Introduction
Colorectal cancer (CRC) is one of the most prevalent
and highly diagnosed types of cancer and is a common cause of
cancer-associated mortality worldwide, despite the availability of
a variety of therapeutic strategies (1,2).
Ulcerative colitis (UC) is a serious inflammatory bowel disease in
humans and has been demonstrated to be a high-risk factor for CRC
(3,4).
Thus, the early diagnosis and treatment of UC may delay its
progression to CRC. Furthermore, lifestyle and diet each serve an
important role in the aetiology of cancer at the majority of sites
(5,6).
Specifically, high intakes of red meat, fat and carbohydrates have
been suggested to increase the risk of CRC (7). By contrast, lactoferrin (LF), fruit,
vegetable and fibre intake may reduce the risk of developing CRC
(8). Therefore, altering dietary
habits and consuming appropriate foods may serve as a novel
therapeutic strategy for the prevention of human cancer.
LF is an 80 kDa iron-binding, single-chain
glycoprotein that was first purified from human milk (9). It is expressed in the secretory granules
of neutrophils and in various secretory fluids, including milk,
tears, nasal fluids, saliva, pancreatic fluids and gastrointestinal
fluids (10). LF has been reported to
exert a wide range of physiological functions, including
anticancer, antimicrobial, anti-inflammatory and immune regulatory
activities (11,12). In addition, previous studies have
observed that bLF induces the suppression of proliferation in
various types of cancer cells in vitro (13–16). The
carcinogen 1,2-dimethylhydrazine (DMH) is widely used to induce CRC
in animal models (17). DMH also
induces the formation of aberrant cryptic foci, which are involved
in the multistep pathogenesis of colon cancer (18). Dextran sulphate sodium (DSS) is a
synthesised sulphated polyglucan that has previously been used to
induce gut inflammation and colitis in animal models (19,20).
In the present study, the aim was to comprehensively
evaluate the effect of liposomal bovine LF (LbLF), which is covered
in soybean lecithin and exhibits improved stability in the stomach
and enhanced absorption by the intestinal tract than bLF, on
DMH-induced colorectal cancer following treatment with DSS in F344
rats.
Materials and methods
Preparation of LbLF
The test sample, which consisted of multi-lamellar
vesicles, was prepared by hydrating dietary soy phosphatidylcholine
with an aqueous solution containing bLF. Briefly, 10.2% (w/w) soy
phosphatidylcholine solubilised in glycerine and 19.8% (w/w) bLF
were mixed at a ratio of 1.00:1.54, and emulsified (R&D
Division, Sunstar Inc., Osaka, Japan). The emulsified solution was
then liposomalised using a high-pressure homogenizer. The diameter
of the liposomes was determined using a particle size analyser, and
the mean diameter was ~70 nm. The control solution (glycerine) was
prepared in a similar manner.
Animals and diet
A total of 36 male 5-week-old F344 rats (weighing
70–90 g) were purchased from Charles River Laboratories Japan, Inc.
(Yokohama, Japan). The animals were cared for in compliance with
the principles and guidelines of the Ethical Committee for Animal
Care of the Prefectural University of Hiroshima (Hiroshima, Japan)
and the Prefectural University of Hiroshima Animal Ethics Committee
in accordance with the Japanese National Law on Animal Care and
Use. The Ethical Committee for Animal Care of the Prefectural
University of Hiroshima (Hiroshima, Japan) approved the experiments
undertaken. The rats were housed in an air-conditioned room at the
Laboratory Animal Research Centre of the Prefectural University of
Hiroshima, Japan. The room provided a 12-h light/dark cycle, a
controlled ambient temperature of 23±2°C and a humidity of 50±10%.
The rats had free access to drinking water and were fed a moderate
fat basal diet (Oriental Yeast Co., Ltd., Tokyo, Japan).
Experimental protocol
DMH (Tokyo Chemical Industry Co., Ltd., Tokyo,
Japan) was dissolved in 0.9% NaCl solution, and the pH was adjusted
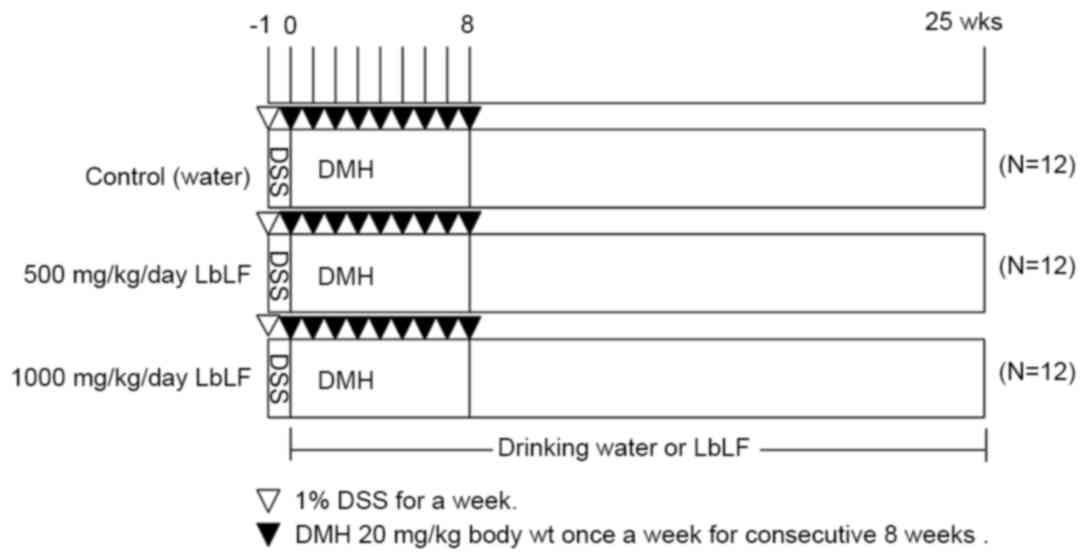
to 6.5 using NaHCO3. As indicated in Fig. 1, the drinking water of all 36 rats was
supplemented with 1% DSS for one week (week-1), starting at 5 weeks
of age. Upon reaching 6 weeks of age (week 0), the rats were
randomly allocated into three groups of 12 rats each. Each group
received water (control), 500 or 1,000 mg/kg/day LbLF from week
0–25. All rats were injected with DMH (20 mg/kg body weight) once
per week for 8 consecutive weeks (weeks 0–8). The body weights and
LbLF dilution intake of the rats were recorded every week to
determine the correct dose of LbLF. All rats were sacrificed by
anaesthesia (45 mg/kg body weight of sodium pentobarbital). 25
weeks following the commencement of DMH administration to allow for
tissue examination.
Analysis of aberrant crypt foci
(ACF)
After the rats were sacrificed, the colons were
quickly removed, flushed with saline solution and opened
longitudinally from the cecum to the anus. The colons were placed
on a paper towel and fixed in 10% buffered formalin for 24 h at
room temperature. They were stained with 0.5% methylene blue for
15–30 min at room temperature and then placed on a glass slide,
with the luminal side facing up. The stained colons were observed
under a light microscope at a magnification of ×20–30. The number
of ACFs was recorded.
Histological analysis
After the ACFs had been recorded, the 10% buffered
formalin-fixed colons were embedded in paraffin, sectioned at a
thickness of 4 µm, stained with haematoxylin and eosin (H&E)
and examined under a light microscope (magnification, ×100; Olympus
Corporation, Tokyo, Japan). The counted tumours were classified
into two types: Adenomas (including mild, moderate or severe
dysplasia categorisations) and adenocarcinomas (including well,
moderately or poorly differentiated tubular adenocarcinoma, signet
ring cell or mucinous carcinoma categorisations).
Cell lines and cell culture
RKO and RCN-9 human CRC cells, provided by the
Japanese Collection of Research Bioresources Cell Bank (Ibaraki,
Japan), were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) and 100 U/ml penicillin-streptomycin at 37°C in a
humidified atmosphere of 5% CO2. For the growth assay,
5×103 cells were plated onto 24-well plates (Falcon;
Corning Incorporated, Corning, NY, USA) and cultured in DMEM with
10% FBS at 37°C in a humidified atmosphere of 5% CO2.
Subsequently, trypsinized cells were counted at 0, 1, 2, and 3 days
using a Cell Counter (Coulter Z1, Coulter Co., Hialeah, FL,
USA).
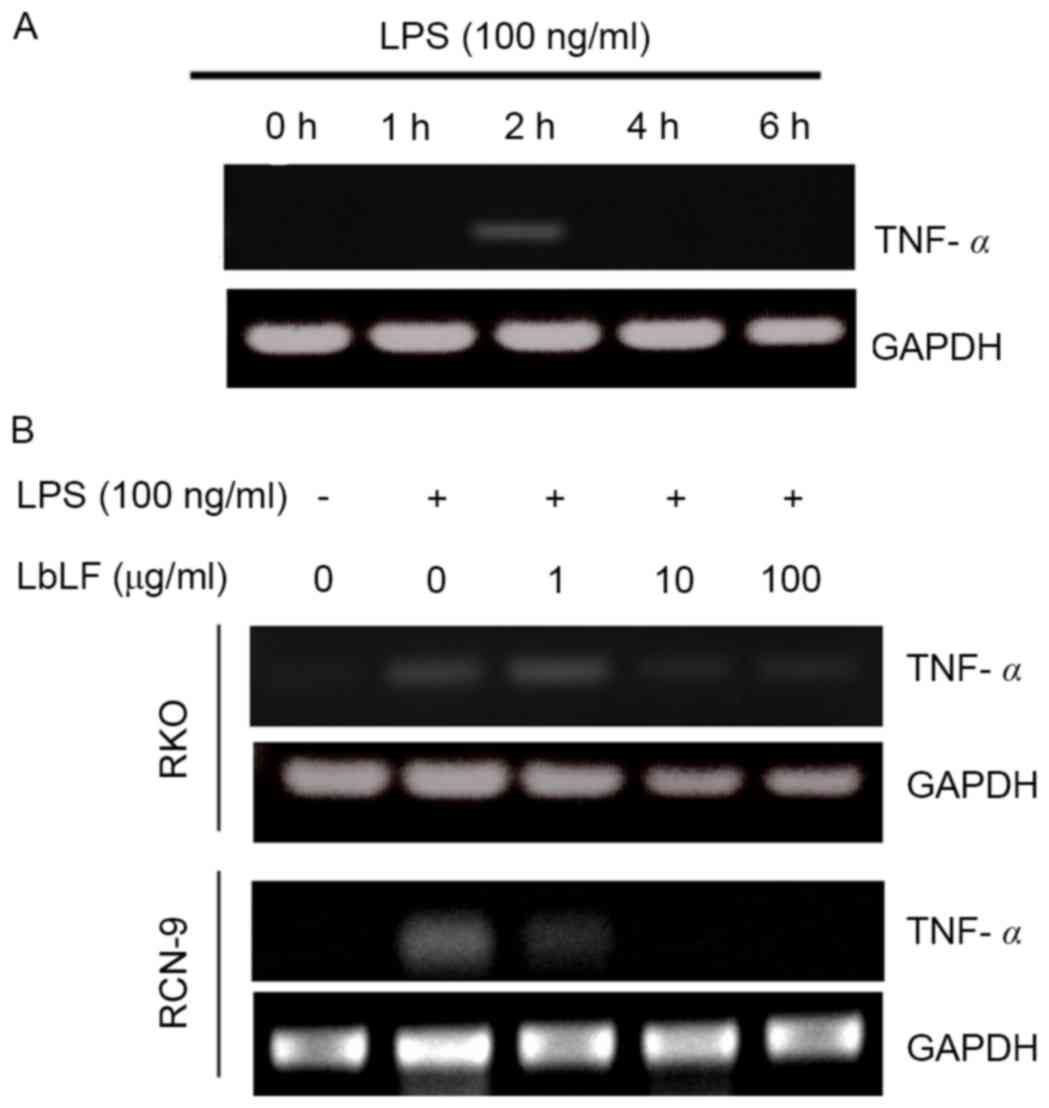
Gene expression experiments
RKO and RCN-9 cells were seeded into 60-mm culture
dishes (5×105 cells/well) and cultured in DMEM
supplemented with 10% FBS as aforementioned. The cells were then
cultured in fresh DMEM supplemented with 10% FBS with or without
lipopolysaccharide (A.a-LPS; 100 ng/ml), LPS from Aggregatibactor
Actinomycetemcomitance (ATCC29522 strain; A.a.-LPS) was provided by
Professor Tatsuji Nishihara of the Kyusyu Dental College (Kyusyu,
Japan). The cultured cells were harvested 0, 2, 4 and 6 h after LPS
stimulation. The expression level of TNFα mRNA was determined.
Furthermore, following a 4-h treatment with LbLF (1, 10 or 100
µg/ml) or a control treatment (no LbLF) at 37°C in a humidified
atmosphere of 5% CO2, the culture plates were briefly
washed twice with PBS and the cells were incubated with A.a.-LPS
(100 ng/ml) with or without a 2-h pre-treatment. The cultured cells
were collected and expression level of TNFα mRNA was evaluated.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total RNA from the harvested fully confluent
cells was isolated using an RNeasy Mini kit (Qiagen GmbH, Hilden,
Germany). An ultramicro spectrophotometer (ND-2000: NanoDrop 2000;
Thermo Scientific, Inc.,) was used to detect the concentration of
RNA. The cDNA was synthesized from 1 µg of total RNA was produced
using a transcriptase PCR kit (ReverTra Dash; Toyobo Biochemicals,
Osaka, Japan) according to the manufacturer's protocol. The
following primers were used: Human tumour necrosis factor α (TNFα):
5′-GCCCATGTTGTAGCAAACC-3′, forward and 5′-CCAAAGTAGACCTGCCCAGA-3′,
reverse (product size, 239 bp); human GAPDH:
5′-TCCACCACCCTGTTGCTGTA-3′, forward and 5′-ACCACAGTCCATGCCATCAC-3′,
reverse. Aliquots of total cDNA (0.05 µg) were amplified with 1.25
U rTaq-DNA Polymerase (Qiagen GmbH) in a thermal cycler (MyCyler;
Bio-Rad Laboratories, Inc., Hercules, CA). The PCR protocol for all
primers consisted of 30 cycles of the following: An initial 30 sec
of denaturation at 94°C, annealing for 30 sec at 60°C and extension
for 1 min at 72°C. The amplification reaction products were
resolved on 1.2% agarose/Tris-acetate-EDTA gels (Nacalai Tesque,
Inc., Kyoto, Japan). The final PCR products were separated by
electrophoresis on 1.2% agarose gels at 100 mV for 20–40 min and
visualised using ethidium bromide.
Statistical analysis
The Statcel software package (KaleidaGraph version
4.1, Reading, PA, USA), was used for statistical analysis. The data
in the current study are presented as means ± standard error. The
significance differences between control group and LbLF group in
the in vivo and in vitro experiments were evaluated
using unpaired Student's t-tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
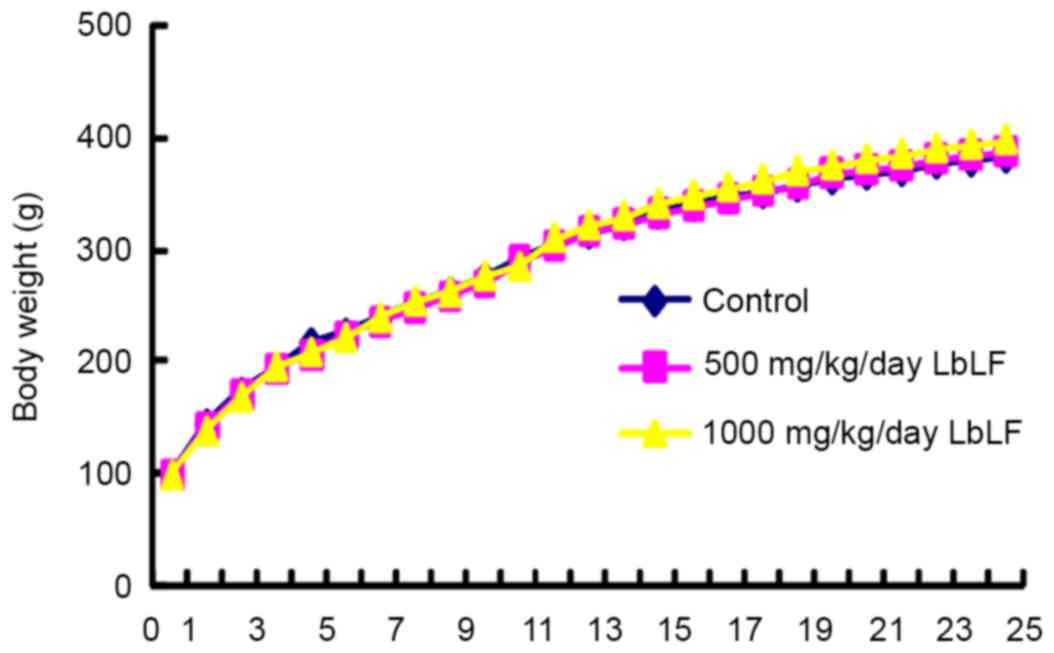
Body weight
Fig. 2 presents the
body weight of rats from the three groups measured during the
experiment. Upon reaching 6 weeks of age (defined as week 0), the
rats were randomly allocated into 3 groups of 12 rats. Each group
received water (control), 500 or 1,000 mg/kg/day LbLF from week
0–25. The body weights of the rats were recorded every week. Groups
of rats were compared for body weight from week 0–25. Body weight
was not observed to significantly differ between any of the groups
of rats at any point of the experiment.
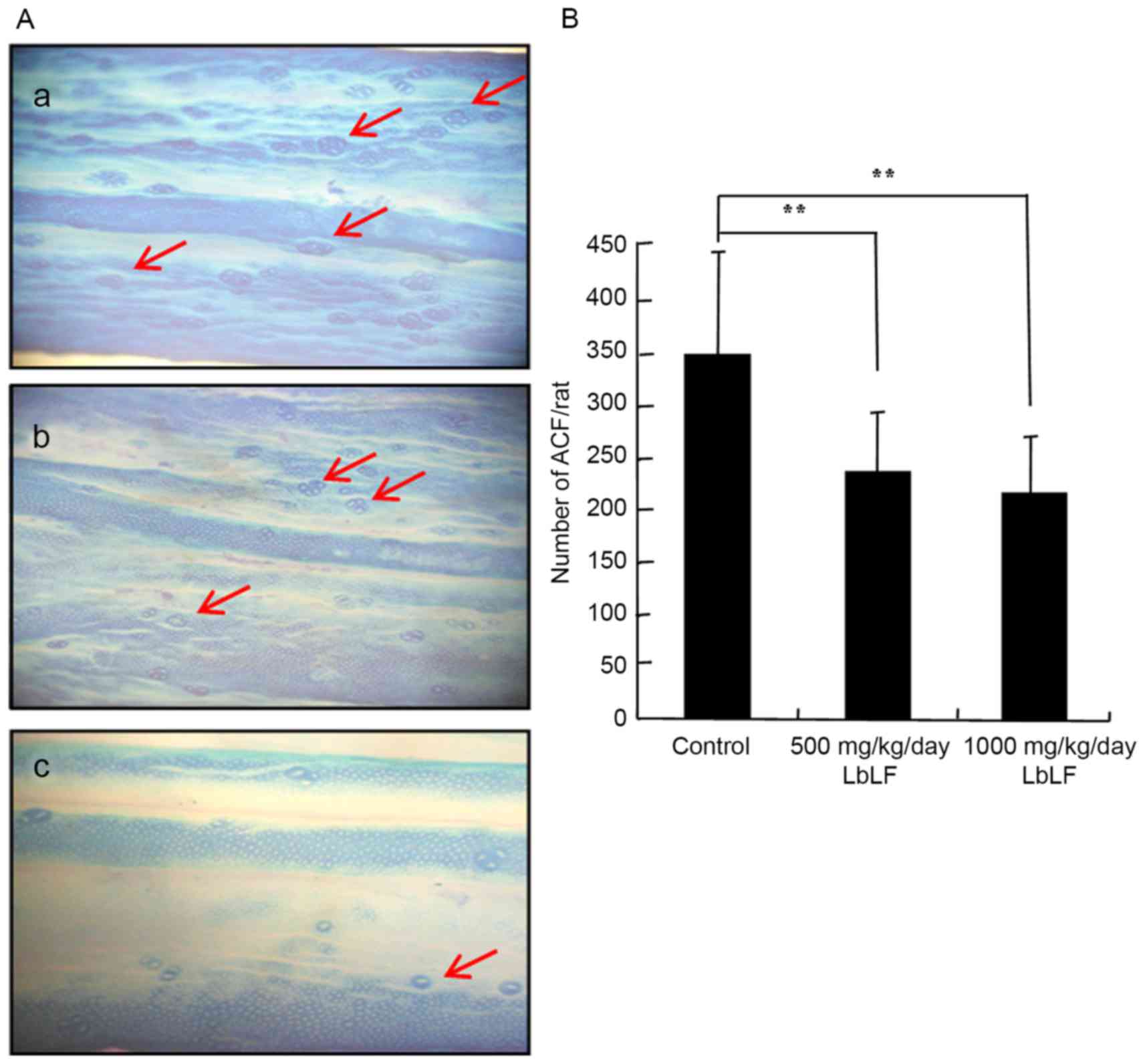
Total number of colonic ACFs
The inhibitory influence of LbLF on the growth and
development of DMH-induced total number of colonic ACF in rats is
presented in Fig. 3 and Table I. ACF expression in the colons of rats
treated with DMH-DSS was analysed using 0.5% methylene blue stain
(Fig. 3A). Rats treated with DMH
exhibited a 100% incidence of ACF. Furthermore, the mean number of
ACF was significantly lower in the 500 and 1,000 mg/kg/day LbLF
groups, as compared with in the control group (Fig. 3B; Table
I; P<0.01). Arrows indicate the ACFs in the colon (Fig. 3A).
 | Table I.Number of macroscopically evident
ACFs. |
Table I.
Number of macroscopically evident
ACFs.
| LbLF dose,
mg/kg/day | Rats, n | ACF (n, mean ±
standard deviation) |
|---|
| 0 (control) | 12 | 352.9±94.3 |
| 500 | 12 |
236.6±57.5a |
| 1,000 | 12 |
215.1±54.2a |
Colon tumours
The colon tissue sections obtained from the rats
were subjected to histopathological investigation, and the colon
epithelial lesions were classified as adenomas or adenocarcinomas
(Fig. 4). Table II indicates that rats from the 500
and 1,000 mg/kg/day LbLF groups harboured significantly fewer colon
adenomas than rats from the control group (500 mg/kg/day,
P<0.05; 1,000 mg/kg/day, P<0.01). The mean ± standard
deviation (SD) of adenomas identified in the control, 500 and 1,000
mg/kg/day LbLF groups were 33.70±12.91, 23.60±7.86 and 17.10±5.81,
respectively. Table II also
demonstrates that rats from the 500 and 1,000 mg/kg/day LbLF groups
harboured significantly fewer colon adenomas with moderate atypia
than rats from the control group (500 mg/kg/day, P<0.05; 1,000
mg/kg/day, P<0.01). Furthermore, rats from the 1,000 mg/kg/day
LbLF group harboured significantly fewer colon adenomas with mild
or severe atypia than rats from the control group (mild, P<0.01;
severe, P<0.05; Table II).
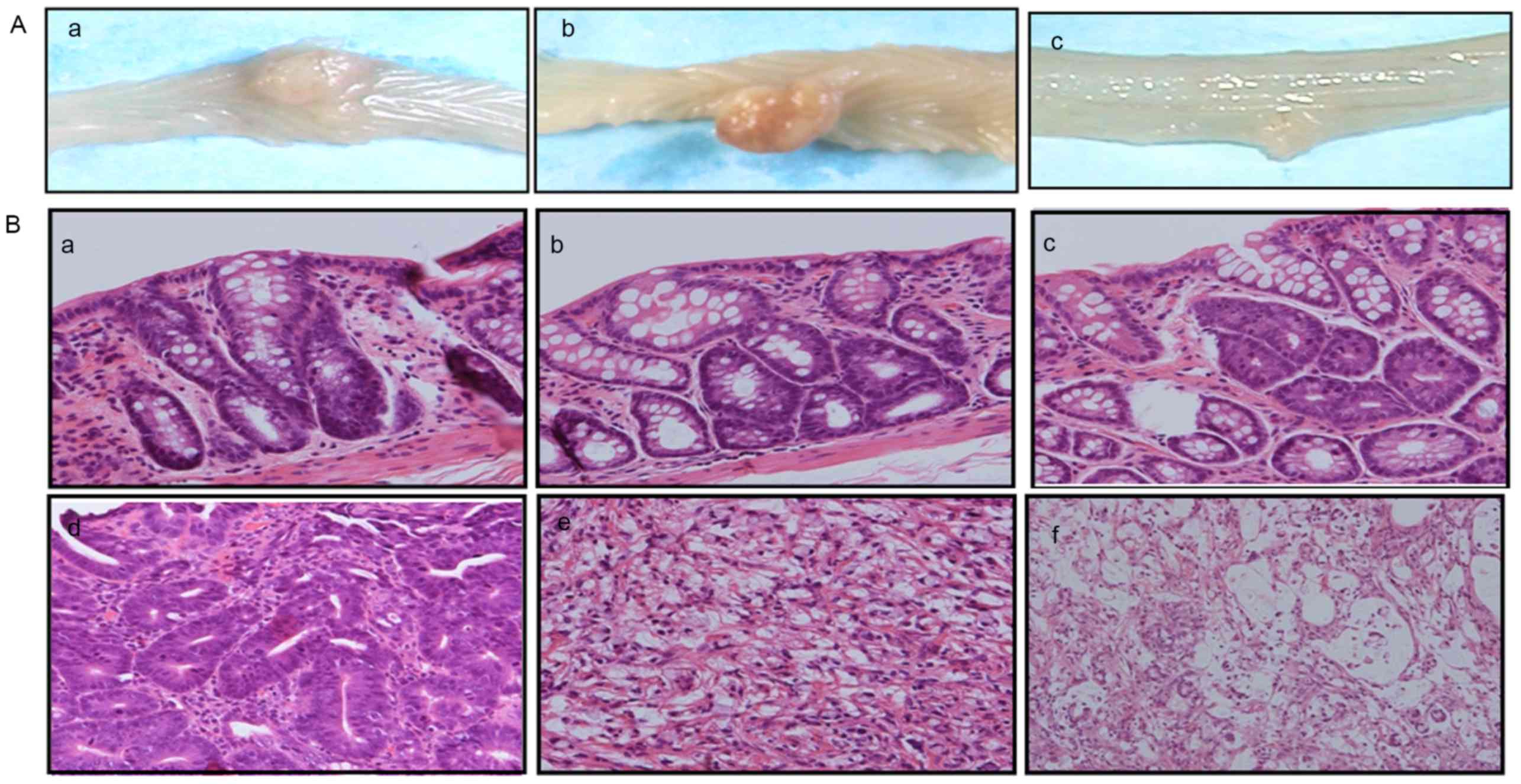 | Figure 4.Macroscopic and histological features
of the DMH-treated rat colons. (A) Gross features of macroscopic
tumours in the colon: a) Tumour in the colon of a rat in the
control group, b) tumour in the colon of a rat in the 500 mg/kg/day
LbLF group, c) tumour in the colon of a rat in the 1,000 mg/kg/day
LbLF group. (B) DMH-induced colorectal tumours in rats with
haematoxylin and eosin staining: a) Adenoma with mild atypia, b)
adenoma with moderate atypia, c) adenoma with severe atypia, d)
well-differentiated tubular adenocarcinomas, e) signet ring cell
carcinomas, f) Mucinous adenocarcinomas. Haematoxylin and eosin
staining (magnification, ×200). DMH, 1,2-dimethylhydrazine; LbLF,
liposomal bovine lactoferrin. |
 | Table II.Number of colon adenomas per rat. |
Table II.
Number of colon adenomas per rat.
|
|
| Adenomas per rat
(mean ± standard deviation) |
|---|
|
|
|
|
|---|
| LbLF dose,
mg/kg/day | Rats, n | Total | Mild atypia | Moderate
atypia | Severe atypia |
|---|
| 0 (control) | 12 | 33.7±12.91 | 22.9±10.39 | 9.2±3.54 | 1.6±1.68 |
| 500 | 12 |
23.6±7.86a | 17.1±5.01 |
5.5±3.26a | 0.9±1.44 |
| 1,000 | 12 |
17.1±5.81b |
13.3±5.10b |
3.6±2.23b |
0.3±0.45a |
Table III indicates
that the 500 and 1,000 mg/kg/day LbLF groups harboured
significantly fewer colon adenocarcinomas than rats from the
control group (P<0.05 and P<0.01, respectively). In addition,
the mean ± SD for adenocarcinomas identified in the control, 500
and 1,000 mg/kg/day LbLF groups were 1.25±0.62, 0.67±0.65 and
0.50±0.67, respectively. Table III
also reveals that rats from the 1,000 mg/kg/day LbLF group
harboured significantly fewer differentiated carcinomas (well and
moderate adenocarcinoma; P<0.05), but not undifferentiated
carcinomas (poorly, mucinous, signet ring cell carcinoma), compared
with rats from the control group. Thus, LbLF for low malignant
grade carcinoma inhibition effect is marked.
 | Table III.Number of colon adenocarcinomas per
rat. |
Table III.
Number of colon adenocarcinomas per
rat.
|
|
| Adenocarcinomas per
rat (n, mean ± standard deviation) |
|---|
|
|
|
|
|---|
| LbLF dose,
mg/kg/day | Rats, n | Total |
Undifferentiateda |
Differentiatedb |
|---|
| 0 (control) | 12 | 15 (1.25±0.62) | 6 (0.50±0.52) | 9 (0.75±0.75) |
| 500 | 12 | 7
(0.67±0.65)c | 4 (0.33±0.49) | 3
(0.25±0.45)c |
| 1,000 | 12 | 6
(0.50±0.67)d | 4
(0.33±0.49)c | 2 (0.17±0.39) |
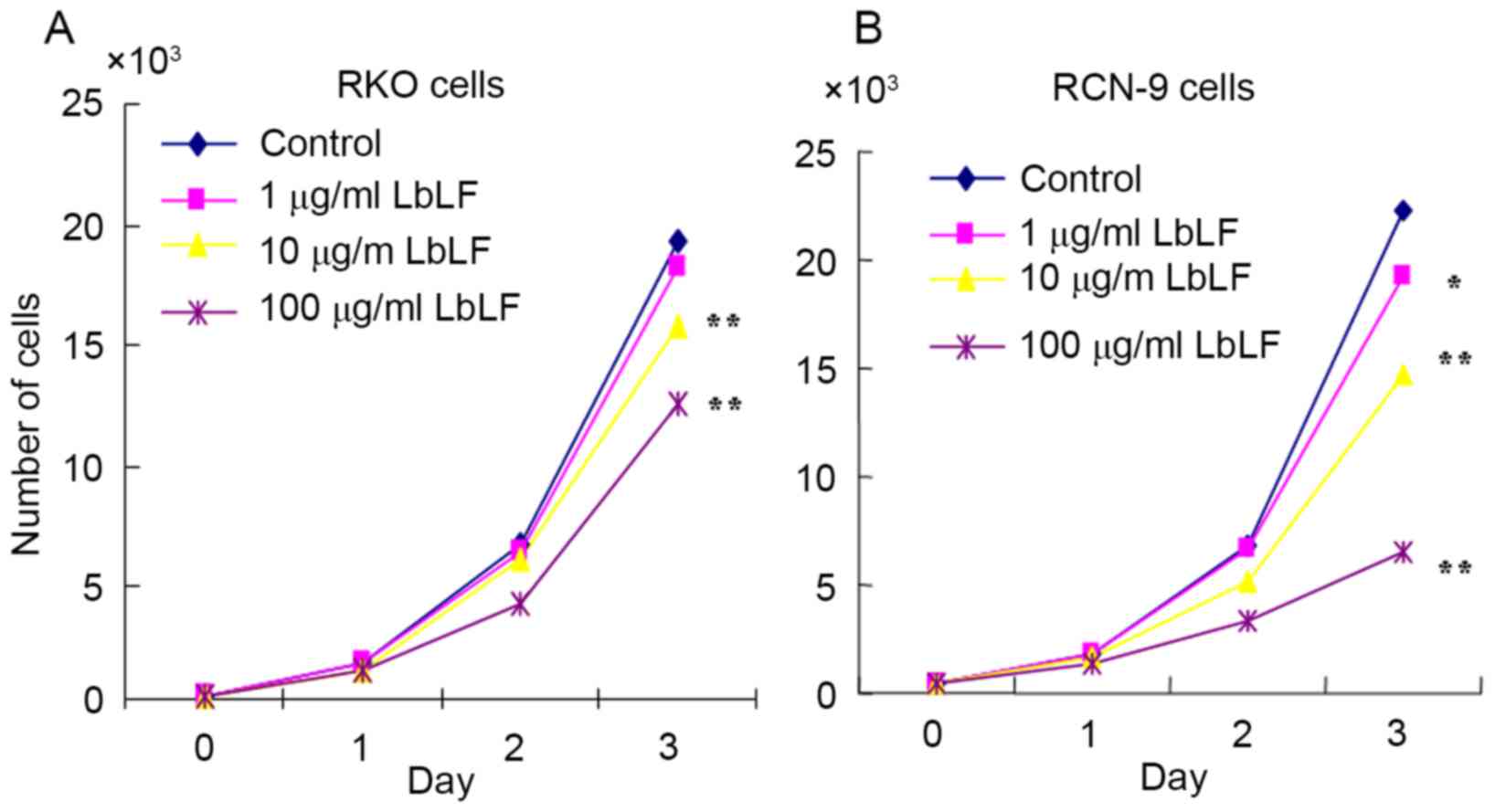
LbLF inhibits CRC cell growth
The cell growth of LbLF-treated RKO and RCN-9 cells
was examined. Compared with the control (no treatment with LbLF),
it was observed that treatment with ≥10 µg/ml LbLF significantly
inhibited the growth of RKO and RCN-9 cells (Fig. 5; P<0.01).
LbLF inhibits TNF-α mRNA expression in
CRC cells
The RKO cells were harvested 0, 2, 4 and 6 h
following LPS stimulation. The expression levels of TNFα mRNA were
determined. LPS was observed to upregulate the expression of TNF-α
mRNA in RKO cells after 2 h (Fig.
6A). Subsequently, treatment with LbLF (1, 10 or 100 µg/ml) was
demonstrated to inhibit TNF-α mRNA expression in CRC cells (RKO and
RCN-9 cells). Pre-treatment with a high concentration LbLF (10 or
100 µg/ml) blocked the LPS-induced upregulation of TNF-α mRNA in
RKO and RCN-9 cells (Fig. 6B).
Discussion
In the present study, LbLF significantly inhibited
colon cancer development, suggesting that it may be an effective
chemopreventive agent. LF has anticancer, anti-inflammatory and
immune regulatory activities (11,12). UC,
one of the two major forms of chronic inflammatory bowel disease,
was first described in the 1800s (21). This disease is characterised by
inflammation-induced chronic destruction and regeneration of
colonic mucosa, and is most common in individuals 25–35 or 55–65
years of age (22). Patients with UC
exhibit a high risk of CRC; specifically, this risk is estimated to
be >2–5 times compared with in the general population (3,23,24). DMH treatment induced oxidative stress
and the early inflammatory and tumour promotion responses in the
colons of Wistar rats (25).
DSS is a synthetic sulphated polyglucan that has
previously been used to induce inflammation in the gut (19,20).
Inflammation serves an important role in tumour initiation and
promotion (26). TNF-α is a cytokine
released by macrophages in response to infection and various other
stress conditions (27). The results
of the present study demonstrate that LbLF inhibits the LPS-induced
upregulation of TNF-α mRNA expression. In our previous study, it
was revealed that orally administered LbLF significantly inhibits
LPS-induced alveolar bone resorption (28). We also previously demonstrated the
anti-inflammatory effect of LF (29),
and other studies have observed that LF may interact with
epithelial and immune cells in the intestinal mucosa (30,31).
Furthermore, the levels of LF increase markedly during inflammation
(32); LF is important as it may
promote or inhibit the inflammatory response (33). Angiogenesis is also associated with
inflammatory disease via similar mechanisms (34), and LF may inhibit angiogenesis
(35). The findings of the present
study suggest that LbLF has an influence against tumour promotion
by DMH-DSS; this may be a cause for the observed anti-carcinogenic
effect of LbLF.
LbLF exhibits not only anti-inflammatory functions,
but also anticancer functions. The results of the present study
revealed that rats from the 500 and 1,000 mg/kg/day LbLF groups
harbour significantly fewer ACF, adenomas and adenocarcinomas of
the colon, compared with rats from the control group. In addition,
a number of previous reports indicated that bLF is associated with
the inhibition of tumour growth and the prevention of
carcinogenesis in vivo and in vitro (13–16). The
initial observation that bLF could inhibit tumorigenesis was made
in 1995; the whey fraction of bovine milk was demonstrated to
inhibit the development of DMH induced colon tumours in rats
(36). Other studies have indicated
that the incidence of adenocarcinomas in the large intestine
induced by azoxymethane in rats was significantly decreased in the
bLF-fed group, as compared with in the control group (14). However, there is currently no reported
evidence that bLF can inhibit the development of colon cancer in
animals.
In a previous human study, a randomised and
controlled clinical trial was conducted in the National Cancer
Center Hospital (Tokyo, Japan) in order to determine whether the
ingestion of bLF had an effect on the growth of colorectal polyps
in humans; daily ingestion of 3 g bLF suppressed the growth of
colorectal polyps and increased the levels of serum human LF in the
trial participants (37). bLF is
hypothesised to inhibit cancer via its ability to bind iron
(38). A previous study indicated
that the immunostimulation of LF, which activates a T helper cell
type 1 response, and the release of anticancer killer cells may be
key factors in the anticancer effect of bLF (25). In addition, LF may also prevent cancer
by regulating the expression of certain cell-cycle proteins
(39). The results of the present
study demonstrated that LbLF inhibits RKO and RCN-9 cell growth. LF
may also serve an important role in delaying the development of
tumours by acting as an inhibitor of angiogenesis (35). Although the mechanisms by which LF
inhibits cancer are not yet fully understood, its anticancer
activity is apparent.
In conclusion, the present study described the
effects of LbLF on colorectal carcinogenesis in rats. Thus, the
present study intended to explore the preventive and therapeutic
value of LbLF in CRC. Nevertheless, additional studies are required
to confirm these findings and explore the potential underlying
mechanisms by which LF affects cancer.
Acknowledgements
This study was supported in part by The National
Natural Science Foundation of China (grant nos. 81460411 and
81160256) and the Guangxi University of Science and Technology
Research Projects (grant no. ZD20140094).
References
|
1
|
Klimczak A, Kempińska-Mirosławska B, Mik
M, Dziki L and Dziki A: Incidence of colorectal cancer in Poland in
1999–2008. Arch Med Sci. 7:673–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jewell DP, Chapman RGW and Mortensen N:
Ulcerative colitis and Crohn's disease, a clinician's guide.
London: Churchill. Livingstone; pp. 791992
|
|
4
|
Efthymiou M, Taylor AC and Kamm MA: Cancer
surveillance strategies in ulcerative colitis. The need for
modernization. Inflmm Bowel Dis. 17:1800–1813. 2011. View Article : Google Scholar
|
|
5
|
Doll R and Peto R: The causes of cancer:
Quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1193–1308. 1981. View Article : Google Scholar
|
|
6
|
Willett WC: Diet, nutrition, and avoidable
cancer. Environ Health Perspect. 103 Suppl 8:165–170. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willett WC, Stampfer MJ, Colditz GA,
Rosner BA and Speizer FE: Relation of meat, fat, and fiber intake
to the risk of colon cancer in a prospective study among women. N
Engl J Med. 323:1664–1672. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan AT and Giovannucc EL: Primary
prevention of colorectal cancer. Gastroenterology.
138:2029–2043.e10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farnaud S and Evans RW: Lactoferrin-a
multifunctional protein with antimicrobial properties. Mol Immunol.
40:395–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fleet JC: A new role for lactoferrin: DNA
binding and transcription activation. Nutr Rev. 53:226–227. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibson RJ and Bowen JM: Biomarkers of
regimen-related mucosal injury. Cancer Treat Rev. 37:487–493. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Mejia EG and Dia VP: The role of
nutraceutical proteins and peptides in apoptosis, angiogenesis, and
metastasis of cancer cells. Cancer Metastasis Rev. 29:511–528.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Sakashita S, Morishita Y, Kano J,
Shiba A, Sato T and Noguchi M: Binding of lactoferrin to IGBP1
triggers apoptosis in a lung adenocarcinoma cell line. Anticancer
Res. 31:529–534. 2011.PubMed/NCBI
|
|
14
|
Tsuda H, Sekine K, Nakamura J, Ushida Y,
Kuhara T, Takasuka N, Kim DJ, Asamoto M, Baba-Toriyama H, Moore MA,
et al: Inhibition of azoxymethane initiated colon tumor and
aberrant crypt foci development by bovine lactoferrin
administration in F344 rats. Adv Exp Med Biol. 443:273–284. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masuda C, Wanibuchi H, Sekine K, Yano Y,
Otan S, Kishimoto T, Tsuda H and Fukushima S: Chemopreventive
effects of bovine lactoferrin on
N-butyl-N-(4-hydroxybutyl)nitrosamine-induced rat bladder
carcinogenesis. Jpn J Cancer Res. 91:582–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ushida Y, Sekine K, Kuhara T, Takasuka N,
Iigo M, Maeda M and Tsuda H: Possible chemopreventive effects of
bovine lactoferrin on esophagus and lung carcinogenesis in the rat.
Jpn J Cancer Res. 90:262–267. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baskar AA, Ignacimuthu S, Paulraj GM and
Al Numair KS: Chemopreventive potential of beta-sitosterol in
experimental colon cancer model-an in vitro and in vivo study. BMC
Complement Altern Med. 10:242010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamiza OO, Rehman MU, Tahir M, Khan R,
Khan AQ, Lateef A, Ali F and Sultana S: Amelioration of 1,2
dimethylhydrazine (DMH) induced colon oxidative stress,
inflammation and tumor promotion response by tannic acid in Wistar
rats. Asian Pac J Cancer Prev. 13:4393–4402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Axelsson LG and Ahlstedt S: Actions of
sulphasalazine and analogues in animal models of experimental
colitis. Inflammopharmacology. 2:219–232. 1993. View Article : Google Scholar
|
|
20
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilks S: Morbid appearances in the
intestine of Miss Bankes. London Medical Times & Gazette.
2:2641859.
|
|
22
|
Hou JK, Kramer JR, Richardson P, Mei M and
El-Serag HB: The incidence and prevalence of inflammatory bowel
disease among U.S, veterans: A national cohort study. Inflamm Bowel
Dis. 19:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ekbom A, Helmick C, Zack M and Adami HO:
Ulcerative colitis and colorectal cancer. A population-based study.
N Engl J Med. 323:1228–1233. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bernstein CN, Blanchard JF, Kliewer E and
Wajda A: Cancer risk in patients with inflmmatory bowel disease: A
population-based study. Cancer. 91:854–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fischer R, Debbabi H, Dubarry M, Boyaka P
and Tomé D: Regulation of physiological and pathological Th1 and
Th2 responses by lactoferrin. Biochem Cell Biol. 84:303–311. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi G, Zeng S, Takashima T, Nozoe K,
Shobayashi M, Kakugawa K, Murakami K, Jikihara H, Zhou L and
Shimamoto F: Inhibitory Effect of Various Breads on DMH-Induced
Aberrant Crypt Foci and Colorectal Tumours in Rats. Biomed Res Int.
2015:8290962015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blackwell TS and Christman JW: Sepsis and
cytokines: Current status. Br J Anaesth. 77:110–117. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawazoe A, Inubushi T, Miyauchi M,
Ishikado A, Tanaka E, Tanne K and Takata T: Orally administered
liposomal lactoferrin inhibits inflammation-related bone breakdown
without interrupting orthodontic tooth movement. J Periodontol.
84:1454–1462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inubushi T, Kawazoe A, Miyauchi M, Kudo Y,
Ao M, Ishikado A, Makino T and Takata T: Molecular mechanisms of
the inhibitory effects of bovine lactoferrin on
lipopolysaccharide-mediated osteoclastogenesis. J Biol Chem.
287:23527–23536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Damiens E, Mazurier J, Yazidi I, Masson M,
Duthille I, Spik G and Boilly-Marer Y: Effects of human lactoferrin
on NK cell cytotoxicity against haematopoietic and epithelial
tumour cells. Biochim Biophys Acta. 1402:277–287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shau H, Kim A and Golub SH: Modulation of
natural killer and lymphokine-activated killer cell cytotoxicity by
lactoferrin. J Leukoc Biol. 51:343–349. 1992.PubMed/NCBI
|
|
32
|
Casado B, Pannell LK, Iadarola P and
Baraniuk JN: Identification of human nasal mucous proteins using
proteomics. Proteomics. 5:2949–2959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Legrand D and Mazurier J: A critical
review of the roles of host lactoferrin in Immunity. Biometals.
23:365–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rajashekhar G, Willuweit A, Patterson CE,
Sun P, Hilbig A, Breier G, Helisch A and Clauss M: Continuous
endothelial cell activation increases angiogenesis: Evidence for
the direct role of endothelium linking angiogenesis and
inflammation. J Vasc Res. 43:193–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parodi PW: A role for milk proteins and
their peptides in cancer prevention. Curr Pharm Des. 13:813–828.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Levay PF and Viljoen M: Lactoferrin: A
general review. Haematologica. 80:252–267. 1995.PubMed/NCBI
|
|
37
|
Iigo M, Alexander DB, Xu J, Futakuchi M,
Suzui M, Kozu T, Akasu T, Saito D, Kakizoe T, Yamauchi K, et al:
Inhibition of intestinal polyp growth by oral ingestion of bovine
lactoferrin and immune cells in the large intestine. Biometals.
27:1017–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
González-Chávez SA, Arévalo-Gallegos S and
Rascón-Cruz Q: Lactoferrin: Structure, function and applications.
Int J Antimicrob Agents. 33:301.e1–8. 2009. View Article : Google Scholar
|
|
39
|
Rodrigues L, Teixeira J, Schmitt F,
Paulsson M and Månsson HL: Lactoferrin and cancer disease
prevention. Crit Rev Food Sci Nutr. 49:203–217. 2009. View Article : Google Scholar : PubMed/NCBI
|