Introduction
Gastric cancer is the fourth most common type of
malignancy, and the fourth and fifth leading cause of
cancer-associated mortality in male and female worldwide,
respectively, and the situation is poor in developing countries
(1). The recognized etiology of
gastric cancer includes host genetic factor, for instance eating
habits and Helicobacter pylori infection. H. pylori
is a gram-negative bacterium that infects 50% of the world's
population and is considered the primary cause of peptic ulcer and
chronic gastritis (2). In 1994, the
International Agency for Research of Cancer defined H.
pylori as a class I carcinogen of gastric cancer which serves
an important function in the early stage of gastric carcinogenesis
(2). Furthermore, patients with
gastric cancer with H. pylori infection may exhibit a poor
prognosis (3); however, the
underlying molecular mechanism of H. pylori infection
leading to gastric cancer remains unknown.
The hepatocyte growth factor (HGF)/c-Met signaling
pathway was considered to be associated with the biological
development, regeneration and malignancy of tumors. HGF, also known
as scatter factor, may regulate cell viability, migration and
angiogenesis by binding to its only high-affinity receptor, c-Met
(4). Under normal conditions,
HGF-induced c-Met tyrosine kinase activation is regulated in
epithelial cells by paracrine ligand delivery (5). In addition, this pathway is
constitutively activated in a number of types of malignant tumor
including ovarian, lung, colon, breast and gastric cancer (6–9). A
previous study revealed that aberrant HGF/c-Met activation occurs
in gastric carcinogenesis and a number of antibody or small
molecules targeting the HGF/c-Met signaling pathway have been
evaluated in a cancer therapy clinical trial (7). However, whether aberrant HGF/c-Met
activation is associated with H. pylori infection remains
unknown. To investigate the function of the HGF/c-Met signaling
pathway in the carcinogenesis of H. pylori infection, in the
present study, human gastric biopsies, following clinical endoscopy
or gastrectomy, were selected and gastric epithelial cell lines
were co-cultured with H. pylori in vitro.
Materials and methods
Patients
Gastric tissue samples were selected from patients
who underwent a gastroduodenoscopy at the First Affiliated Hospital
of Nanchang University (Nanchang, China) between January 2011 and
January 2013. A total of 160 patients were enrolled in the present
study, which included 40 chronic gastritis (CNGS), 40 intestinal
metaplasia (IM), 40 dysplasia (Dysp) and 40 gastric cancer (GC)
samples. Each group of patients (CNGS, TM, Dysp and GC) contained
20 H. pylori-positive and 20 H. pylori-negative
samples. There was no significant difference in the age and sex
distribution between these groups. The present study was approved
by the Ethics Committee of the First Affiliated Hospital of
Nanchang University and written informed consent was obtained from
all patients for participation in the present study.
Detection of H. pylori infection
An ‘in-house’ rapid urease test (RUT) and modified
Giemsa staining were employed to determine H. pylori
infection. The effectiveness of RUT was >95% (data not shown).
The modified Giemsa staining was carried out in a double-blind
fashion. The tissues used for this staining analysis were fixed in
10% formaldehyde in Ca2+ and Mg2+-free PBS
overnight at 4°C prior to paraffin embedding. Paraffin-embedded
sections of 4 µm were cut with a microtome and stored at room
temperature. The specimens were then stained with 20% Giemsa stain
for 20 min at room temperature. Each specimen was reviewed under
the light microscope (magnification, ×400). H. pylori
infection was diagnosed as positive only if the two methods
produced positive results and a H. pylori-negative diagnosis
was classified if the two methods yielded negative results.
Histological examinations
All biopsies from patients with distinct gastric
lesions were obtained from the gastric antrum and individual
lesions from patients. The tissues used for histological analysis
were fixed as aforementioned: In 10% formaldehyde in
Ca2+ and Mg2+-free PBS overnight at 4°C prior
to paraffin embedding. Paraffin-embedded sections of 4 µm were cut
with a microtome and stored at room temperature. Pathological
diagnosis and classification were carried out according to the
criteria of the World Health Organization and the updated Sydney
system (10).
Immunohistochemistry (IHC)
Paraffin sections were prepared from the gastric
tissues or biopsy specimens. For immunohistochemistry, tissue
sections were first de-waxed in xylene and sequentially dehydrated
in 100, 95 and 85% ethanol. The sections were immunostained using
the PV-9000 Polymer Detection System (Origene Technologies, Inc.,
Beijing, China) according to the standard staining protocol.
Sections were first washed in PBS and endogenous peroxidase was
blocked using 3% H2O2. Subsequently, the
specimens were incubated with the primary antibody overnight at
4°C. Primary antibodies used were a rabbit polyclonal anti-human
HGF (cat. no. ab83760; dilution, 1:400) or a rabbit monoclonal
anti-human c-Met (cat. no. ab51067; dilution, 1:800), and were
purchased from Abcam (Cambridge, UK). Specimens were washed with
PBS, followed by incubation with polymer helper for 30 min and
polyperoxidase-conjugated anti-rabbit immunoglobulin G (IgG;
ready-to-use; PV-9000 Detection System; Origene Technologies, Inc.)
for 30 min. Subsequently, specimens were washed three times with
PBS and incubated with 3,3-diaminobenzidin (OriGene Technologies)
for developing the positive color. The negative control sections
were incubated with PBS only, without primary antibodies. The
sections were counterstained with hematoxylin and mounted with
coverslips.
Review and scoring of immunostained
sections
The stained sections were reviewed and scored under
a light microscope by two pathologists, without knowing the
clinicopathological data. The concordance rates were typically high
and data with any grading discrepancies were re-reviewed to
finalize the score. Epithelial cells stained yellow or brown in the
nucleus and/or cytoplasm were defined as positively stained. Each
section was selected, reviewed and scored between five randomly
selected fields (magnification, ×200). The H-score system was used
to evaluate the expression of HGF or c-Met. Combining staining
intensity (score, between 0 and 4) with the proportion of positive
cells (score, between 0 and 100%). Each intensity level was
multiplied by the proportion of positive cells and all values were
summed to obtain the final IHC score (range, between 0 and 400).
Scores between 0 and 200 were considered to be associated with
negative expression, whereas scores between 201 and 400 were
considered to demonstrate positive expression (11).
Cell lines and H. pylori strains
The immortalized gastric epithelial mucosa cell line
GES-1, established by the Beijing Institute for Cancer Research
(Beijing, China), was a kind gift from Professor Y. Ke of Beijing
Institute for Cancer Research, and the human gastric cancer AGS
cell line were kind gifts from Professor D.M. Fan of Xijing
Hospital (Xi'an, China). These two cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare,
Logan, UT, USA), supplemented with 10% fetal bovine serum, 100
µg/ml penicillin and 100 µg/ml streptomycin, at 37°C in an
atmosphere containing 5% CO2. The cytotoxin-associated
gene A (CagA+) and VacA+ H. pylori
type strain ATCC43504 was cultured on Campylobacter agar
plates (Diarrheal Disease Research Center, Shanghai, China),
containing 10% sheep blood, and incubated at 37°C under
microaerophilic conditions for 24 h. The bacteria were suspended in
DMEM. The density was estimated using a spectrophotometer
(A660). GES-1 and AGS cells (2.5×106
cells/flask, 25 cm2 flask) were incubated at 37°C in 5%
CO2 with H. pylori (multiplicity of infection,
50).
Protein extraction and western blot
analysis
At exponential growth phase, ~5×106 cells
were selected and lysed in a buffer containing 0.5% Lubrol-PX, 50
mM KCl, 2 mM CaCl2, 20% glycerol, 50 mM Tris-HCl (pH
7.4), 0.1% protease and 1% phosphatase inhibitors (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The concentrations of protein
samples were determined using the bicinchoninic acid assay (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein (50 µg)
was separated by SDS-PAGE (10% gel), with 4X sample buffer (250 mM
Tris-HCl, pH 6.8, 4% SDS, 10% glycerol, 0.006% bromophenol blue and
2% 2-mercaptoethanol), and denatured by boiling for 5 min in a
water bath. Subsequently, proteins were transferred onto
nitrocellulose membranes (Whatman GmbH, Dassel, Germany) and
blocked with 5% non-fat milk in Tris-buffered saline (TBS)
containing 0.1% Tween-20 (TBST). The membranes were incubated
overnight with the primary antibody at 4°C. Primary antibodies used
were a rabbit polyclonal anti-human HGF (cat. no. ab83760;
dilution, 1:1,000; Abcam) or a rabbit monoclonal anti-human c-Met
(cat. no. ab51067; dilution, 1:1,000; Abcam), and a rabbit
polyclonal anti-human β-actin antibody (cat. no. sc-1615-R;
dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was used as a reference protein. Subsequently, a horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. ZB-2301;
dilution, 1:10,000; Origene Technologies, Inc.) secondary antibody
was incubated with the membrane at 4°C for 4 h. The reactions were
subjected to incubation with the enhanced chemiluminescence
detection system (Pierce; Thermo Fisher Scientific, Inc.) and
exposed to X-ray film for visualization. The blots were quantified
by Gel-Pro analyzer (version 4.0; Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
or as a proportion of the control. The χ2 test (SPSS
software, version 16.0 for Windows; SPSS, Inc., Chicago, IL, USA)
was performed to evaluate the difference between categorical
variables, including sex, among the distinctly defined groups. The
one-way analysis of variance test and least significant difference
test (SPSS software, version 16.0) was used to determine the
differences, including the age of patients in numerical variables
among the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HGF and c-Met in
distinct gastric lesions, in association with H
pylori infection
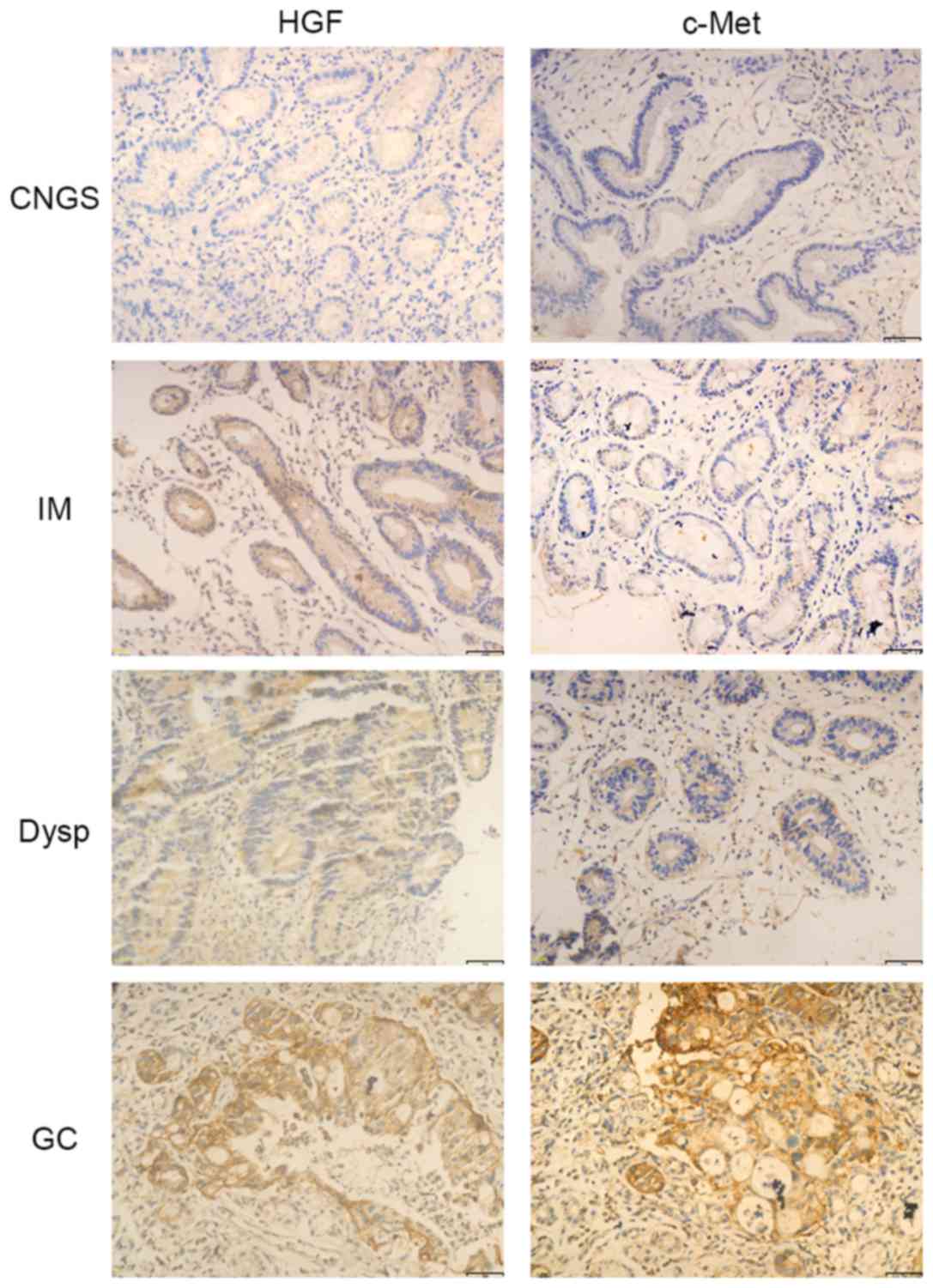
The expression of c-Met was significantly increased
in GC compared with precancerous lesions (P<0.001; Table I; Fig.
1). The expression of HGF was observed in only 32.5% of
patients with CNGS, whereas between weak and strong expression of
HGF was identified in 52.5, 37.5 and 57.5% of patients with IM,
Dysp and GC, respectively, although there was no significant
difference in the expression of HGF between the groups (P>0.05;
Table I; Fig. 1).
 | Table I.Differential expression of HGF and
C-Met in distinct histological gastric tissue specimens. |
Table I.
Differential expression of HGF and
C-Met in distinct histological gastric tissue specimens.
|
|
| Overall score of
protein expression |
|---|
|
|
|
|
|---|
|
|
| HGF | c-Met |
|---|
|
|
|
|
|
|---|
| Group | n | − | + | PR, % | P-value | − | + | PR, % | P-value |
|---|
| Overall | 160 | 88 | 72 | 45.0 | 0.078 | 105 | 55 | 34.4 |
|
| CNGS | 40 | 27 | 13 | 32.5 |
| 33 | 7 | 17.5 |
|
| IM | 40 | 19 | 21 | 52.5 |
| 33 | 7 | 17.5 |
|
| Dysp | 40 | 25 | 15 | 37.5 |
| 28 | 12 | 30.0 |
|
| GC | 40 | 17 | 23 | 57.5 |
| 11 | 29 | 72.5 |
<0.001a,b,c |
| H.
pylori+ | 80 | 34 | 46 | 57.5 |
| 44 | 36 | 45.0 |
|
| CNGS | 20 | 12 | 8 | 40.0 |
| 16 | 4 | 20.0 |
|
| IM | 20 | 8 | 12 | 60.0 |
| 15 | 5 | 25.0 |
|
| Dysp | 20 | 9 | 11 | 55.0 |
| 11 | 9 | 45.0 |
|
| GC | 20 | 5 | 15 | 75.0 |
| 2 | 18 | 90.0 |
|
| H.
pylori− | 80 | 54 | 26 | 32.5 |
| 61 | 19 | 23.8 |
|
| CNGS | 20 | 15 | 5 | 25.0 |
| 17 | 3 | 15.0 |
|
| IM | 20 | 11 | 9 | 45.0 |
| 18 | 2 | 10.0 |
|
| Dysp | 20 | 16 | 4 | 20.0 | 0.022d | 17 | 3 | 15.0 | 0.038d |
| GC | 20 | 12 | 8 | 40.0 | 0.025e | 9 | 11 | 55.0 | 0.013e |
In the presence or absence of H. pylori
infection, the expression of c-Met was significantly increased in
GC samples (P<0.001; Table I;
Fig. 1). However, there was no
significant difference in the expression of HGF between the groups
in the presence or absence of H. pylori infection
(P>0.05; Table I; Fig. 1).
The expression of the proteins was compared between
H. pylori-positive and H. pylori-negative patients at
distinct pathological stages. In patients with Dysp and GC, the
expression of HGF and c-Met was significantly increased in the
presence of H. pylori infection, compared with that in the
absence of the infection (P<0.05; Table I). However, in patients with CNGS and
IM, there was no significant difference determined in the
expression of HGF and c-Met between patients with and patients
without H. pylori infection (P>0.05; Table I).
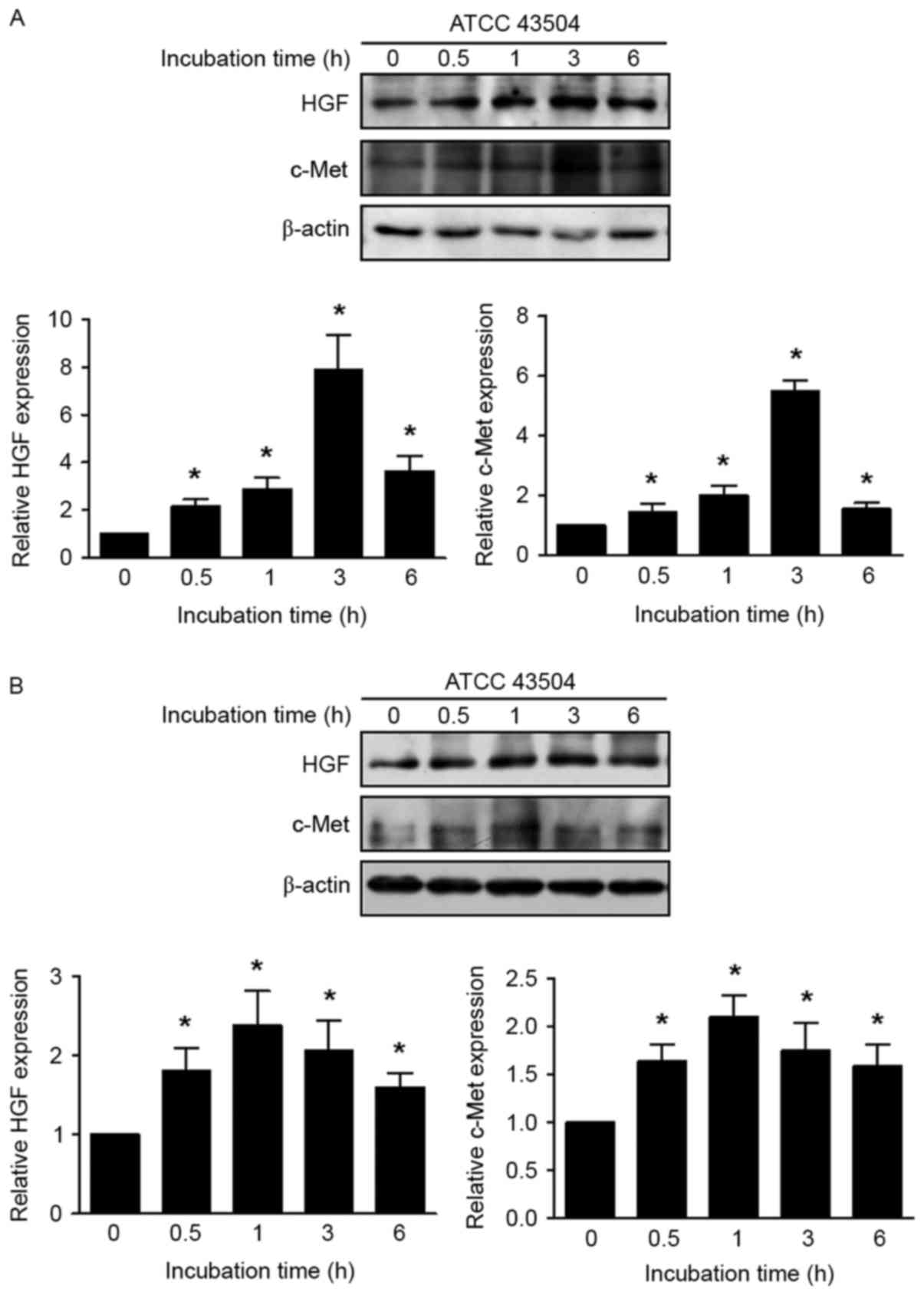
Induced expression of HGF and c-Met by
H. pylori in non-malignant and malignant gastric cell lines
Non-malignant GES-1 and AGS cells were incubated
with H. pylori (multiplicity of infection, 50) and the
expression of HGF and c-Met was significantly increased at all time
points (0.5, 1, 3 and 6 h), compared with that in the absence of
infection (P<0.05; Fig. 2).
Discussion
The abnormal activation of the HGF/c-Met signaling
pathway has been studied in a variety of types of human epithelial
cancer. The underlying molecular mechanism includes gene
overexpression, focal gene amplification, gene copy-number gain and
activation mutations (11). A
previous study revealed that overexpression of c-Met was observed
in 46.1% of gastric carcinoma cases and gene amplification of c-Met
was detected in 10.2% of gastric carcinoma cases (12). Furthermore, the increased expression
of c-Met was an indicator of liver metastasis and independent
prognostic factors in patients with GC (12,13). The
results of the present study identified similar results, as the
increased expression of c-Met was observed in the GC group,
compared with precancerous lesions. However, no significant
difference was identified in the CNGS, IM and Dysp lesions,
suggesting that the activation of HGF/c-Met was activated in the
later stage of gastric carcinogenesis.
The positive rate of c-Met expression was identified
to be increased in the H. pylori-positive Dysp and GC group,
compared with that in the H. pylori-negative group. The
results identified in gastric tissue was validated by the in
vitro study. The results of the present study indicated that
H. pylori infection may activate c-Met expression. The
underlying molecular mechanism may be attributed to CagA, which is
translocated via a type IV secretion system into epithelial cells,
intracellularly modulating the receptor tyrosine kinase c-Met,
leading to the activation of extracellular-signal-regulated kinase
(ERK) 1/2 in AGS cells (14). Franke
et al (15) performed a
logical statistical model revealing the differences and
commonalities of the response of the network upon HGF and H.
pylori-induced c-Met signaling, and the results predicted an
effect on ERK1/2 signaling induced by the H. pylori
stimulus, but not by HGF treatment.
A previous study identified that the serum HGF
levels were markedly increased in the group of patients with
gastric cancer and the expression of HGF was positive in 72% of GC
tissues (16). Whether HGF is an
important factor in H. pylori pathogenesis remains unknown.
The results of the present study demonstrated that the expression
of HGF increased in GC samples, compared with that in the
precancerous lesions, but with no significant difference. However,
the positive rate of HGF was increased in the H.
pylori-positive Dysp and GC group, which was identified by
cytological research in vitro. The controversial results may
be due to the complex regulatory mechanisms of HGF in local and
systemic physical endocrine systems. In addition, the limitation of
gastric samples may have implications for the present study, as it
may not comprehensively reflect the level of HGF in entirety.
Evaluation of the serum levels of HGF may improve the understanding
of the function of HGF in gastric carcinogenesis.
HGF/c-Met activation were identified to be late
molecular events in gastric carcinogenesis, which was markedly
observed in Dysp and GC with the presence of H. pylori
infection. H. pylori infection may activate the HGF/c-Met
signaling pathway in the in vitro study; however, the
underlying molecular mechanism remains to be elucidated.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460116), the Youth
Natural Science Foundation of Jiangxi Province (grant no.
20122BAB215009) and the Science and Technology Project of Jiangxi
Provincial Education Department (grant no. GJJ10230).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wroblewski LE, Peek RM Jr and Wilson KT:
Helicobacter pylori and gastric cancer: Factors that
modulate disease risk. Clin Microbiol Rev. 23:713–739. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li G, Wang Z, Wang Z, Xu J, Cui J, Cai S,
Zhan W and He Y: Gastric cancer patients with Helicobacter
pylori infection have a poor prognosis. J Surg Oncol.
108:421–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gherardi E and Stoker M: Hepatocyte growth
factor-scatter factor: Mitogen, motogen, and met. Cancer Cells.
3:227–232. 1991.PubMed/NCBI
|
|
5
|
Prat M, Narsimhan RP, Crepaldi T, Nicotra
MR, Natali PG and Comoglio PM: The receptor encoded by the human
c-MET oncogene is expressed in hepatocytes, epithelial cells and
solid tumors. Int J Cancer. 49:323–328. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou HY, Pon YL and Wong AS: HGF/MET
signaling in ovarian cancer. Curr Mol Med. 8:469–480. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hack SP, Bruey JM and Koeppen H:
HGF/MET-directed therapeutics in gastroesophageal cancer: A review
of clinical and biomarker development. Oncotarget. 5:2866–2880.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gelsomino F, Rossi G and Tiseo M: MET and
small-cell lung cancer. Cancers (Basel). 6:2100–2115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sipos F and Galamb O:
Epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions
in the colon. World J Gastroenterol. 18:601–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated Sydney
System. International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marano L, Chiari R, Fabozzi A, De Vita F,
Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F and
Patriti A: c-Met targeting in advanced gastric cancer: An open
challenge. Cancer Lett. 365:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakajima M, Sawada H, Yamada Y, Watanabe
A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T and
Nakano H: The prognostic significance of amplification and
overexpression of c-met and c-erb B-2 in human gastric carcinomas.
Cancer. 85:1894–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amemiya H, Kono K, Itakura J, Tang RF,
Takahashi A, An FQ, Kamei S, Iizuka H, Fujii H and Matsumoto Y:
c-Met expression in gastric cancer with liver metastasis. Oncology.
63:286–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Churin Y, Al-Ghoul L, Kepp O, Meyer TF,
Birchmeier W and Naumann M: Helicobacter pylori CagA protein
targets the c-Met receptor and enhances the motogenic response. J
Cell Biol. 161:249–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franke R, Muller M, Wundrack N, Gilles ED,
Klamt S, Kähne T and Naumann M: Host-pathogen systems biology:
Logical modelling of hepatocyte growth factor and Helicobacter
pylori induced c-Met signal transduction. BMC Syst Biol.
2:42008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi E, Saito N, Kobayashi M and
Kameoka S: Clinical significance of hepatocyte growth factor/c-Met
expression in the assessment of gastric cancer progression. Mol Med
Rep. 11:3423–3431. 2015.PubMed/NCBI
|