascarenhas RD:
Cryopreservation of domestic animal sperm cells. Cell Tissue Bank.
10:49–62. 2009. View Article : Google Scholar : PubMed/NCBI
|