Introduction
Acute lymphoblastic leukemia (ALL) is the most
common type of childhood malignancy, and is characterized by
uncontrolled clonal proliferation of lymphoid blasts with reduced
capacity to differentiate into mature cells (1). The therapeutic outcome for children with
newly diagnosed (ND) ALL (ALL-ND) has been markedly improved by
risk-adapted treatments and supportive care over the past decades
(2). The cure rates have increased to
>80% in a number of study groups (3–6). However,
15–20% of patients eventually relapse, which has become the main
obstacle to further improve treatment (7). The complexity of the mechanism of ALL
progression, together with limited knowledge about the biological
features of this disease, presents a challenge to developing novel
therapeutic approaches.
Evidence indicates that leukemia stem cells (LSCs),
a small population of leukocytes, are responsible for the relapse
of ALL (8,9). LSCs have a primitive cell origin and
share a number of immunophenotypic characteristics with normal
hematopoietic cells (10). LSCs
possess the characteristics of self-renewal, proliferation and drug
resistance, and have been revealed to express certain LSCs markers,
including cluster of differentiation (CD)90, CD96, CD117, CD123 and
CD133 (11). CD133, also termed
prominin-1, is a five-transmembrane protein, which was originally
identified as one marker of hematopoietic stem cells (12). A previous study revealed that CD133 is
a more specific marker of hematopoietic stem cells than CD34
(13). The expression of the CD133
antigen in acute leukemia was associated with a more immature
phenotype of the blast population and a poor prognosis. However,
studies about the expression of CD133 in ALL are conflicting,
particularly in pediatric ALL. A number of studies have revealed a
high level of CD133 expression in particular cases (14), while others have demonstrated either
low level (15) or absent (16) expression.
The regulation of stem cell self-renewal and
differentiation requires a specific microenvironment, which is
termed the stem cell niche (17).
Adhesion molecules are known to mediate interactions between
hemopoietic cells and the cellular and extracellular stromal
microenvironment (18). These
interactions are important for maintenance, proliferation,
differentiation and homing of hemopoietic progenitors, as well as
for LSCs (19). In leukemia, adhesion
molecules have been revealed to be differentially expressed and to
have an effect on prognosis (20).
The CD82 gene, also termed KAI1, is a member of the
tetraspanin superfamily (TM4SF) (21). It is widely accepted that CD82 is
associated with cell growth, differentiation and proliferation,
T-cell activation, regulation activity and adhesion of natural
killer cells. In the context of cancer, CD82 is associated with
integrins on the surfaces of various tumor cells, and its
expression is associated with metastasis suppression (22). Numerous clinical studies have
demonstrated that CD82 is a valid metastasis suppressor gene. Loss
of CD82 protein and mRNA was associated with a poor prognosis in a
number of solid malignancies, including prostate (23), colon (24), lung (25) and breast (26) cancer. However, there are few studies
on the expression level of CD82 in malignant blood disease.
Burchert et al (27) reported
that CD82 was overexpressed in peripheral blood isolated from
patients with acute myeloid leukemia (AML), in leukemic cells from
patients with chronic myeloid leukemia (CML) in the accelerated or
blastic phase and in chronic lymphocytic leukemia (CLL). It has
been suggested that CD82 is abundantly expressed on primitive and
hemopoietic progenitor cells (27).
Nishioka et al (28)
identified that CD82 is aberrantly expressed in
CD34+CD38− acute myelogenous leukemia cells.
Subsequently, the study demonstrated that CD82 regulates adhesion
and survival of LSCs. However, little is known regarding the
expression and roles of CD82 in the bone marrow (BM) of pediatric
patients with ALL, and the association between CD82 expression and
its clinical characteristics. To date, to the best of our
knowledge, no previous study has reported data about the
association between CD133 and CD82 expression in pediatric ALL.
In the present study, CD133 and CD82 expression
levels were measured in the BM of pediatric patients with ALL, and
the association between the expression of CD133 and CD82 was
determined, as well as the associations with clinical pathological
characteristics.
Patients and methods
Patients and controls
A total of 59 pediatric patients with ALL (18
females and 41 males; median age, 5 years; age range, 1–13 years)
were enrolled in the present study. All patients were diagnosed
according to the World Health Organization classification (29). A total of 37 cases were patients with
newly diagnosed ALL (ALL-ND), while 22 patients had complete
remission ALL (ALL-CR). Among the patients with ALL-ND, 30 patients
(81.08%) were diagnosed with B-ALL and 7 patients (18.92%) with
T-ALL. BM samples were obtained from ALL-ND patients prior to any
treatment. The BM of ALL-CR was aspirated during morphological
remission. A total of 16 hematologically normal age-matched BM
samples were obtained from patients undergoing a BM aspiration as a
part of routine investigation for non-malignant hematological
disease (such as idiopathic thrombocytopenia) or lymphoma for
staging (proven to be uninvolved on BM biopsy). The karyotype
analyses of all patients were performed as part of the routine
investigations. Hyperdiploidy karyotype was defined by the presence
of 51–68 chromosomes in a karyotype. All patients were treated
according to the Chinese Childhood Leukemia Group-Acute
Lymphoblastic Leukemia 2008 protocol (30). Enrollment occurred between October
2014 and July 2015 at the Department of Pediatrics, Shandong
Provincial Hospital Affiliated to Shandong University (Jinan,
China). Written informed consent was obtained from parents on
behalf of the children enrolled. The present study was approved by
the Medical Ethical Committee of Shandong Provincial Hospital
Affiliated to Shandong University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of CD133 and CD82
Total RNA was obtained from BM mononuclear cells
(BMMCs) of patients and healthy controls and isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
accordance with the manufacturer's protocol. For the reverse
transcription reaction, the Prime Script RT reagent kit (Takara
Biotechnology, Inc., Dalian, China) was used according to the
manufacturer's protocol. Reverse transcription was performed at
37°C for 15 min, followed by 85°C for 5 sec. RT-qPCR was conducted
using an ABI Prism 7500 Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. In the final 10-µl reaction volume, the qPCR contained 5
µl 2X SYBR-Green Real-time PCR Master mix (Toyobo Biotechnology
Co., Ltd., Osaka, Japan), 3.2 µl ddH2O, 1 µl cDNA sample and 0.4 µl
forward and reverse primers. All RT-qPCR was conducted on the Roche
LightCycler® 480 PCR system (Roche Diagnostics, Basel,
Switzerland) and performed in triplicate. PCR thermocycling
conditions for all genes were as follows: 95°C initial activation
for 15 min followed by 45 cycles of 95°C for 15 sec, 60°C for 15
sec and 72°C for 30 sec. The primers were as follows: CD133
forward, 5′-GCATTGGCATCTTCTATGGTT-3′ and reverse,
5′-CGCCTTGTCCTTGGTAGTGT-3′; and CD82 forward,
5′-TGTCCTGCAAACCTCCTCCA-3′ and reverse,
5′-CCATGAGCATAGTGACTGCCC-3′. The results are expressed relative to
the number of β-actin transcripts, which were used as an internal
control. β-actin was analyzed using the following primers: Forward,
5′-CCTTCCTGGGCATGGAGTCCTG-3′ and reverse,
5′-GGAGCAATGATCTTGATCTTC-3′. Relative gene expression levels (the
amount of target, normalized to endogenous control gene) were
calculated using the comparative Cq method formula,
2−ΔΔCq (19).
Flow cytometry analysis of CD133- and
CD82-expressing cells
BM samples from all patients were collected into
EDTA-containing tubes. BMMCs were isolated by Ficoll-Hypaque (GE
Healthcare, Chicago, IL, USA) gradient centrifugation at 1,000 × g
for 20 min at 20°C and analyzed using three-color flow cytometric
analysis. BMMCs (1×106) were incubated with Fc receptor
saturation reagent (Beckman Coulter, Inc., Brea, CA, USA) for 20
min at 20°C. Subsequently, BMMCs cells were stained with a
fluorescein isothiocyanate-conjugated monoclonal antibody (mAb)
against CD34 (cat. no. 348053; BD Biosciences, San Jose, CA, USA),
a phycoerythrin-conjugated mAb against CD133 (cat. no. 130-098-826;
Miltenyi Biotec, Inc., Auburn, CA, USA) and an Alexa Fluor
647-conjugated anti-CD82 antibody (cat. no. 342108; BioLegend,
Inc., San Diego, CA, USA), followed by incubation at room
temperature in the dark for 20 min. Immunoglobulin G (IgG) isotype
staining was used as a negative control. In all cases, 30,000
events were analyzed. All samples were assayed using a Beckman
Gallios Flow Cytometer. Data were analyzed using FlowJo software
(version 7.6; Tree Star, Inc., Ashland, OR, USA).
ELISA
BM samples were collected into heparin-anticoagulant
vacutainer tubes, including 22 ALL-ND, 16 ALL-CR and 12 control
samples. Plasma was obtained by centrifugation at 650 × g for 5 min
at 20°C and stored at −80°C for determination of cytokines. The
level of CD82 was detected by a human CD82 ELISA kit (cat. no.
CSB-E13037h; Cusabio Biotech Co., Ltd., Wuhan, China), according to
the manufacturer's protocol. Serum samples or standard (made
according to the manufacturer's protocol) (100 µl) were separately
added into each well of a 96-well plate and incubated for 2 h at
37°C. Biotin antibody (100 µl; 1:100 dilution) from the ELISA kit
was added and incubated for 1 h at 37°C. Subsequently, 100 µl of
horseradish peroxidase-conjugated avidin from the ELISA kit was
added and incubated for 1 h at 37°C. Tetramethylbenzidine substrate
(90 µl) was then added and incubated for 15 min at 37°C. Finally,
50 µl of stop solution was added to each well, and absorbance was
read at 450 nm. During the procedure, washing of the plate was
according to the ELISA routine method. The lower detection limits
were 0.156 ng/ml.
Statistical analysis
Results are expressed as the mean ± standard
deviation, or as the median and range. Student's t-test,
χ2 test and Wilcoxon test were performed to assess the
differences between two groups. One-way analysis of variance with
Tukey's post hoc test was used to assess the difference between
three or more groups. Pearson's or Spearman's correlation test was
used for correlation analysis depending on data distribution. SPSS
software 19.0 (IBM SPSS, Armonk, NY, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Aberrant mRNA expression of CD133 and
CD82 in pediatric patients with ALL
CD133 and CD82 mRNA expression levels were
determined by RT-qPCR. CD82 mRNA expression level was observably
elevated in pediatric patients with ALL-ND (median, 0.0032; range,
0.0003–0.0340) compared with controls (median, 0.0020; range,
0.0002–0.0053) (P=0.0063). However, no significant difference was
observed between ND and CR patients (median, 0.0021; range,
0.0003–0.0129) (Fig. 1A). In B-ALL,
CD82 mRNA expression in ND patients (median, 0.0031; range,
0.0003–0.0340) was significantly increased compared with CR
patients (median, 0.0014; range, 0.0003–0.0092) (P=0.0217) and
controls (median, 0.0022; range, 0.0018–0.0053) (P=0.018) (Fig. 1B). In T-ALL, CD82 expression in ND
patients (median, 0.0044; range, 0.0012–0.0087) was markedly higher
than that in controls (median, 0.0020; range, 0.0002–0.0053)
(P=0.0110; Fig. 1C). No significant
difference in CD82 expression was observed between CR (median,
0.0071; range, 0.0007–0.0129) and controls in patients with T-ALL.
CD133 mRNA expression in all ND patients (median, 0.0013; range,
0.0001–0.0594) was increased compared with that in all CR patients
(median, 0.0008; range, 0.0002–0.0098; P=0.012) and controls
(median, 0.0010; range, 0.0001–0.0028; P=0.007). In B-ALL, the
CD133 mRNA expression level was markedly higher in pediatric
patients with ALL-ND (median, 0.0027; range, 0.0001–0.0595)
compared with controls (median, 0.0010; range, 0.0000–0.0028;
P=0.0571). CD133 mRNA expression in ND patients was also
significantly higher than that in CR patients (median, 0.0005;
range, 0.0003–0.0027; P=0.0140; Fig.
1D). In T-ALL, there was no difference between ND (median,
0.0003; range, 0.0001–0.0338) and controls in the mRNA expression
of CD133. The difference in CD133 mRNA expression between ND and CR
(median, 0.0018; range, 0.0002–0.0098) was not significant
(P=0.082). No significant difference in CD133 and CD82 mRNA
expression was observed between patients with B-ALL and T-ALL. In
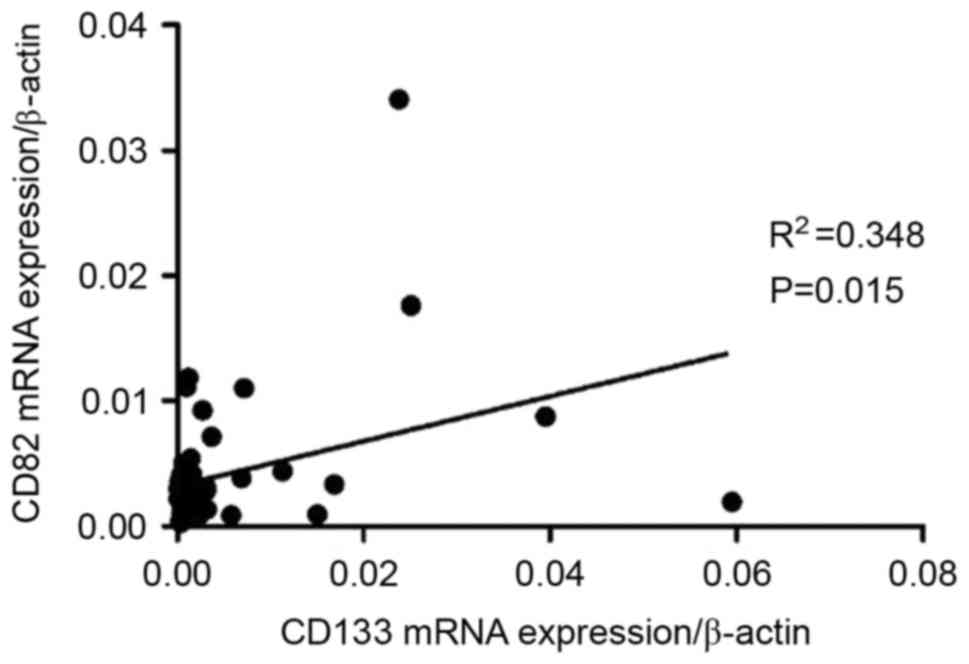
patients with B-ALL, a positive correlation was observed between
CD133 mRNA expression and CD82 mRNA expression in BM (r=0.3174;
P=0.0316; Fig. 2), however, in
patients with T-ALL, the correlation was not significant (data not
shown).
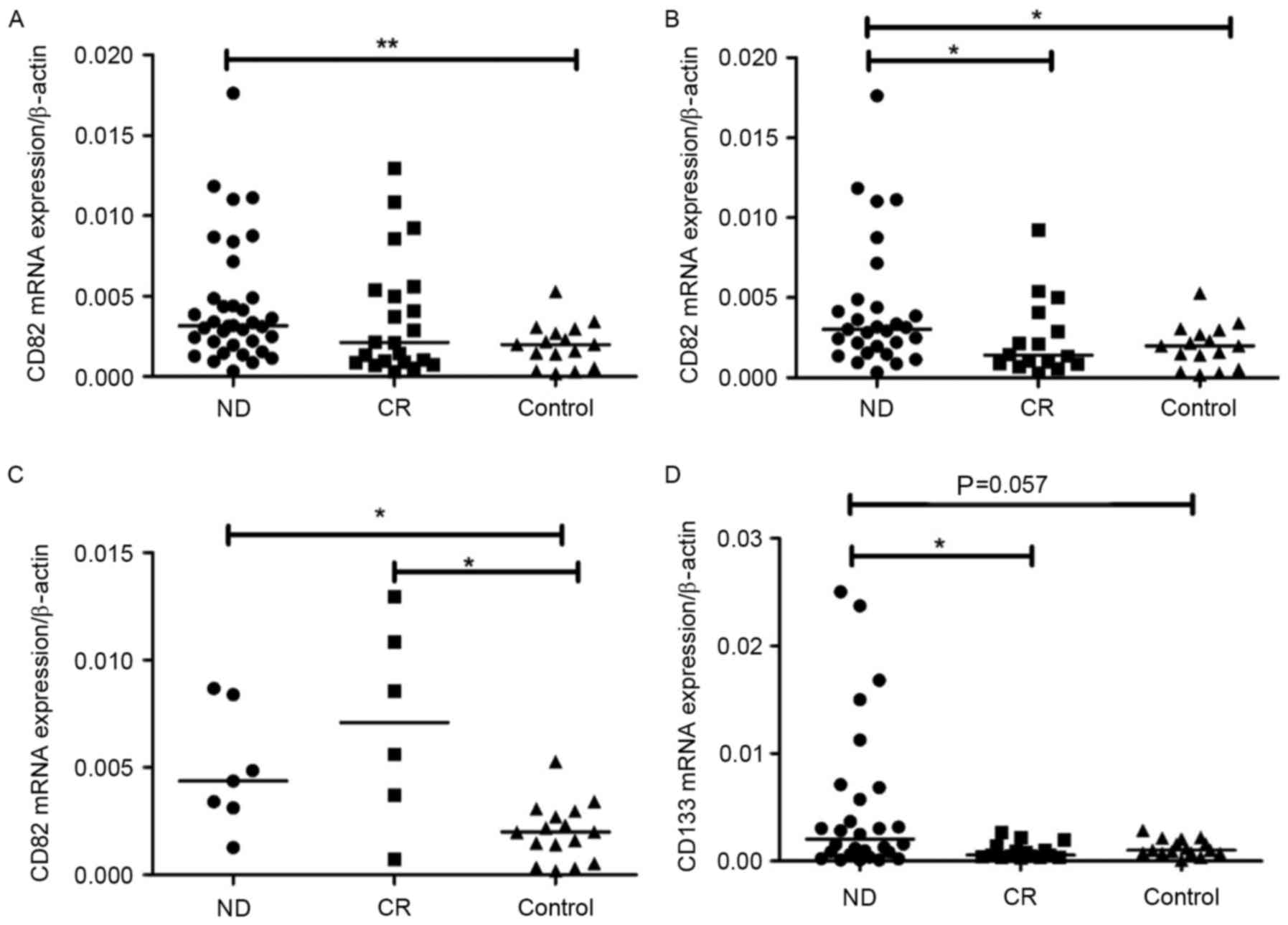 | Figure 1.mRNA expression of CD133 and CD82.
(A) CD82 mRNA expression in ND was significantly higher than in
controls. **P<0.001, ND vs. control (B) CD82 mRNA expression in
B-ALL ND was significantly higher than in CR and controls.
*P<0.05, ND vs. CR or ND vs. control (C) CD82 mRNA expression in
T-ALL ND and CR was significantly higher than in controls.
*P<0.05, ND vs. control or CR vs. control (D) CD133 mRNA
expression was markedly higher in B-ALL ND compared with controls,
and CD133 mRNA expression was significantly higher than in CR.
*P<0.05, ND vs. CR. ND, newly-diagnosed; CR, complete remission;
CD, cluster of differentiation; B-ALL, B cell-acute lymphoblastic
leukemia; T-ALL, T cell-acute lymphoblastic leukemia. |
Increased CD34-, CD133- and
CD82-positive cells in the BM of pediatric ALL
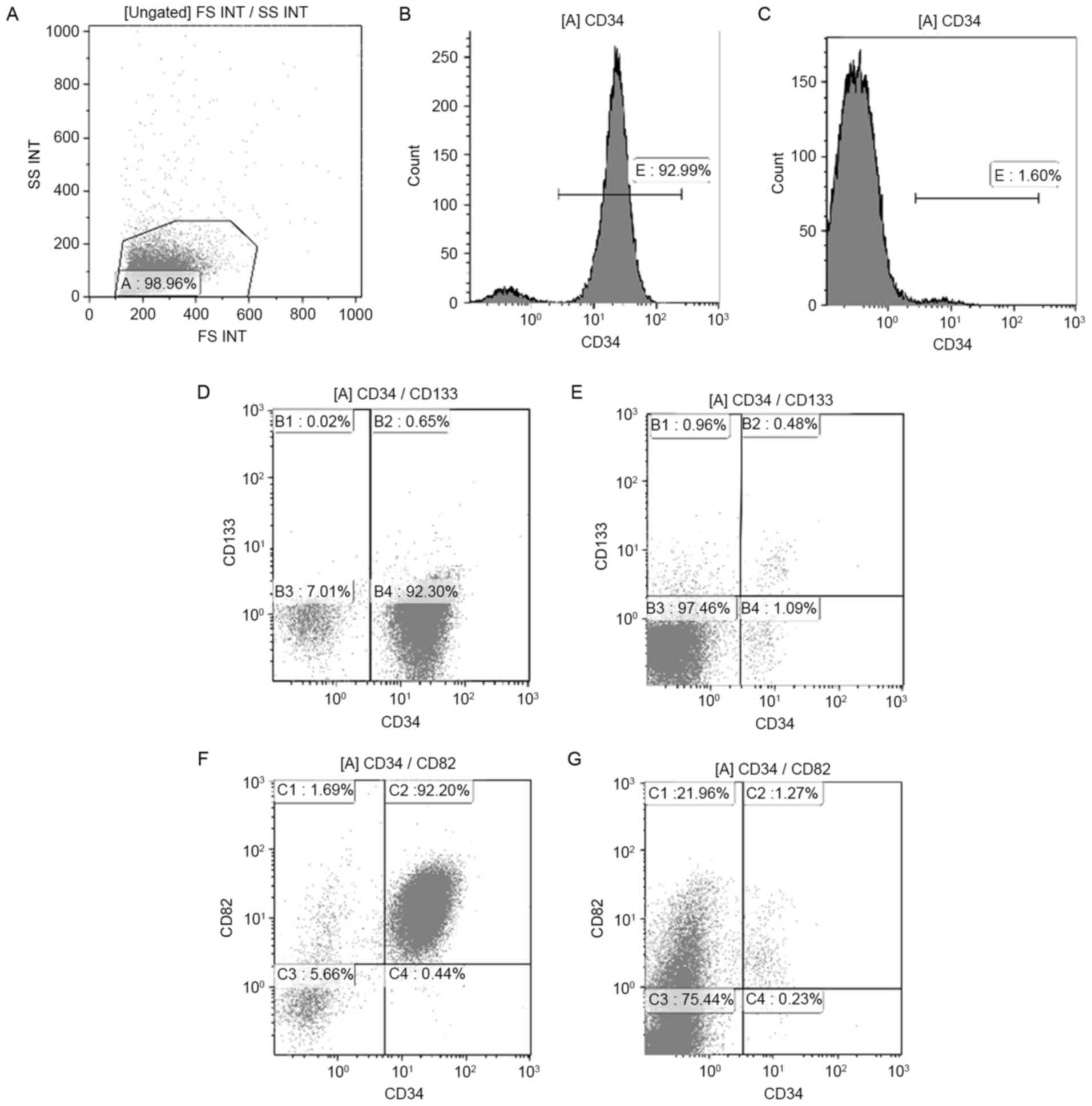
BMMCs of 30 samples (17 ND and 13 control samples)
were stained with CD34, CD133 and CD82 antibodies. The typical
histogram of CD34 and dot-plot of CD133 and CD82 in pediatric
patients with ALL and controls is shown in Fig. 3. In samples from ND patients, the
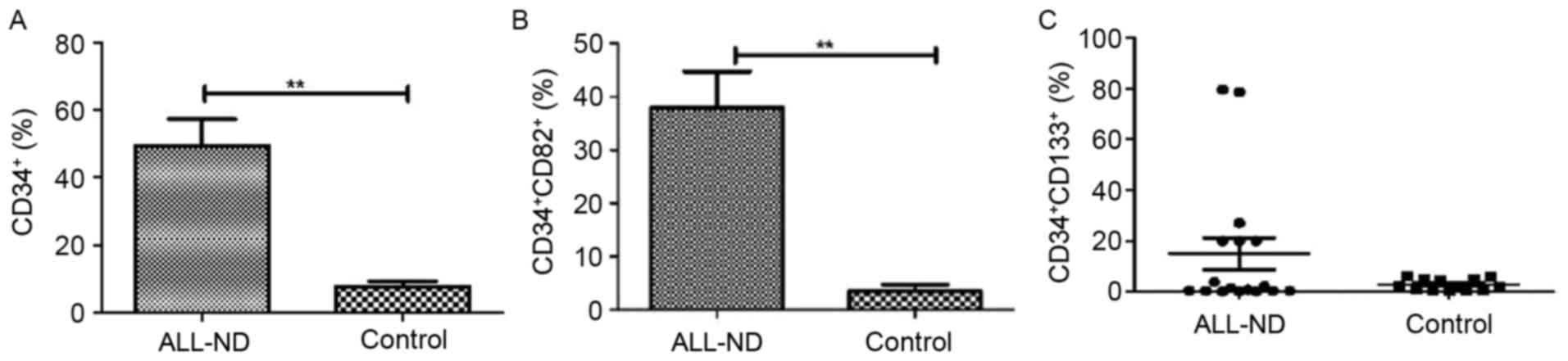
percentage of total CD34+ cells within the BMMC fraction
was highly heterogeneous, with a median of 44.67% (range,
0.50–95.22%). Such levels were significantly higher than those
observed in controls, in which a median of 6.42% (range,
1.81–18.52%) was observed for total CD34+ cells
(P<0.01) (Fig. 4A). Compared with
those in controls (median, 1.41%; range, 0.07–12.89%),
CD34+CD82+ cells in patients with ALL-ND were
elevated (median, 39.77%; range, 0.37–82.74%; P<0.01; Fig. 4B). In the present study, samples were
considered positive when ≥10% of cells expressed
CD34+CD133+. In patients with ALL-ND, 6 out
of 17 (35.29%) patients, the CD34+CD133+
frequency was <10%, but in all the controls the frequency was
>10% (P=0.024; Fig. 4C). The
difference in CD34+CD133+ frequency between
ALL and controls was statistically significant (P<0.001).
BM plasma CD82 level in patients with
pediatric ALL
To investigate the expression levels of BM plasma
CD82 in patients with pediatric ALL, the levels of CD82 in BM were
detected by ELISA. The expression level of plasma CD82 in ND
patients (373.2±39.7 pg/ml) was significantly higher than that in
controls (249.4±24.5 pg/ml) (P=0.0373). The difference between ND
and CR (385.7±87.26 pg/ml), and CR and control was not
statistically significant (P>0.05).
Positive correlation between
percentage of CD133- and CD82-positive cells with mRNA
expression
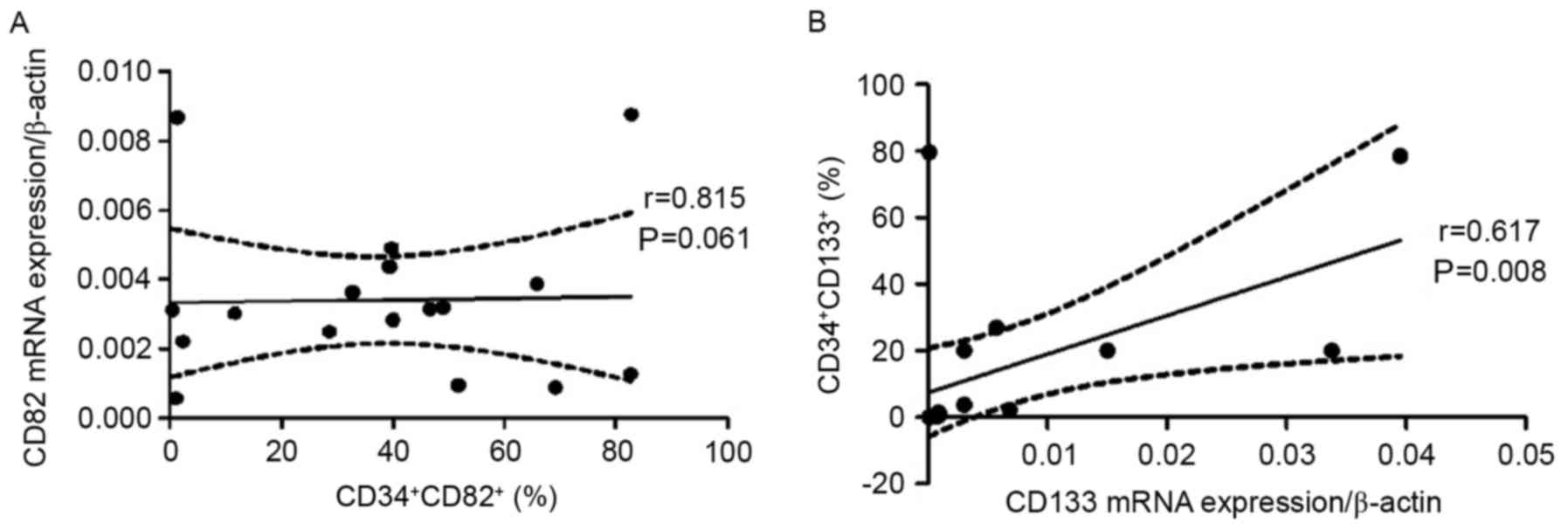
A positive correlation was observed between the
frequency of CD34+CD82+ cells and CD82 mRNA
expression (r=0.549; P=0.022; Fig.
5A), and between the percentage of
CD34+CD133+ cells and CD133 mRNA expression
(r=0.617; P=0.008; Fig. 5B).
Clinical relevance of CD133 and CD82
expression in patients with ALL
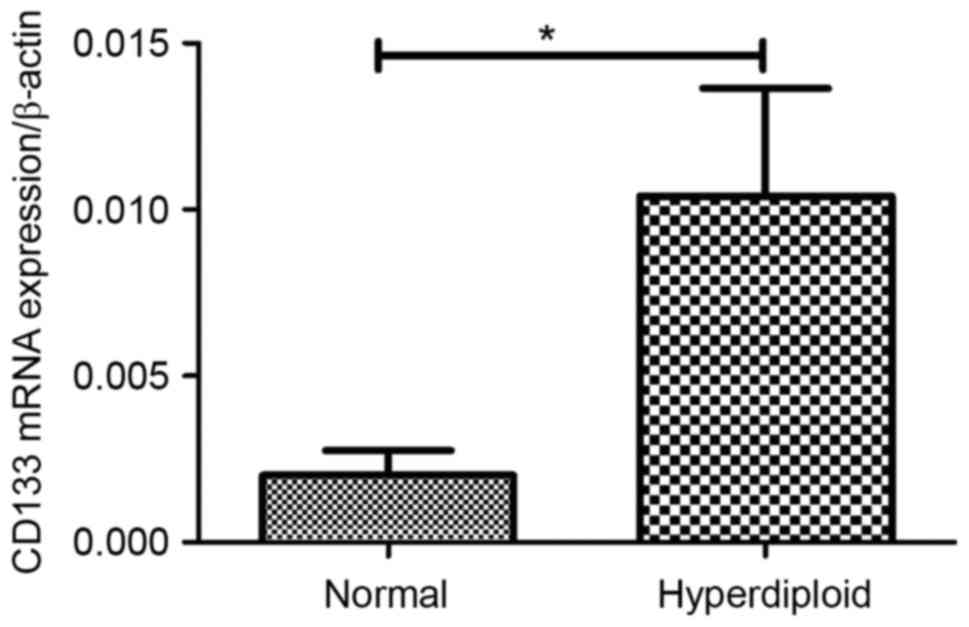
In the present study, it was demonstrated that CD133
mRNA expression in ALL patients with the hyperdiploid karyotype was
significantly increased compared with that of those patients with
the normal karyotype (P=0.003; Fig.
6). The expression of CD133 and CD82 exhibited no association
with other clinical factors, including sex, age, white blood cells,
infusion genes and the risk stage, in patients with ALL (Table I).
 | Table I.Associations between CD133 and CD82
mRNA expression and clinicopathological characteristics of
pediatric patients with newly-diagnosed acute lymphoblastic
leukemia. |
Table I.
Associations between CD133 and CD82
mRNA expression and clinicopathological characteristics of
pediatric patients with newly-diagnosed acute lymphoblastic
leukemia.
|
|
| CD133 mRNA | CD82 mRNA |
|---|
|
|
|
|
|
|---|
| Characteristic | Patients, n | Relative
expressiona | P-value | Relative
expressiona | P-value |
|---|
| Sex |
|
| 0.1676 |
| 0.2850 |
|
Male | 24 | 0.0052±0.0019 |
| 0.0060±0.0015 |
|
|
Female | 13 | 0.0115±0.0049 |
| 0.0038±0.0006 |
|
| Age |
| | 0.2752 |
| 0.7855 |
| 1–10
years | 33 | 0.0083±0.0024 |
| 0.0051±0.0011 |
|
| <1
or >10 years | 4 | 0.0006±0.0002 |
| 0.0060±0.0023 |
|
| WBCs,
×109 |
|
| 0.0769 |
| 0.6873 |
|
<10 | 14 | 0.0116±0.0044 |
| 0.0065±0.0024 |
|
|
10–50 | 13 | 0.0024±0.0007 |
| 0.0045±0.0010 |
|
|
>50 | 4 | 0.0006±0.002 |
| 0.0041±0.0016 |
|
| Risk stage |
|
| 0.5818 |
| 0.9045 |
|
High | 5 | 0.0131±0.0116 |
| 0.0045±0.0012 |
|
|
Middle | 28 | 0.0064±0.0019 |
| 0.0052±0.0012 |
|
|
Stander | 4 | 0.0078±0.0058 |
| 0.0064±0.0037 |
|
| Karyotype |
|
| 0.0341 |
| 0.2720 |
|
Hyperdiploid | 11 | 0.0020±0.0007 |
| 0.0040±0.0011 |
|
|
Normal | 14 | 0.0104±0.00132 |
| 0.0073±0.0024 |
|
Discussion
It has been well accepted that LSCs, which possess
the characteristics of self-renewal, proliferation and drug
resistance, perform an important role in leukemia progression
(31,32). LSCs are responsible for the relapse of
acute leukemia. CD34 has been used to distinguish between immature
and mature cells (33). In the
present study, it was demonstrated that in patients with ALL-ND,
the percentage of total CD34+ cells within the BMMC
fraction was significantly higher than that observed in the
controls, which was consistent with previous studies (15,34,35).
Previous studies demonstrated that LSCs express
CD133. CD133 has been used for cancer stem cell identification in
several types of cancer, including glioblastoma (36), melanoma (37), liver cancer (38), osteosarcoma (39) and colon cancer (40). There are numerous studies on the
expression of CD133 in acute leukemia, however, a few studies on
the expression of CD133 in ALL, particularly in pediatric ALL, are
contradictory. The expression of CD133 antigen in acute leukemia
was associated with a more immature phenotype of the blast
population and a bad prognosis. Mak et al suggested that
pro-B-ALL cell samples with 11q23-anomalies and mixed lineage
leukemia (MLL) gene translocations were positive for CD133
(41). The expression of
11q23-anomalies and MLL were high-risk factors in pediatric ALL.
Crucially, it was demonstrated that CD133+ cells were
more resistant to treatment with the key components in pediatric
ALL therapy, such as dexamethasone and vincristine, than the bulk
leukemia population. Therefore, the poor clinical outcomes
associated with positive CD133 expression in ALL cases could be
explained by evidence that CD133+ cells show high
resistance to chemotherapy. Furthermore, high resistance to
chemotherapy is attributed to evidence that there is increased
expression of the multidrug resistance genes and DNA mismatch
repair genes, as well as genes that inhibit apoptosis in the
CD133-expressing LSCs. In addition, it was demonstrated that the
associations between expression of cancer stem cell specificity are
maintained by tight regulation of CD133 expression at
transcriptional and post-translational levels (42). Therefore, evaluating the expression
level of CD133 is important in clinical diagnosis and
treatment.
The CD133 expression level of BM was measured in
pediatric patients with ALL-ND, patients with ALL-CR and a control
group in the present study. The results demonstrated that CD133
expression in pediatric ALL-ND was significantly increased compared
with that in the controls, and notably, that it decreased when
patients achieved CR. Regarding correlations between CD133
expression and a number of the studied standard prognostic factors,
a highly significant association between CD133 mRNA expression and
the hyperdiploid karyotype was demonstrated. The CD133 mRNA
expression level was increased in patients with the hyperdiploid
karyotype compared with that in patients with the normal chromosome
karyotype. In addition, a patient who presented with the
MLL/ALL-1-fused gene on chromosome 4 (AF4) mutation expressed a
higher CD133 mRNA level compared with other patients. This
observation was consistent with the hypothesis by Mak et al
which stated that MLL fusion-associated gene AF4 promotes CD133
transcription (41).
Originally identified as a tumor metastasis
suppressor in prostate carcinoma (37), the CD82 gene is a member of the TM4SF
that is located on human chromosome 11p11.2. CD82 performs an
important function in cell fusion, migration, adhesion, signaling,
fertilization, differentiation and invasion. A previous study
demonstrated that CD82 gene expression is under-regulated in the
majority of metastatic cancer types, which was in contrast to CD82
expression in malignant hematological disease. It had been revealed
that CD82 was overexpressed in patients with CML in the accelerated
or blastic phase, as well as in patients with AML and CLL. Nishioka
et al (43) detected aberrant
expression of CD82 in CD34+CD38− AML cells.
Importantly, it was revealed that downregulation of CD82 in
CD34+CD38− AML cells could inhibit the
adhesion and colony forming ability of these cells. In addition, in
CD34+/CD38− AML cells, CD82 downregulation
significantly impaired engraftment of the cells in severely
immunocompromised mice. Taken together, the results suggested that
aberrant CD82 expression may serve a role in the adhesion of LSCs
to the BM microenvironment and in LSC survival (28). Subsequently, Nishioka et al
(43) demonstrated that the
CD82/signal transducer and activator of transcription
5/interleukin-10 signaling pathway is involved in the survival of
CD34+/CD38− AML cells, while Nishioka et
al (44) demonstrated the same
for the p38-mitogen-activated protein kinase signaling pathway.
Therefore, CD82 performs an important role in leukemogenesis. The
aforementioned studies were all performed in AML cell lines, and to
the best of our knowledge, no data has been reported about the
expression level and role of CD82 BM in pediatric patients with
ALL. In the present study, the expression level of CD82 in the BM
of pediatric ALL patients was evaluated. It was demonstrated that
CD82 expression in the BM of all pediatric patients with ALL-ND
increased compared with that in the controls. In B-ALL, CD82
expression in ND patients was significantly higher than that in the
CR patients and controls at the mRNA and protein levels. The level
of CD82 in BM plasma in patients with ND B-ALL was also higher than
that in controls, which was consistent with the RT-qPCR and flow
cytometry results. The results were consistent with those of
patients with AML and CML. This may indicate that CD82 is also
involved in the development of ALL progression in pediatrics. The
mechanism of the role of CD82 in ALL development requires
additional study in the future.
In conclusion, the present study revealed that CD133
and CD82 were aberrantly expressed in pediatric patients with ALL.
In addition, a significant positive correlation existed between
CD133 and CD82 expression in the BM. Furthermore, there was a
significant correlation between CD133 mRNA expression and a
hyperdiploid karyotype. Taken together, the results of the present
study indicated that CD133 and CD82 may represent important
potential immunotherapeutic targets in pediatric ALL. Although the
precise molecular mechanism involved in this process is unclear,
the results have potential clinical benefits. CD133 and CD82
expression, which could be detected by fluorescence-activated cell
sorting, may be a useful molecular marker to evaluate disease
progress in patients with ALL. The combined detection of CD133 and
CD82 can reflect the biological behavior of ALL, to a certain
extent, and may be used for molecular targeting therapy. However,
the number of samples in the present study was relatively small.
Additional larger prospective studies are required to verify the
present observations.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of Shandong Province (grant no.
ZR2015PH060) and the Outstanding Young Scientist Research Award
Foundation of Shandong Province (grant no. BS2010YY004).
References
|
1
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: An update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Möricke A, Zimmermann M, Reiter A, Henze
G, Schrauder A, Gadner H, Ludwig WD, Ritter J, Harbott J, Mann G,
et al: Long-term results of five consecutive trials in childhood
acute lymphoblastic leukemia performed by the ALL-BFM study group
from 1981 to 2000. Leukemia. 24:265–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conter V, Aricò M, Basso G, Biondi A,
Barisone E, Messina C, Parasole R, De Rossi G, Locatelli F, Pession
A, et al: Long-term results of the Italian Association of Pediatric
Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for
childhood acute lymphoblastic leukemia. Leukemia. 24:255–264. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pui CH, Campana D, Pei D, Bowman WP,
Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu
M, et al: Treating childhood acute lymphoblastic leukemia without
cranial irradiation. N Engl J Med. 360:2730–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhojwani D and Pui CH: Relapsed childhood
acute lymphoblastic leukaemia. Lancet Oncol. 14:e205–e217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Styczynski J and Drewa T: Leukemic stem
cells: From metabolic pathways and signaling to a new concept of
drug resistance targeting. Acta Biochim Pol. 54:717–726.
2007.PubMed/NCBI
|
|
9
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Makino S: The role of tumor stem-cells in
regrowth of the tumor following drastic applications. Acta Unio Int
Contra Cancrum. 15 Suppl 1:S196–S198. 1959.
|
|
11
|
Chávez-González A, Dorantes-Acosta E,
Moreno-Lorenzana D, Alvarado-Moreno A, Arriaga-Pizano L and Mayani
H: Expression of CD90, CD96, CD117, and CD123 on different
hematopoietic cell populations from pediatric patients with acute
myeloid leukemia. Arch Med Res. 45:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong D, Gupta R, Ancliff P, Atzberger A,
Brown J, Soneji S, Green J, Colman S, Piacibello W, Buckle V, et
al: Initiating and cancer-propagating cells in TEL-AML1-associated
childhood leukemia. Science. 319:336–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toren A, Bielorai B, Jacob-Hirsch J,
Fisher T, Kreiser D, Moran O, Zeligson S, Givol D, Yitzhaky A,
Itskovitz-Eldor J, et al: CD133-positive hematopoietic stem cell
‘stemness’ genes contain many genes mutated or abnormally expressed
in leukemia. Stem cells. 23:1142–1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wuchter C, Ratei R, Spahn G, Schoch C,
Harbott J, Schnittger S, Haferlach T, Creutzig U, Sperling C,
Karawajew L and Ludwig WD: Impact of CD133 (AC133) and CD90
expression analysis for acute leukemia immunophenotyping.
Haematologica. 86:154–161. 2001.PubMed/NCBI
|
|
15
|
Miraglia S, Godfrey W, Yin AH, Atkins K,
Warnke R, Holden JT, Bray RA, Waller EK and Buck DW: A novel
five-transmembrane hematopoietic stem cell antigen: Isolation,
characterization, and molecular cloning. Blood. 90:5013–5021.
1997.PubMed/NCBI
|
|
16
|
Horn PA, Tesch H, Staib P, Kube D, Diehl V
and Voliotis D: Expression of AC133, a novel hematopoietic
precursor antigen, on acute myeloid leukemia cells. Blood.
93:1435–1437. 1999.PubMed/NCBI
|
|
17
|
Morrison SJ and Scadden DT: The bone
marrow niche for haematopoietic stem cells. Nature. 505:327–334.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheppard D: In vivo functions of
integrins: Lessons from null mutations in mice. Matrix Biol.
19:203–209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Möhle R, Murea S, Kirsch M and Haas R:
Differential expression of L-selectin, VLA-4, and LFA-1 on
CD34+ progenitor cells from bone marrow and peripheral
blood during G-CSF-enhanced recovery. Exp Hematol. 23:1535–1542.
1995.PubMed/NCBI
|
|
20
|
Baer MR, Stewart CC, Lawrence D, Arthur
DC, Byrd JC, Davey FR, Schiffer CA and Bloomfield CD: Expression of
the neural cell adhesion molecule CD56 is associated with short
remission duration and survival in acute myeloid leukemia with
t(8;21)(q22;q22). Blood. 90:1643–1648. 1997.PubMed/NCBI
|
|
21
|
Gil ML, Vita N, Lebel-Binay S, Miloux B,
Chalon P, Kaghad M, Marchiol-Fournigault C, Conjeaud H, Caput D,
Ferrara P, et al: A member of the tetra spans transmembrane protein
superfamily is recognized by a monoclonal antibody raised against
an HLA class I-deficient, lymphokine-activated killer-susceptible,
B lymphocyte line. Cloning and preliminary functional studies. J
Immunol. 148:2826–2833. 1992.PubMed/NCBI
|
|
22
|
Miranti CK: Controlling cell surface
dynamics and signaling: How CD82/KAI1 suppresses metastasis. Cell
Signal. 21:196–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Iiizumi-Gairani M, Okuda H,
Kobayashi A, Watabe M, Pai SK, Pandey PR, Xing F, Fukuda K, Modur
V, et al: KAI1 gene is engaged in NDRG1 gene-mediated metastasis
suppression through the ATF3-NFkappaB complex in human prostate
cancer. J Biol Chem. 286:18949–18959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu DH, Liu L, Chen LH and Ding YQ: KAI1
gene expression in colonic carcinoma and its clinical
significances. World J Gastroenterol. 10:2245–2249. 2004.PubMed/NCBI
|
|
25
|
Goncharuk VN, del-Rosario A, Kren L, Anwar
S, Sheehan CE, Carlson JA and Ross JS: Co-downregulation of PTEN,
KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in
non-small cell lung cancer. Ann Diagn Pathol. 8:6–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mooez S, Malik FA, Kayani MA, Rashid R,
Zahid A and Khan A: Expressional alterations and transcript
isoforms of metastasis suppressor genes (KAI1 and KiSS1) in breast
cancer patients. Asian Pac J Cancer Prev. 12:2785–2791.
2011.PubMed/NCBI
|
|
27
|
Burchert A, Notter M, Dietrich Menssen H,
Schwartz S, Knauf W, Neubauer A and Thiel E: CD82 (KAI1), a member
of the tetraspan family, is expressed on early haemopoietic
progenitor cells and up-regulated in distinct human leukaemias. Br
J Haematol. 107:494–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishioka C, Ikezoe T, Furihata M, Yang J,
Serada S, Naka T, Nobumoto A, Kataoka S, Tsuda M, Udaka K and
Yokoyama A: CD34+/CD38− acute myelogenous
leukemia cells aberrantly express CD82 which regulates adhesion and
survival of leukemia stem cells. Int J Cancer. 132:2006–2019. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vardiman JW: The World Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Zou Y, Wang H, Chen X, Ruan M, Chen
Y, Yang W, Guo Y, Liu T, Zhang L, et al: Treatment outcome of
childhood standard-risk and median-risk acute lymphoblastic
leukemia with CCLG-2008 protocol. Zhonghua Er Ke Za Zhi.
52:449–454. 2014.(In Chinese). PubMed/NCBI
|
|
31
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guenova M and Balatzenko G: CD133-2
(AC141) expression analysis in acute leukemia immunophenotyping in
correlation to CD34 and P-glycoprotein. Hematology. 13:137–141.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bene MC, Castoldi G, Knapp W, Ludwig WD,
Matutes E, Orfao A and van't Veer MB: Proposals for the
immunological classification of acute leukemias. European Group for
the Immunological Characterization of Leukemias (EGIL). Leukemia.
9:1783–1786. 1995.PubMed/NCBI
|
|
35
|
Gallacher L, Murdoch B, Wu DM, Karanu FN,
Keeney M and Bhatia M: Isolation and characterization of human
CD34(−)Lin(−) and CD34(+)Lin(−) hematopoietic stem cells using cell
surface markers AC133 and CD7. Blood. 95:2813–2820. 2000.PubMed/NCBI
|
|
36
|
Yamamuro S, Okamoto Y, Sano E, Ochiai Y,
Ogino A, Ohta T, Hara H, Ueda T, Nakayama T, Yoshino A and Katayama
Y: Characterization of glioma stem-like cells from human
glioblastomas. Int J Oncol. 47:91–96. 2015.PubMed/NCBI
|
|
37
|
Welte Y, Davies C, Schäfer R and
Regenbrecht CR: Patient derived cell culture and isolation of
CD133+ putative cancer stem cells from melanoma. J Vis
Exp. e502002013.PubMed/NCBI
|
|
38
|
Bahnassy AA, Fawzy M, El-Wakil M, Zekri
AR, Abdel-Sayed A and Sheta M: Aberrant expression of cancer stem
cell markers (CD44, CD90 and CD133) contributes to disease
progression and reduced survival in hepatoblastoma patients: 4-year
survival data. Transl Res. 165:396–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Zhong XY, Li ZY, Cai JF, Zou L, Li
JM, Yang T and Liu W: CD133 expression in osteosarcoma and
derivation of CD133+ cells. Mol Med Rep. 7:577–584.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mak AB, Nixon AM and Moffat J: The mixed
lineage leukemia (MLL) fusion-associated gene AF4 promotes CD133
transcription. Cancer Res. 72:1929–1934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pellacani D, Packer RJ, Frame FM, Oldridge
EE, Berry PA, Labarthe MC, Stower MJ, Simms MS, Collins AT and
Maitland NJ: Regulation of the stem cell marker CD133 is
independent of promoter hypermethylation in human epithelial
differentiation and cancer. Mol Cancer. 10:942011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishioka C, Ikezoe T, Yang J, Nobumoto A,
Kataoka S, Tsuda M, Udaka K and Yokoyama A: CD82 regulates
STAT5/IL-10 and supports survival of acute myelogenous leukemia
cells. Int J Cancer. 134:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishioka C, Ikezoe T, Yang J and Yokoyama
A: Tetraspanin family member, CD82, regulates expression of EZH2
via inactivation of p38 MAPK signaling in leukemia cells. PLoS One.
10:e01250172015. View Article : Google Scholar : PubMed/NCBI
|