Introduction
Hemangioma is a type of congenital vascular
dysplasia common among infants and young children, with a high
incidence rate of ~10–12%; (1). The
primary pathological features of hemangioma include the excessive
proliferation of vascular endothelial cells and the formation of an
abnormal vascular cavity (2).
Kasabach-Merritt syndrome (K-MS; large hemangioma with
thrombocytopenia syndrome) is a serious and life-threatening
hemangioma (3–7), which is often associated with comorbid
conditions such as hemangioendothelioma (8–10) or
plexiform hemangioma (tufted angiomas) (11). In addition to the rapid increase in
the size of the tumor, other features of the syndrome include the
development of a low platelet count, microvascular disease, anemia
and blood coagulation dysfunctions (12). In the present study, it was generally
considered that vasculogenesis and angiogenesis are involved in the
formation of new blood vessels in the hemangioma (13). The potential underlying molecular
mechanisms include vascular endothelial growth factor (VEGF) and
receptors (VEGFR) (14), angiogenin
and receptors (15), the Notch
signaling pathway (16), mammalian
target of rapamycin (mTOR) signaling pathway (17), β-adrenergic receptors and the
renin-angiotensin system (18), among
others.
Artesunate is an important derivative of
artemisinin, and its metabolite dihydro-artemisinin has been used
to treat malaria (19). In recent
years, studies have identified that artesunate has anti-tumor
potential (20). Artesunate has a
variety of mechanisms as an anti-tumor agent: It can influence
tumor cell apoptosis, the iron-mediated formation of free radicals,
anti-angiogenesis, block the cell cycle, anti-immunosuppressive
(21), reverse drug resistance and
restore chemosensitivity (22,23). A
large number of studies are underway, evaluating the anti-cancer
activity of artemisinin derivatives; however, artesunate has not
yet been studied for hemangioma.
The aim of the current experiment was to explore the
effect of artesunate on hemangioma. Thus, EOMA cells were used in
an established hemangioma nude mouse model (24,25).
Subsequently, the mechanisms of the artesunate-mediated inhibition
of EOMA cell proliferation and invasion in vitro, and of
hemangioma growth in vivo, were investigated.
Materials and methods
Antibodies and main reagents
Hypoxia-inducible factor (HIF-1α; cat. no. ab82832),
caspase-3 (cat. no. ab13847), VEGFR1 (cat. no. ab32152) and β-actin
(cat. no. ab8227) were purchased from Abcam (Cambridge, UK). VEGF-A
(cat.no. 31274-1) and VEGFR-2 (cat. no. 21079-1) were purchased
from Signalway Antibody LLC., (College Park, Maryland, USA). The
EOMA cell line was purchased from the American Type Culture
Collection, (ATCC, Manassas, VA, USA). Fetal calf serum (FCS) and
Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA); artesunate was
purchased from Guilin Pharmaceutical Co. Ltd., (Guilin, China); MTT
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Matrigel-coated Transwell chambers were purchased from Qiagen GmbH
(Hilden, Germany). Apoptosis kits, including propidium iodide (PI)
and fluorescein isothiocyanate (FITC)-Annexin V work solution, were
purchased from Thermo Fisher Scientific, Inc. PrimeScript™ RT
reagent kit with gDNA Eraser and SYBR® Premix Ex Taq™ II
were purchased from Takara Bio, Inc., (Otsu, Japan). TRIzol Reagent
was purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture
Cells were cultured in DMEM supplemented with 10%
FCS and incubated at 37°C with 5% CO2, prior to being
used at the logarithmic growth phase.
Growth inhibition assay
An MTT assay was used to assess cell proliferation.
The EOMA cells (1×105/ml) were seeded onto 96-well
plates. Artesunate was diluted in 1 ml 5% sodium bicarbonate
solution, shaken for 2–3 min producing 0, 100, 200, 300, 400 and
500 µg/ml concentrations and then 100 µl was added to the EOMA
cells in each well. At 0, 24, 48 and 72 h, an equal volume of MTT
solution was added to each well and cultured for another 4 h. The
incubation conditions in this assay were all 37°C in an atmosphere
containing 5% CO2. MTT-treated cells were fixed with 150
µl dimethyl sulfoxide for 30 min at room temperature and then
assayed with an Evolution™ 201/220 UV-Vis spectrophotometer at 490
nm.
Apoptosis assessment
After 24 and 48 h, artesunate-treated (300 µg/ml)
cells were collected and washed with cold PBS. Stained cells were
assessed with a FACSCalibur™ flow cytometer and analyzed using BD
CellQuest™ software (version 5.1; BD Biosciences, Franklin Lakes,
NJ, USA). The percentage of early apoptotic cells (stained with
Annexin V only) and late apoptotic cells (stained with Annexin V
and PI) was recorded. Furthermore, Hoechst 33342 staining solution
was used to identify the apoptotic cells, and later detected with
fluorescence microscopy. Unstained cells were included as the
control.
Transwell invasion assay
A Transwell assay was performed by using
Matrigel-coated Transwell chambers (pore size of 8.0 µm). A total
of 1×105 cells were re-suspended in 200 µl serum-free
medium and seeded into the upper compartment of the chamber. The
lower compartment was loaded with 800 µl DMEM containing 10% FCS.
After incubation at 37°C for 36 h, the membranes were fixed with
formaldehyde at room temperature for 5 min, and counter-stained
with DAPI at room temperature for 30 min in the dark. The staining
was examined under a vertical fluorescence microscope.
Trans-membrane migrated cells from each sample were counted in
three random fields at ×200 magnification. The assay was performed
in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
An RT-qPCR assay was used for the quantitative
estimation of the mRNA expression of VEGF-A, VEGFR-1, VEGFR-2 and
HIF-1α in EOMA cells, in the artesunate and in the control groups.
Total RNA from ~1×107 cells was extracted using
TRIzol® reagent, according to the manufacturer's
protocol. Total RNA then underwent RT using the PrimeScript™ RT
reagent kit with gDNA Eraser. The expression of VEGF-A, VEGFR-1,
VEGFR-2 and HIF-1α mRNA was determined using the CFX96 Real-Time
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using SYBR® Premix Ex Taq™ II. β-actin was used as the
internal reference. Data were analyzed using the 2−ΔΔCq
method (26). Three separate
experiments were performed for each clone. Primers used for PCR
analysis were as follows: VEGF-A forward,
5′-CAGGCTGCTGTAACGATGAA-3′ and reverse, 5′-TTTCTTGCGCTTTCGTTTTT-3′;
VEGFR-1 forward, 5′-GAGGAGGATGAGGGTGTCTATAGGT-3′ and reverse,
5′-GTGATCAGCTCCAGGTTTGACTT-3′; VEGFR-2 forward,
5′-TTCTGGACTCTCCCTGCCTA-3′ and reverse, 5′-AAGGACCATCCCACTGTCTG-3′;
HIF-1α forward, 5′-TGAGCTTGCTCATCAGTTGC-3′ and reverse,
5′-CCATCTGTGCCTTCATCTCA-3′; β-actin forward,
5′-AAGATGACCCAGATCATGTTTGAGACC-3′ and reverse,
5′-GCCAGGTCCAGACGCAGGAT-3′.
Western blot analysis
EOMA cells were treated with 300 µg/ml artesunate
for 24, 48 and 72 h, washed with cold PBS, harvested and extracted
using lysis buffer. The protein concentration was detected using a
NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Inc.). A
total of 100 µg protein were separated using SDS-PAGE (10% gel) and
then transferred to polyvinylidene fluoride membranes. To eliminate
background disturbance, membranes were blocked by incubation in
blocking solution (5% low fat milk and 0.1% Tween 20 in TBS) at
room temperature for 1 h. Cells were then incubated with a rabbit
monoclonal primary antibody specific for VEGF-A (dilution, 1:500),
VEGFR-1 (dilution, 1:1,000), VEGFR-2 (dilution, 1:500), HIF-1α
(dilution, 1:1,000), caspase-3 (dilution, 1:500) and β-actin
(dilution, 1:2,000) at 4°C overnight, followed by incubation with a
secondary antibody for 1 h at room temperature. Following this,
enhanced chemiluminescence protein analysis was performed.
Animals
To observe efficacy of intra-tumor injection of
artesunate for mouse K-MS and later a comparison with pingyangmycin
(PYM), a total of 20 five-week-old female BALB/c nude mice (weight,
between 18 and 20 g) were obtained from Chongqing Medical
University (Chongqing, China). The mice were housed under specific
pathogen-free conditions in an animal facility with free access to
pelleted regular rodent diet (Experimental Animal Centre of the
Children's Hospital of Chongqing Medical University, Chongqing,
China) and water ad libitum. The room temperature was between
22–25°C, humidity was between 45–55%, and a 12-h light-dark diurnal
cycle (lights on from 7:00 to 19:00) was used. The Experimental
Animal Centre of Children's Hospital of Chongqing Medical
University approved the experimental protocols. All procedures were
carried out according to the Institutional Animal Care and Use
Committee Guide in Child Development and Disorders' Laboratories
(Chongqing, China; license no. SYXK (YU)2012-0001; sydwzx.cqmu.edu.cn).
An inoculation of 1×107 EOMA cells in 100
µl PBS was injected into the subcutaneous tissues of the right
flank of the female nude mice. The female nude mice were randomly
divided into the following four groups of five each: Artesunate
group, PYM group, PBS group and no treatment group. Tumor-bearing
mice were treated with intra-tumoral injections every three days
until the tumor was visible to the naked eye. The PBS group
(intra-tumoral injection with the same dose of PBS every three
days) was used as the negative control group and the no treatment
group (tumor growth was not modified) was considered as the blank
control group. As PYM is typically used as a treatment modality for
hemangioendothelioma, the curative effect of artesunate was
compared with that of PYM. The tumors were measured on alternate
days using tissue calipers. Tumor volume was determined using the
following formula: (Width)2x length ×0.52. Mice were
sacrificed by cervical dislocation when difficulty with ambulation
and lethargy had set in.
Statistical analysis
SPSS 13.0 software was used for all data analysis
(SPSS, Inc., Chicago, IL, USA). Numerical data are represented as
the mean ± standard deviation. Differences between the control and
treated groups were determined using the Student's t-test and
one-way analysis of variance. Each experiment was performed in
triplicate and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of artesunate on the
proliferation of EOMA cells
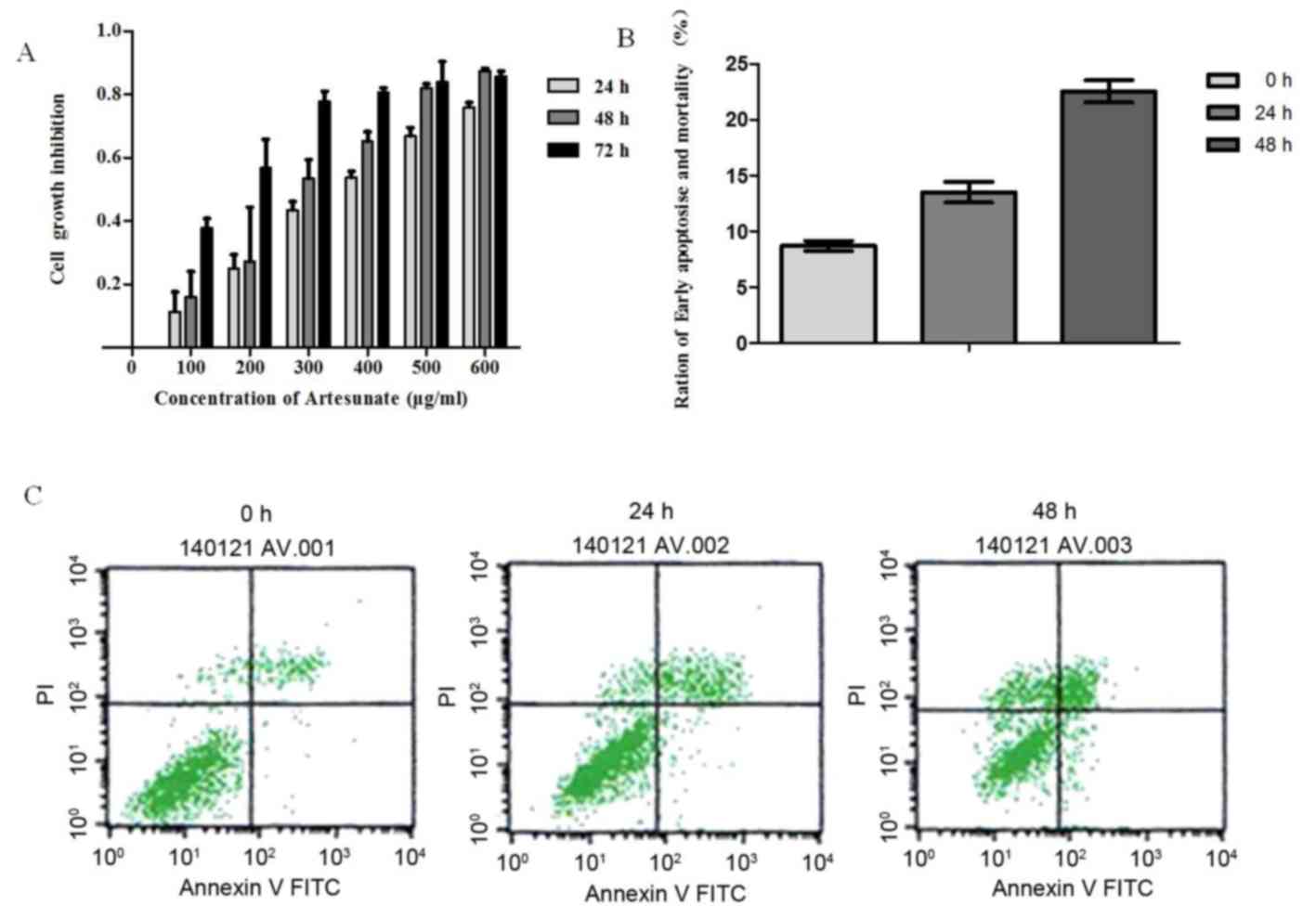
Artesunate treatment significantly inhibited the
growth of EOMA cells in a time- and dose-dependent manner (Fig. 1A). The inhibition of cell
proliferation increased correspondingly with the increase in drug
concentration during the same time. Also, on extension of exposure
time, there was an increase in cell proliferation inhibition with
the same concentration. However, this inhibitory effect became
apparent as ≤50% (IC50) at a concentration of 300 µg/ml
artesunate; therefore, this concentration was used throughout the
study.
Effect of artesunate on the apoptosis
of EOMA cells
Based on the results of the MTT assay, 300 µg/ml of
artesunate was used for determining apoptosis. To elucidate the
mechanism by which artesunate exerts its anti-proliferative effect
on EOMA cell lines, an Annexin V/PI binding assay was performed.
Results demonstrated an increased percentage of early apoptotic
cells (Annexin V/PI) after treatment of EOMA cells with 300 µg/ml
artesunate for 24 h (24 h, P<0.05 vs. 0 h), and this percentage
of early apoptotic cells increased significantly following
incubation for 48 h (48 h, P<0.01 vs. 0 h; Fig. 1B and C). The rate of apoptosis for
EOMA cells treated with artesunate increased in a time-dependent
manner.
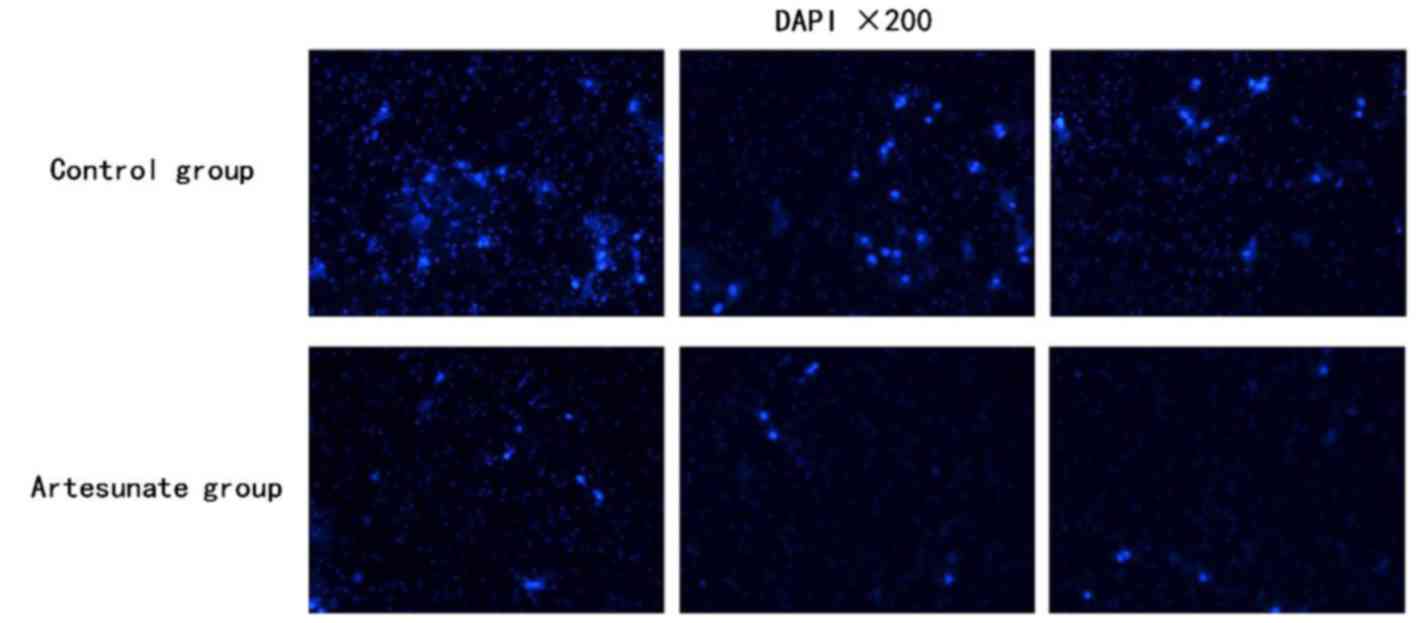
Effect of artesunate on the invasion
of EOMA cells
To investigate the inhibitory effect of artesunate
on the invasion of EOMA cells in vitro. The invasion
efficiency of EOMA cells in response to artesunate was tested using
a Transwell invasion system. The invasion efficiency was calculated
as the number of invaded cells to the control. In the presence of
artesunate (300 µg/ml), the number of invaded EOMA cells was
significantly decreased compared with that of the control group,
which has the same number of EOMA cells in the initial stage of
this experiment (Fig. 2;
P<0.05).
Effect of artesunate on VEGF-A,
VEGFR-1, VEGFR-2 and HIF-1α mRNA expression
RT-qPCR was performed to examine the expression of
VEGF-A, VEGFR-1, VEGFR-2 and HIF-1α mRNA in EOMA cells treated with
artesunate at 300 µg/ml for 24, 48 and 72 h. The results
demonstrate that VEGF-A, VEGFR-2 and HIF-1α mRNA expression levels
in EOMA cells treated with artesunate were significantly decreased
in a time-dependent manner (*P<0.05, #P<0.01;
Fig. 3A). There was no statistically
significant difference in the VEGFR-1 mRNA expression level among
the treatment groups (Fig. 3A).
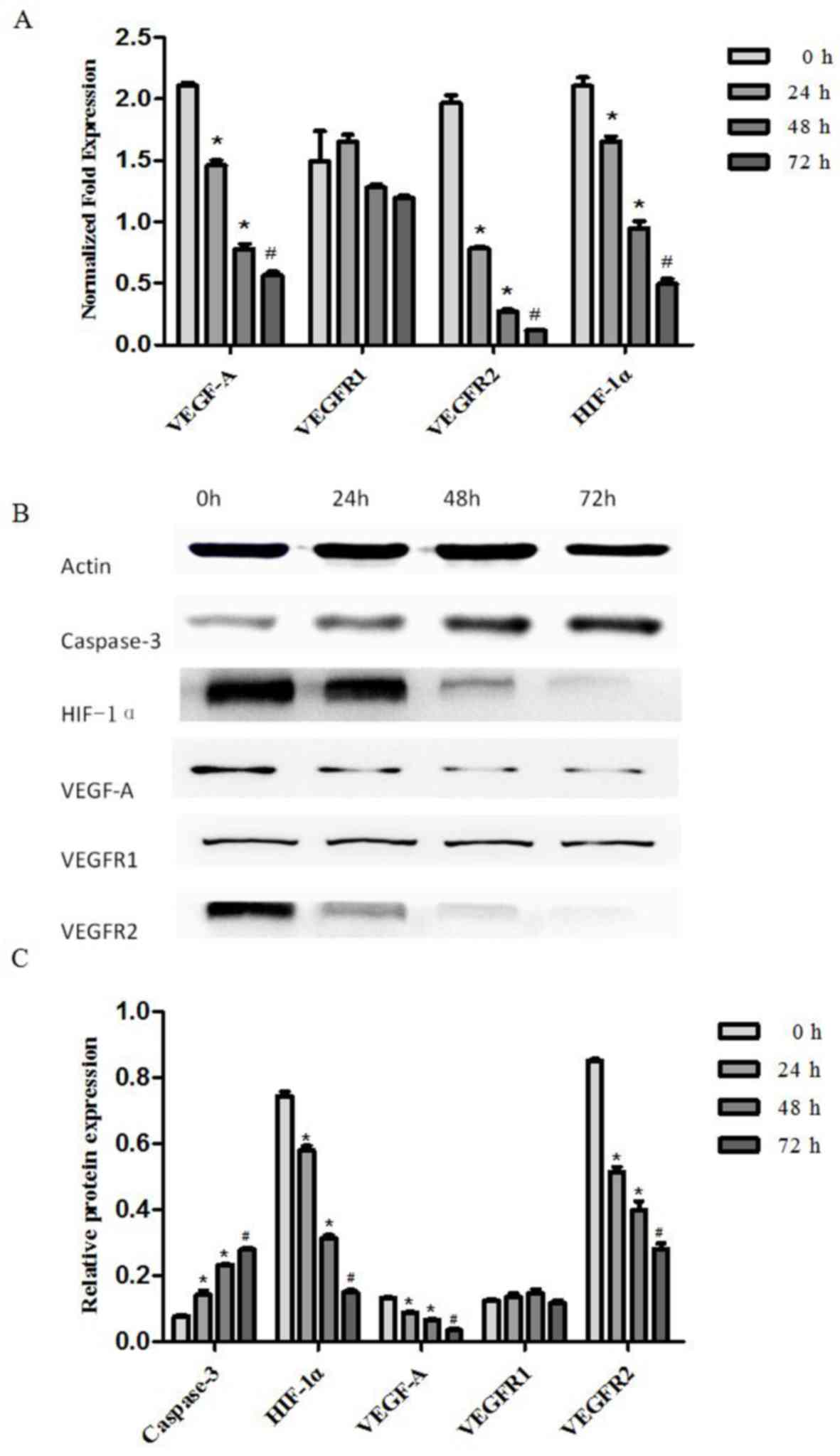 | Figure 3.Artesunate suppressed the gene and
protein expression of VEGF-A, VEGFR-2 and HIF-1α, and increased the
protein expression of caspase-3 in mouse EOMA cell lines. (A) The
transcript levels of VEGF-A, VEGFR-1, VEGFR-2 and HIF-1α mRNA were
analyzed using RT-qPCR; β-actin was included as the loading
control. VEGF-A, VEGFR-2 and HIF-1α expression was significantly
downregulated by artesunate. The results are expressed as the mean
± standard deviation from three separate experiments (*P<0.05,
#P<0.01, vs. control). (B) Western blot analysis of
the protein expression in EOMA cells treated with artesunate for
24, 48 and 72 h, to evaluate VEGFA, VEGFR-1, VEGFR2, HIF-1α and
caspase 3 expression. (C) Results presented are representative data
of at least three independent experiments (*P<0.05,
#P<0.01, vs. control). VEGFR, vascular endothelial
growth factor receptor; HIF-1α, hypoxia inducible factor-1α. |
Effect of artesunate on VEGF-A, VEGFR-1, VEGFR-2,
HIF-1 and, caspase-3 protein level. Western blotting was performed
to examine the expression of VEGF-A, VEGFR-1, VEGFR-2, HIF-1α and
caspase-3 protein in EOMA cells treated with artesunate at 300
µg/ml for 24, 48 and 72 h. The results demonstrated that HIF-1α,
VEGF-A and VEGFR-2 protein expression levels in EOMA cells were
significantly decreased, but caspase 3 protein expression levels
were significantly increased (*P<0.05, #P<0.01;
Fig. 3B). There were no statistically
significant differences in VEGFR-1 protein expression levels among
the treatment groups (Fig. 3C).
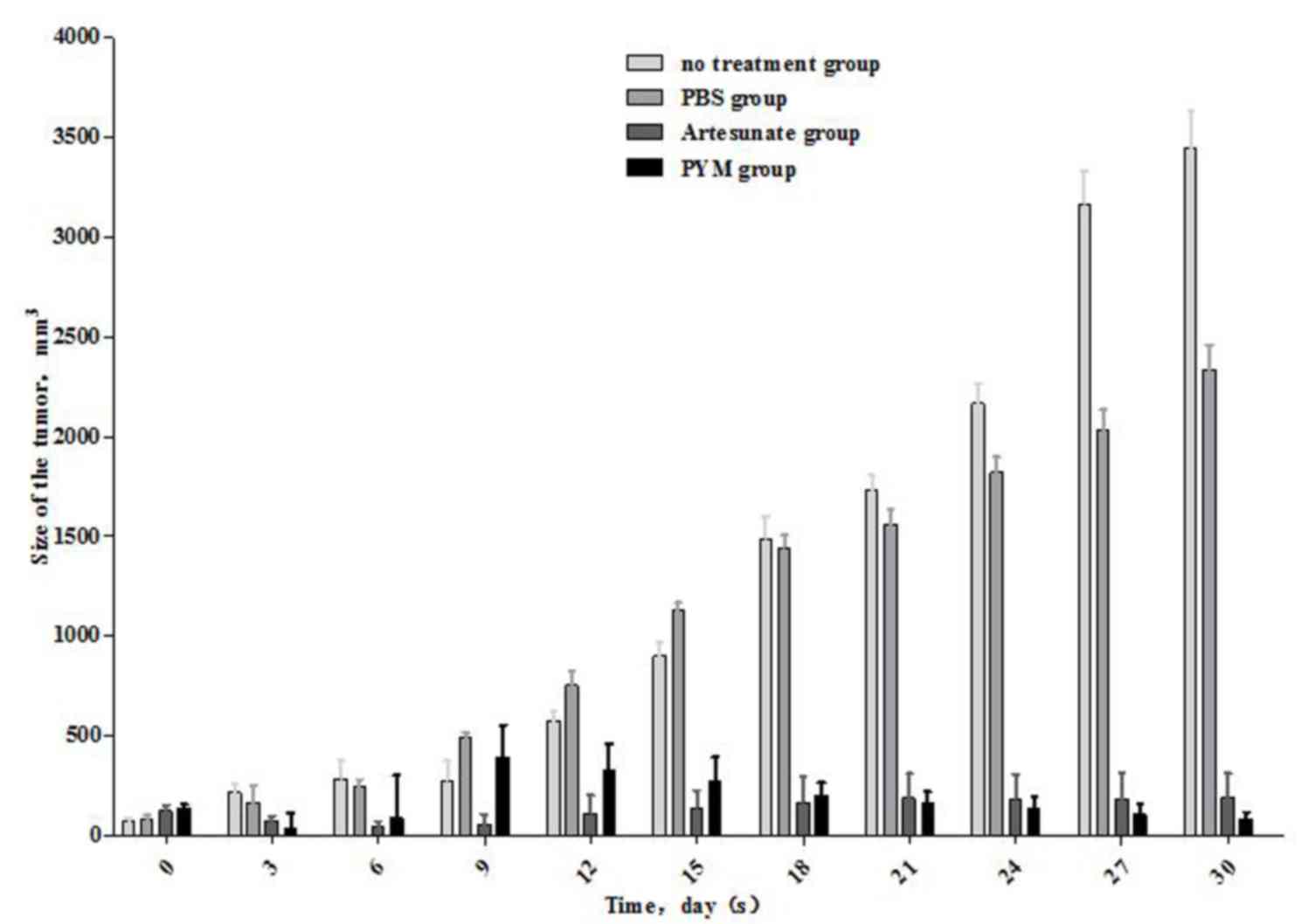
Effect of artesunate on the growth of
Kasabach-Merritt tumors in nude mice
The outcome of artesunate expression on tumor growth
in mice was examined by establishing a subcutaneous K-MS
transplantation model. The tumor size was measured every three days
(Table I and Fig. 4). The results demonstrated that the
volume of the tumor in the artesunate-treated group had
significantly reduced, and the effect was approximately equal to
that recorded for PYM treatment. Artesunate and PYM can
significantly reduce Kasabach-Merritt tumor growth compared with
the no treatment and PBS groups (P<0.05). There were no
statistically significant differences in tumor size between the
artesunate and PYM groups, which was the same between the no
treatment and PBS groups (P>0.05).
 | Table I.The volume of tumor in every group at
various times (mm3; n=5, mean ± standard error of the
mean). |
Table I.
The volume of tumor in every group at
various times (mm3; n=5, mean ± standard error of the
mean).
| Time | No treatment
group | PBS group | PYM group | Artesunate
group |
|---|
| 0 day |
68.9±19.5 |
80.0±21.2 |
132.6±23.7 |
122.4±15.2 |
| 3 days |
215.3±145.4 |
161.9±90.4 |
32.6±79.9 |
71.4±13.2 |
| 6 days |
284.9±190.1 |
241.5±138.4 |
87.1±213.4 |
44.4±12.7 |
| 9 days |
274.3±199.5 |
492.3±225.1 |
391.6±161.2 |
57.4±25.1 |
| 12 days |
575.8±418.1 |
751.8±370.9 |
323.7±135.4 |
111.1±45.3 |
| 15 days |
900.3±637.5 |
1,129.4±319.4 |
273.9±119.9 |
139.8±43.0 |
| 18 days |
1,489.7±1,018.6 |
1,442.2±604.2 |
202.2±61.6 |
167.3±62.9 |
| 21 days |
1,728.7±975.7 |
1,559.1±718.2 |
161.4±58.8 |
186.8±60.9 |
| 24 days |
2,165.8±1,041.8 |
1,819.0±789.3 |
136.3±58.9 |
183.4±61.6 |
| 27 days |
3,163.8±1,688.2 |
2,033.3±1,021.8 |
105.6±50.9 |
184.1±64.3 |
| 30 days |
3,445.7±1,889.8 |
2,337.4±1,282.7 |
84.1±33.3 |
188.6±61.5 |
Discussion
K-MS is a life-threatening complication of
hemangioma; its most common histological appearance is as
Kaposiform hemangioendothelioma (46% cases), followed by plexiform
vascular (31%) and capillary tumors (23%) (27). The primary clinical manifestations are
a rapid increase in tumor size, progressive decrease in platelet
count, microvascular anemia, blood coagulation dysfunction and
serious internal bleeding (28). EOMA
cells were originally derived from 129/J mice spontaneous
hemangioendothelioma (29). In
vitro, EOMA cells exhibit the typical characteristics of the
endothelial cells; these can grow in the matrix with tubular
structure rearrangement and their biological actions are similar to
those of microvascular endothelial cells (30). Studies have identified that EOMA cells
administered to 129/J mice subcutaneously or nude mice can form
histological and hematological characteristics similar to
hemangioma with a K-MS animal model (24,25). EOMA
cells have been demonstrated to be useful in constructing K-MS
models with high rates of quick forming tumors; therefore, EOMA
cells are widely used for the study of angiogenesis inhibitors
(31).
A number of positive and negative regulators control
the angiogenic process, particularly the VEGF family and
angiopoietins (32). VEGF-A is one of
the most potent angiogenic factors, which induces migration and
proliferation in endothelial cells, correlates with vascular
density and influences prognosis (33). VEGFR-1 and VEGFR-2 are VEGFA receptors
that mediate numerous biological activities, including:
Proliferation, migration, tyrosine phosphorylation of downstream
targets, Ca2+ mobilization, prostacyclin production,
extracellular signal regulated kinase activation, nitric oxide
production and phosphatidylinositol-3-kinase/protein kinase B
activity (34). Hypoxia can induce
the expression of HIF-1 and HIF-2, which may then upregulate the
transcription factors for VEGF (35).
Thus, VEGF secretes under hypoxic conditions and binds to its
receptors, which are located on the surface of vascular endothelial
cells (36). Hypoxic conditions can
upregulate the expression of VEGF, while tissues can increase their
oxygenation in order to induce blood vessel growth (37). By contrast, normoxia downregulates the
expression of VEGF and can degenerate newly formed blood vessels
(38). The vasculature exactly
satisfies the metabolic requirements of the tissue through these
opposing processes (39). Though
hemodynamic forces, such as shear stress, are fundamental for
remodeling, tissue hypoxia appears to serve an essential role in
the coordinated expression of all angiogenic factors. Once
oxygenation has reached normal levels, they are rapidly
downregulated and HIF levels decrease (40).
Various treatment modalities are available for K-MS,
the commonly used drugs including triamcinolone acetate and PYM
(41,42). A disadvantage is that the long-term
adverse effects are problematic to assess and the mechanism of
action is also unclear. Another study has identified that rapamycin
acts by inhibiting the mTOR pathway, thus suppressing HIF-1α and
VEGF and subsequently inhibiting vascular endothelial cell
proliferation (43). A study also
identified that propranolol decreases HIF-1α, reduces downstream
VEGF, VEGFR-1 and VEGFR-2 and the expression of monocyte
chemoattractant protein-1, thus inhibiting angiomatous
proliferation; however, there were no significant differences in
the expression of matrix metalloproteinases (44).
Artesunate, a compound isolated from a traditional
Chinese medicine plant, is currently being evaluated for anti-tumor
activity (45,46). Chinese medicine workers first
identified artemisinin in the early 1970s as an effective
antimalarial ingredient, extracted from sweet wormwood
(Artemisia annua) (47). The
history of artemisinin drugs used in the clinical treatment of
malaria is decades old, thus it has become a well-established
clinical drug and no severe systemic or local adverse drug
reactions have been identified to date (48). With the development of scientific
technology, the understanding of pharmaceutical ingredients present
in traditional Chinese medicine has increased, and the
pharmacological effects of the effective components are under
further study (49).
Targeting angiogenesis is one of the treatment
modalities in cancer management (50–52).
Dihydro-artemisinin can decrease the expression of VEGF, inhibit
the formation of new blood vessels and promote the apoptosis of
tumor cells in leukemia cell line K562 animal models (53). Studies have identified that
artemisinin has inhibitory effect on A549 human lung adenocarcinoma
cells, and that its mechanism of action may be to inhibit cell
proliferation, induce apoptosis and cell cycle arrest (54,55). Thus,
the co-administration of artemisinin derivatives with other
anti-cancer agents may increase the concentration of those
anti-cancer drugs in the cell (56).
In the present study, it was identified that artesunate can inhibit
the proliferation, apoptosis and invasion of EOMA cells in
vitro by reducing the expression of HIF-1α, VEGF-A and VEGFR-2.
It was also determined that artesunate can inhibit the growth of a
vascular tumor in vivo, with a curative effect similar to
that of PYM.
In conclusion, the traditionally used anti-malarial
drug artesunate has a significant inhibitory effect on hemangioma
growth. This property should be evaluated further and may become a
potential treatment option for hemangioma, with the advantages of
fewer toxic side effects and higher cost-efficiency.
Acknowledgements
This project was supported by the National Natural
Science Foundation of China (grant no. 30973136) and the Project of
Children's Hospital of Chongqing Medical University (grant no.
7000003).
References
|
1
|
Bruckner AL and VFrieden IJ: Hemangiomas
of infancy. J Am Acad Dennatol. 48:477–496. 2003. View Article : Google Scholar
|
|
2
|
Xu D, O TM, Shartava A, Fowles TC, Yang J,
Fink LM, Ward DC, Mihm MC, Waner M and Ma Y: Isolation,
characterization, and in vitro propagation of infantile hemangioma
stem cells and an in vivo mouse model. J Hematol Oncol. 4:542011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yasui N, Koh K, Kato M, Park MJ, Tomizawa
D, Oshima K, Uchisaka N, Gocho Y, Arakawa A, Seki M, et al:
Kasabach-Merritt phenomenon: A report of 11 cases from a single
institution. J Pediatr Hematol Oncol. 35:554–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernandez-Pineda I, Lopez-Gutierrez JC,
Chocarro G, Bernabeu-Wittel J and Ramirez-Villar GL: Long-term
outcome of vincristine-aspirin-ticlopidine (VAT) therapy for
vascular tumors associated with Kasabach-Merritt phenomenon.
Pediatr Blood Cancer. 60:1478–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Regan GM, Irvine AD, Yao N, O'Marcaigh
A, Sheridan-Pereira M, Phelan E, McDermott MB, Twomey A, Russell J
and Watson R: Mediastinal and neck kaposiform hemangioendothelioma:
Report of three cases. Pediatr Dermatol. 26:331–337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarkar M, Mulliken JB, Kozakewich HP,
Robertson RL and Burrows PE: Thrombocytopenic coagulopathy
(Kasabach-Merritt phenomenon) is associated with kaposiform
hemangioendothelioma and not with common infantile hemangioma.
Plast Reconstr Surg. 100:1377–1386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Li K, Yao W, Dong K, Xiao X and
Zheng S: Steroid-resistant kaposiform hemangioendothelioma: A
retrospective study of 37 patients treated with vincristine and
long-term follow-up. Pediatr Blood Cancer. 62:577–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Enjolras O, Wassef M, Mazoyer E, Frieden
IJ, Rieu PN, Drouet L, Taïeb A, Stalder JF and Escande JP: Infants
with Kasabach-Merritt syndrome do not have ‘true’ hemangiomas. J
Pediatr. 130:631–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper JG, Edwards SL and Holmes JD:
Kaposiform haemangioendothelioma: Case report and review of the
literature. Br J Plast Surg. 55:163–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enjolras O, Mulliken JB, Wassef M, Frieden
IJ, Rieu PN, Burrows PE, Salhi A, Léauté-Labreze C and Kozakewich
HP: Residual lesions after Kasabach-Merritt phenomenon in 41
patients. J Am Acad Dermatol. 42:225–235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hervella M and Iqlesias ME: Vascular
tumors as syndromic indicators. An Sist Sanit Navar. 27(Suppl 1):
S33–S44. 2004.(In Spanish).
|
|
12
|
Haisley-Royster C, Enjolras O, Frieden IJ,
Garzon M, Lee M, Oranje A, de Laat PC, Madern GC, Gonzalez F,
Frangoul H, et al: Kasabach-Merritt phenomenon: A retrospective
study of treatment with vincristine. J Pediatr Hematol/Oncol.
24:459–462. 2002. View Article : Google Scholar
|
|
13
|
Greenberger S and Bisehoff J: Pathogenesis
of infantile haemangioma. Br J Dermatol. 169:12–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Przewratil P, Sitkiewicz A and
Andrzejewska E: Local serum levels of vascular endothelial growth
factor in infantile hemangioma: Intriguing mechanism of endothelial
growth. Cytokine. 49:141–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koh GY: Orchestral actions of
angiopoietin-1 in vascular regeneration. Trends Mol Med. 19:31–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phng LK and Gerhardt H: Angiogenesis: A
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sagara I, Beavogui AH, Zongo I, Soulama I,
Borghini-Fuhrer I, Fofana B, Camara D, Somé AF, Coulibaly AS,
Traore OB, et al: Safety and efficacy of re-treatments with
pyronaridine-artesunate in African patients with malaria: A sub
study of the WANECAM randomised trial. Lancet Infect Dis.
16:189–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang LX, Liu ZN, Ye J, Sha M, Qian H, Bu
XH, Luan ZY, Xu XL, Huang AH, Yuan DL, et al: Artesunate exerts an
anti-immunosuppressive effect on cervical cancer by inhibiting PGE2
production and Foxp3 expression. Cell Biol Int. 38:639–646. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong DE, Song HJ, Lim S, Lee SJ, Lim JE,
Nam DH, Joo KM, Jeong BC, Jeon SS, Choi HY and Lee HW: Repurposing
the anti-malarial drug artesunate as a novel therapeutic agent for
metastatic renal cell carcinoma due to its attenuation of tumor
growth, metastasis, and angiogenesis. Oncotarget. 6:33046–33064.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukanganyama S, Naik YS, Widersten M,
Mannervik B and Hasler JA: Proposed reductive metabolism of
artemisinin by glutathione transferases in vitro. Free Radic Res.
35:427–434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Quevedo ME, Lannutti BJ, Gordon
KB, Guo D, Sun W and Paller AS: In vivo gene therapy with
interleukin-12 inhibits primary vascular tumor growth and induces
apoptosis in a mouse model. J Invest Dermatol. 112:775–781. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sidbury R, Neuschler N, Neuschler E, Sun
P, Wang XQ, Miller R, Tomai M, Puscasiu E, Gugneja S and Paller AS:
Topically applied imiquimod inhibits vascular tumor growth in vivo.
J Invest Dermatol. 121:1205–1209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alvarez-Mendoza A, Lourdes TS,
Ridaura-Sanz C and Ruiz-Maldonado R: Histopathology of vascular
lesions found in Kasahach-Merritt syndrome: Review based on 13
cases. Pediatr Dev Pathol. 3:556–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammill AM, Wentzel M, Gupta A, Nelson S,
Lucky A, Elluru R, Dasgupta R, Azizkhan RG and Adams DM: Sirolimus
for the treatment of complicated vascular anomalies in children.
Pediatr Blood Cancer. 57:1018–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gordillo GM, Atalay M, Roy S and Sen CK:
Hemangioma model for in vivo angiogenesis: Inducible oxidative
stress and MCP-1 expression in EOMA cells. Methods Enzymol.
352:422–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lannutti BJ, Gately ST, Quevedo ME, Soff
GA and Paller AS: Human angiostatin inhibits murine
hemangioendothelioma tumor growth in vivo. Cancer Res.
57:5277–5280. 1997.PubMed/NCBI
|
|
31
|
O'Reilly MS, Brem H and Folkman J:
Treatment of murine hemangioendotheliomas with the angiogenesis
inhibitor AGM-1470. J Pediatr Surg. 30:325–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Breier G, Albrecht U, Sterrer S and Risau
W: Expression of vascular endothelial growth factor during
embryonic angiogenesis and endothelial cell differentiation.
Development. 114:521–532. 1992.PubMed/NCBI
|
|
33
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
36
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L,
Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional
regulation of vascular endothelial cell responses to hypoxia by
HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iruela-Arispe ML and Dvorak HF:
Angiogenesis: A dynamic balance of stimulators and inhibitors.
Thromb Haemost. 78:672–677. 1997.PubMed/NCBI
|
|
41
|
Chowdri NA, Darzi MA, Fazili Z and Iqbal
S: Intralesional corticosteroid therapy for childhood cutaneous
hemangiomas. Ann Plast Surg. 33:46–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao X, He N, Sun J, Wang S, Ji X, Wang J,
Zhang C, Yang J, Lu T, Li J and Zhang G: Interventional treatment
of huge hepatic cavernous hemangioma. Chin Med J (Engl).
113:927–929. 2000.PubMed/NCBI
|
|
43
|
Medici D and Olsen BR: Rapamycin inhibits
proliferation of hemangioma endothelial cells by reducing
HIF-1-dependent expression of VEGF. PLoS One. 7:e429132012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chim H, Armijo BS, Miller E, Gliniak C,
Serret MA and Gosain AK: Propranolol induces regression of
hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann
Surg. 256:146–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang ND, Tan SH, Ng S, Shi Y, Zhou J, Tan
KS, Wong WS and Shen HM: Artesunate induces cell death in human
cancer cells via enhancing lysosomal function and lysosomal
degradation of ferritin. J Biol Chem. 289:33425–33441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
47
|
Chemical studies on qinghaosu
(artemisinine), . China cooperative research group on qinghaosu and
its derivatives as antimalarials. J Tradit Chin Med. 2:3–8.
1982.PubMed/NCBI
|
|
48
|
Douglas NM, Anstey NM, Angus BJ, Nosten F
and Price RN: Artemisinin combination therapy for vivax malaria.
Lancet Infect Dis. 10:405–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chan K: Chinese medicinal materials and
their interface with Western medical concepts. J Ethnopharmacol.
96:1–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aggarwal C, Somaiah N and Simon G:
Antiangiogenic agents in the management of non-small cell lung
cancer: Where do we stand now and where are we headed? Cancer Biol
Ther. 13:247–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Imai K and Takaoka A: Comparing antibody
and small-molecule therapies for cancer. Nat Rev Cancer. 6:714–727.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhou HJ, Wang WQ, Wu GD, Lee J and Li A:
Artesunate inhibits angiogenesis and downregulates vascular
endothelial growth factor expression in chronic myeloid leukemia
K562 cells. Vascul Pharmacol. 47:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liao K, Li J and Wang Z:
Dihydroartemisinin inhibits cell proliferation via
AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung
cancer cells. Int J Clin Exp Pathol. 7:8684–8691. 2014.PubMed/NCBI
|
|
55
|
Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu
J, Ou R, Zhang G, Li F, Hu M, et al: Artemisinin and its
derivatives can significantly inhibit lung tumorigenesis and tumor
metastasis through Wnt/β-catenin signaling. Oncotarget.
7:31413–31428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma H, Yao Q, Zhang AM, Lin S, Wang XX, Wu
L, Sun JG and Chen ZT: The effects of artesunate on the expression
of EGFR and ABCG2 in A549 human lung cancer cells and a xenograft
model. Molecules. 16:10556–10569. 2011. View Article : Google Scholar : PubMed/NCBI
|