Introduction
As one of the basic techniques utilized to study
tumor cell biology, the continual development of tumor cell culture
techniques is vital. Traditional cell culture methods use a
two-dimensional (2D) monolayer. With continuous improvements being
made, this method has become a standard technology in life sciences
at present. However, due to the inherent flaws of traditional 2D
culture, it fails to correctly imitate the architecture and
microenvironments of in vivo, which makes 2D-cultured cells
different from cells growing in vivo in terms of morphology,
proliferation, cell-cell and cell-matrix inter-connections, signal
transduction, differentiation and other aspects (1,2). In order
to improve these simulations of cell microenvironments in
vivo, 3D culture has become the next frontier of cell biology
research.
As the intersection between tumor cell biology and
tissue engineering, 3D in vitro tumor models simulate the
in vivo physiological microenvironment, and may be useful at
the pre-clinical development stage to identify potentially
successful prototypes and eliminate failures at an early stage.
This means that it has potential to bridge the gap between
traditional monolayer cell culture and tumor cytology experiments
in vivo. Therefore, an increasing number of tumor biologists
have begun to emphasize the importance of 3D tumor cell culture
(3). Subsequently, the advent of 3D
culture has seen rapid advancement in the past few decades, as
evidenced by the increasing number of studies in this area
(4,5),
including preclinical drug screening, cancer stem cell maintenance
and differentiation, signal abnormal transduction and other aspects
(6–8).
For example, compared with 2D monolayer cultures, cells in 3D
culture generally exhibit a reduced sensitivity to certain
chemotherapeutic agents (9). These
results and the exponentially increasing number of studies
surrounding this topic convey the importance of 3D tumor cell
culture, one of the primary reasons for the present review.
Thus, in the present review, the current preclinical
models in cancer research are briefly described in order to promote
understanding of the necessity of novel cell culture system. In
addition to this, the advantages and challenges of 3D in
vitro tumor models are discussed in terms of a mechanistic
understanding of tumor cell physiology and in therapeutic
evaluations of anti-tumor drug discovery.
Current preclinical tumor models
At present, in vitro anti-tumor drugs tests
are mainly performed by detecting the reaction of tumor cells in a
2D monolayer culture dish. It is estimated that >80% of cancer
biologists still rely on 2D culture techniques to obtain results
prior to in vivo testing because of its convenience
(10). However, in clinical trials,
it has become apparent that effective drugs in in vitro
experiments have no or weak efficacy in real patients with tumors
(11). This phenomenon, at least
partially, has been attributed to the fact that cells grown in 2D
culture lack the complex 3D tissue architecture and cell-cell or
cell-extracellular matrix (ECM) interactions which exist in the
body (12). These models therefore
fail to fully reflect the pathophysiology of tumor cells, and the
real level of resistance to radiation or drugs in the in
vivo niche (6,13). For example, hepatic cancer cells in 3D
culture may tolerate drug treatment, similar to the resistance
characteristics of solid tumors in vivo (14). Breast cancer MCF-7 cells in 3D
scaffolds demonstrated a stronger resistance to tamoxifen in
endocrine therapy than those in monolayer culture (7). Additionally, drug resistance induced by
cancer stem cells (CSCs) has received substantial attention in
previous decades. However, traditional 2D culture fails to provide
an adequate CSC enrichment culture (6). These studies suggest that the
microenvironment of tumor cells may notably alter the
susceptibility of cancer cell drugs. Traditional 2D culture should,
therefore, be subject to further development.
Animal models are an important tool for tumor
research. Animal model testing is primarily conducted to monitor
drug bioavailability, therapeutic efficacy and dose-limiting
toxicity (15). Any novel drugs must
undergo preclinical testing in animal models prior to human
clinical trials. However there remain a number of issues with these
models, including higher costs, species differences, limited
availability and feasibility (16).
Furthermore, ethical issues in relation to the use of animals in
tumor research are highly controversial. The first guideline of the
animal model is that animals should be replaced with other methods
wherever possible (17). Therefore,
novel in vitro cell culture models are encouraged by the
majority of funding agencies (4), in
order to reduce the number of animals used in tumor research and
drug evaluation.
To resolve these issues, 3D tumor cell culture
methods have been developed where the culture environment takes
into account the spatial organization and ECM of the cell. The
common goal for these methods is to restructure a biomimetic 3D
multicellular tumor model, which may bridge the gap between the
conventional 2D in vitro and the animal testing models.
Tumor cells in 3D models have physiological properties similar to
those in vivo (5). Thus, 3D
culture may be a powerful tool in tumor and relevant drug research.
Marked advances have been made in the basic development of 3D tumor
models so far, and prominent studies using 3D tumor cell models to
simulate the tumor microenvironment or assess drug delivery in the
past 5 years are summarized in Table
I (6,8,18–35). Considering the advantages of 3D tumor
models, the present review provides an overview of the methods and
techniques successfully devised for practicing 3D tumor cell
culture.
 | Table I.Previous 3D cell culture models to
simulate the tumor microenvironment and assess drug. Arranged
according to tumor type. |
Table I.
Previous 3D cell culture models to
simulate the tumor microenvironment and assess drug. Arranged
according to tumor type.
| Author, year | Model | Cells | 3D model | Results | (Refs.) |
|---|
| Talukdar and Kundu,
2012 | Breast cancer | MDA-MB-231 | Non-mulberry silk
fibroin protein scaffolds | Notably higher drug
concentrations are required to achieve a comparable reduction in
cell viability and invasive potential in 3D cultures than 2D
cultures | (18) |
| Chen et al,
2012 |
| MCF-7 | Collagen
scaffolds | High-level
expression of CSC-associated properties of MCF-7 cells cultured in
3D were confirmed | (6) |
| Dunne et al,
2014 |
| MCF-7, BT474,
SKBR3 | Scaffolds | Breast cancer cells
grown in 3D on the decellularized scaffolds demonstrated reduced
sensitivity to doxorubicin relative to 2D cell culture | (19) |
| Maguire et
al, 2016 |
| MCF-10 | Multicellular tumor
spheroids | Genetic
dependencies can be uncovered when cells are grown in 3D conditions
similar to in vivo | (20) |
| Sha et al,
2015 | Gastric cancer | BGC-823 | Multicellular tumor
spheroids | Anti-EGFR-iRGD may
improve anticancer drugs, such as doxorubicin, bevacizumab,
nanoparticle permeability and efficacy throughout the tumor
mass | (21) |
| Kundu et al,
2013 |
Hepatocarcinoma | HepG2 | Tasar silk fibroin
scaffolds | 3D multicellular
model demonstrates insight into hepatocarcinoma progression and
offers a prediction of cellular response to transfection efficacy,
drug treatment and therapeutic intervention | (22) |
| Xu et al,
2013 | Lung cancer | Primary lung cancer
cells, SPCA-1 | Spheroids in
device-assisted culture | Developed a
high-throughput model for assessing drug sensitivities in
vitro. There was a large discrepancy between drug sensitivity
levels in 2D versus 3D. | (23) |
| Simon et al,
2016 |
| A549 | Hydrogels | 3D model can help
regulate the exposure of oxygen to subpopulations of cells in a
tissue-like construct either prior to or following therapy | (24) |
| Stratmann et
al, 2014 |
| HCC827, A549 | Decellularized
scaffolds | Quantitative
read-outs for proliferation, apoptosis and invasion were
established in the complex 3D tumor model | (25) |
| Lee et al,
2013 | Ovarian cancer | 1847, A2780, CaOV3,
COV644, EFO27, ES-2, FUOV1, HEY, IGROV1 | Multicellular tumor
spheroids | 3D cell culture is
an improved reflection of the histological, biological and
molecular features of tumors compared with primary cultures in 2D.
In terms of chemosensitivity, 7 out of 11 cell lines were more
resistant in 3D models. 7 out of 11 cell lines also demonstrated
increased resistance to paclitaxel | (26) |
| Shin et al,
2013 |
| HEY | Multicellular tumor
spheroids | Taxol-induced
apoptosis was detected in monolayer conditions but not in spheroid
cultures. | (27) |
| Loessner et
al, 2013 |
| OV-MZ-6 | Multicellular tumor
spheroids | Combinatorial
approaches of paclitaxel and KLK/MAPK inhibition may be more
efficient than chemotherapeutics alone | (28) |
| Yang and Zhao,
2011 |
| A2780, A2780/DDP,
SK-OV-3 | Nanofiber
scaffolds | Ovarian cancer
cells demonstrated between two and five-fold higher drug resistance
(fluorouracil, paclitaxel and curcumin) relative to 2D culture | (29) |
| Fitzgerald et
al, 2015 | Prostate
cancer | PC3, LNCaP | Collagen-based
scaffold | The two cell lines
in 3D demonstrated higher resistance to docetaxel. Non-viral
delivery vectors, lipofectamine and a modified cyclodextrin,
mediated gene knockdown in this 3D model | (30) |
| Xu et al,
2014 |
| LNCaP | HA-based gels | The hydrogel serves
as a diffusion barrier for nanoparticles. Cells cultured in 3D were
more resistant to docetaxel treatment relative to 2D | (31) |
| Lv et al,
2016 | Glioma | Primary cells,
U87 | Collagen
scaffolds | 3D-cultured cells
also demonstrated enhanced resistance to chemotherapeutic
alkylating agents, with a much higher proportion of glioma stem
cells and upregulation of MGMT | (32) |
| Ma et al,
2016 |
| U251 | Polystyrene
scaffolds coated with laminin | The results
indicate the influence of 3D context on integrin expression,
specifically, the upregulation of the laminin-binding integrin's
alpha 6 and beta 4 | (33) |
| Pedron et
al, 2013 |
| U87 | HA gels | Clustering of
glioma cells was observed exclusively in HA gels and expression of
malignant genes was revealed to vary bi-phasically with
incorporated HA content. | (34) |
| Munson et
al, 2013 |
| RT2, U87, C6,
9L | HA-collagen
gels | Interstitial flow
in this 3D model increases glioma invasion by a CXCR4-dependent
mechanism | (35) |
| Fong et al,
2013 | Ewing sarcoma | TC-71 | Electrospun
poly(e-caprolactone) scaffolds | Ewing sarcoma cells
cultured in 3D were more resistant to traditional cytotoxic drugs,
and exhibited remarkable differences in the expression pattern of
the IGF-1R/mammalian target of rapamycin pathway | (8) |
The methods of 3D cell culture
Multicellular tumor spheroids
(MCTS)
MCTS are aggregates of cancer cells grown in
suspension or embedded in gels using 3D culture methods. This model
partly recapitulates in vivo tumor microenvironments
(36). For example, larger MCTS
(critical size, 400 µm) sustain oxygen and nutrient gradients that
often result in the formation of a necrotic core similar to those
in poorly vascularized tumors (37).
There are several different methods used to create MCTS models
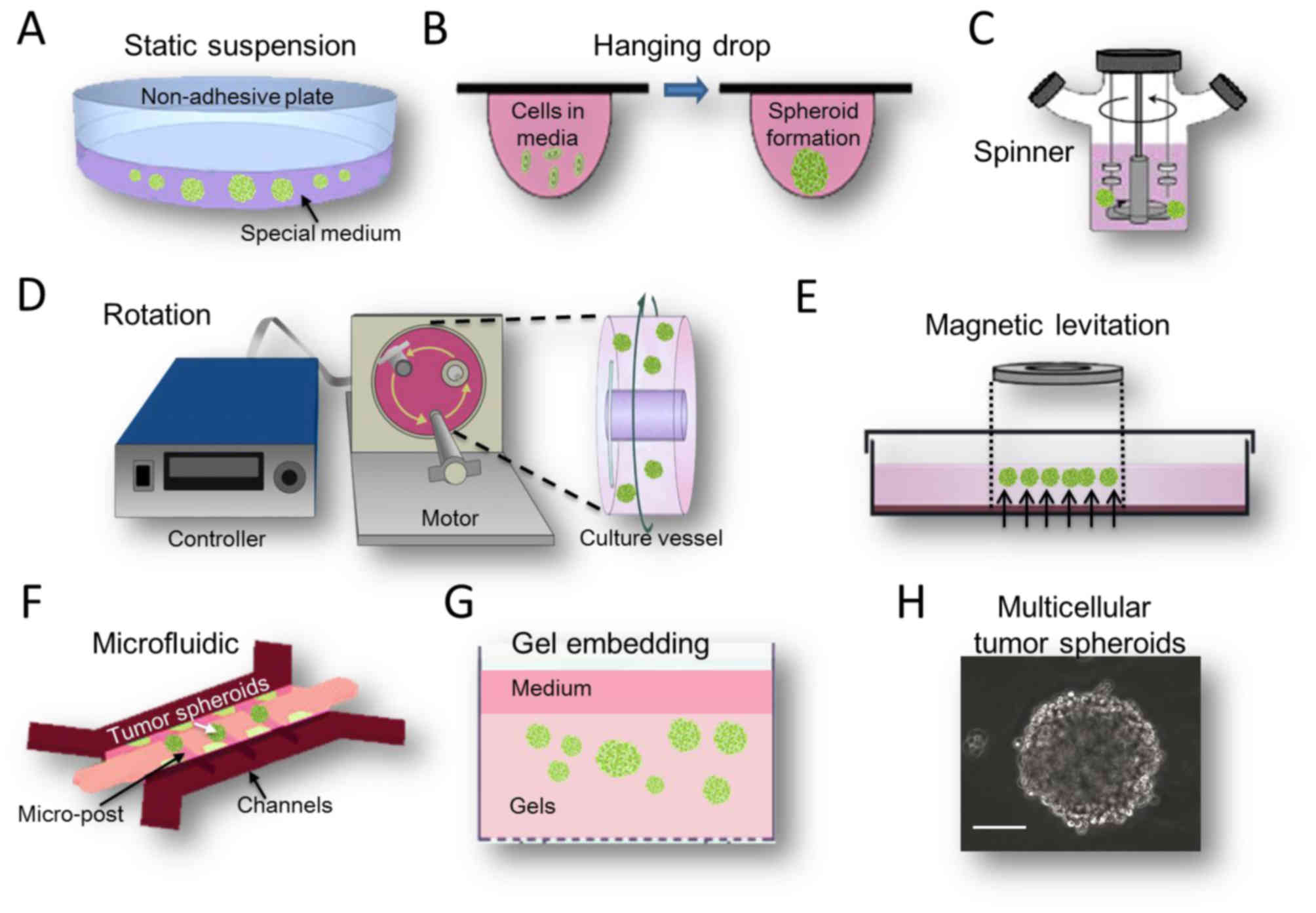
(Fig. 1), each with their distinct
advantages and disadvantages.
Suspension culture
The suspension culture method was invented to
isolate and culture neural stem cells from rat striatal cells in
1970s (38). Subsequently it became a
widely used 3D cell culture method. The main features of this
method are a serum-free and artificially low adhesion cell growth
microenvironment (Fig. 1A).
Specifically, the culture medium has no serum but contains a high
concentration of growth factors, which are differ slightly between
cell lines. For example, glioma suspension MCTS medium contains
Dulbecco's modified Eagle's medium, Nutrient Mixture F12, B27,
recombinant human epidermal growth factor and basic fibroblast
growth factor (39). Modifying cell
culture surfaces by using 1.5% agar-coated plates (40) promotes spheroid culture formation by
preventing cells attaching to their surface. According to the above
conditions, the majority of tumor cells may grow and aggregate into
a sphere with a diameter ranging from 20 µm-1 mm (Fig. 1H). These suspension MCTS demonstrate
not only numerous characteristics of solid tumor cells, but also
tend to exhibit more drug-resistance to either conventional
chemotherapy or monoclonal antibody drugs (41,42). In
addition, the suspension MCTS culture method is widely used for
enriching CSC subpopulations. Under a serum-free environment,
highly differentiated cells gradually die and cells with stemness
potential proliferate and survive. Following several passages, CSC
subpopulations may be enriched and purified (43). The enrichment of CSCs leads to
increased drug resistance, accounting for the traditional view that
drug penetration into the tumor spheroids may be poor. As the
suspension culture model is simple and easy, it may be generated
from a wide range of tumor cell types and is easily accessible for
relevant experimentation, making this method compatible with
high-throughput drug screening (44).
However, the cells in suspension culture have no migratory
movement. Furthermore, the success rate of long-term passages is
low (45) and real in vivo
tumor cells are not inaccessible with serum. Another important
problem with drug testing is lack of defined endpoints and an
accurate means for testing cell viability in 3D culture. There are
no accurate cell viability assays to assess drug response
throughout MCTS so far (46).
The hanging drop method is a special type of
suspension culture (47). It uses a
small quantity of cell suspension dropped onto a culture plate, and
then the plate is inverted to create droplets, as the micro-liquid
adhesion with the substrate surface is greater than its own weight.
Cells will aggregate, proliferate and grow into a spheroid at the
liquid-air interface of the medium drop tip (Fig. 1B). This method is relatively simple,
and it has a ~90% reproducibility rate for producing 1 MCTS per
droplet for numerous tumor cell lines (45). At present, dedicated commercial
384-well droplet suspension plates have emerged (48). But, similar to the suspension culture,
its limitations are apparent. For example, to prevent the droplet
falling, the recommended drop volume is only 10–20 µl. In this
case, the general number of cells in MCTS is not >500. In
addition, once the cell culture is initiated, the medium cannot be
replaced and it is difficult to add the drug in the middle of
culture. These inherent limitations confine the widespread use of
this technique in drug discovery.
Device-assisted culture
Device-assisted suspension culture is an improvement
of the static suspension culture, depending on several biological
devices, including magnetic levitation, spinner or rotational
bioreactors and microfluidic devices. The main feature of these
bioreactors is to prevent tumor cell adherence or to suspend
movement, so that they may grow into MCTS. Additionally,
microcarriers or microcapsules are often used in combination to
increase the efficiency of cell growth and enhance the protection
of the moving cells (49).
The magnetic levitation method is a novel suspension
culture technology invented by n3D Bioscience (Fig. 1E). In this method are magnetized with
NanoShuttle-PL during an overnight incubation and cultured on
magnetic nanoparticles in dedicated plates, where they are
levitated off the bottom by a magnet placed above the plate. This
method does not require any artificial substrate or specialized
media or equipment. Additionally, 3D cultured cells may also be
picked up and transferred using magnetic tools, including the
MagPen (50). Using this approach, Su
et al (51) studied the niches
and conducted a pharmaceutical synergism analysis of myeloma stem
cells.
The spinner bioreactor culture method was derived
from the study of tumor cells in terms of radiotherapy resistance
by Durand and Sutherland (52) in
1970s. This system includes a container to hold the cells culture
and an impeller or paddle stirring continuously to ensure the cells
suspended and medium mixed (Fig. 1C).
The liquid flow not only prevents cell adhesion, but also ensures
the uniform distribution of various nutrients and oxygen. It is
conducive to cellular sphere formation and metabolism. A
disadvantage of this method is that the foam and fluid shear force
generated by the agitation process may cause cell damage, but if
the stirring speed is too low, the cells would drop and adhere
easily. The rotational bioreactor culture method was adapted from
the former method by the National Aeronautics and Space
Administration in 1992. It is designed to use the culture
container's self-rotating ability to generate microgravity
(Fig. 1D), and to be used for the
purpose of researching the biological characteristics of the cells
in this case (53). The system is now
widely used in the 3D culture of tumor cells. It may reduce damage
more effectively by the fluid shear force than the former device,
but it still fails to avoid mechanical damage by collision between
the cells and the bioreactor wall. These bioreactor systems may
produce a large number of uniform cellular spheres. Certain
researchers have reported that using these two types of bioreactors
may improve amplification efficiency by 8–30× greater than that of
the 2D method on the culture of stem cells (54,55).
Various tumor models, including hepatocellular carcinoma (56), neuroblastoma (57), breast adenocarcinoma (58) and melanoma (59) have been successfully engineered using
spinner/rotational bioreactors.
Microfluidic devices, which allow spatial control
over fluids in micrometer-sized channels, have become a valuable
tool to further increase the physiological relevance of 3D tumor
models (Fig. 1F). These devices
process or manipulate micro-liquid, using microchannels with
dimensions of 1–1,000 µm (60). MCTS
are generated within microfluidic channels. Spheroid formation may
be controlled precisely in the microfluidic device. Continuous
perfusion under physiological conditions during spheroid formation
allows for faster formation and increased uniformly in size
(61). Microfluidic platforms also
allow the formation, maintenance, and testing of spheroids within a
single device.
Gel embedding culture
MCTS in suspension culture lose their complicated
ECM microenvironments. The solid tumor cells in vivo should
not form suspended spheroids, they associate with other cells and
the ECM (62). As the ECM affects
cellular organization and cell function, novel 3D culture methods
that incorporate ECM arguably mimic in vivo situations more
accurately, as they allow for cell-ECM interactions. Gel is used as
a substrate for 3D cell culture. It is a type of highly hydrophilic
polymer with a soft tissue-like stiffness which aims to mimic the
ECM. Tumor cells grown in the gel usually form a spheroid-like
structure spontaneously (Fig. 1G).
This structure contains not only cell-cell adhesions, but also
supports contact between the cells and artificial ECM. When tumor
cells are allowed to grow in gels, there is architectural support
and important signaling motifs to aid in 3D cancer growth, which
often leads to higher resistance to chemotherapy. For example, when
human epithelial ovarian cancer cells were cultured on 3D hydrogel
system as MCTS, a higher survival rate was observed following
exposure to paclitaxel (63). A
number of commonly used gel methods are described below.
Collagen is a widely used material in gel embedding
culture (64). As a key ECM
component, collagen has excellent biocompatibility for the vast
majority of tumor cells. The earliest application of collagen is in
tumor tissue culture. Certain tumor tissues are embedded in
collagen gel for culture following cutting (diameter ~1 mm), and
researchers have demonstrated that the tumor tissue structure and
cell viability are maintained (65).
Subsequently, certain researchers selected collagen for tumor
tissue culture and drug sensitivity testing (66). Tumor cell culture with collagen gel
was originally applied in breast cancer research. It has been
revealed that the breast cancer cells in collagen gel embedding
culture may form tube-like structures, which are similar to typical
breast cancers in vivo (67).
In CSC research, a novel colorectal cancer stem cell-enriched cell
line was established by 3D culture in Collagen I (68). For drug sensitivity testing of
hepatocellular carcinoma lines, cancer cells in collagen gel
presented with increased drug-resistance compared with those in
monolayer culture (69). With the
improvement of gel technologies, complex ECM mimetic materials
consisting of collagen and hyaluronic acid (HA) have been
developed. These are suitable for investigating the behaviors and
drug testing of glioblastoma cells (70).
Alginate is a natural polymer derived from brown
seaweed. It gelates in the presence of calcium ions, and is often
used for the encapsulation of various types of cells. The main
advantage of alginate gel culture is that gelation may be
accomplished at room temperature following addition of the cells to
the polymer. It allows the cells to mix into the gel-liquid
uniformly and grow nondestructively through the process of
gelation. The hepatocytes in alginate gel may grow and maintain the
function of albumin synthesis (71).
Thus, this culture gel substrate may maintain hepatocytes function
well. Breast cancer cells grown in alginate gel 3D culture form
MCTS, which have stronger chemotherapy resistance compared with
breast cancer cells grown in in 2D culture (72). In addition, a previous study
successfully enriched multiple CSCs by culturing them in alginate
gel beads (73), which suggested that
alginate gel is a useful biomaterial for enriched CSCs culture.
Matrigel derives from Engelbreth-Holm-Swarm mouse
tumor cell-derived basement membrane proteins which include
collagen IV, laminin, entactin, perlecan, multiple cytokines and
growth factors (74). It has wider
commercial applications in numerous tumor cellular experiments
including 3D culture and tumor invasion models. 3D cultured breast
cancer cells in Matrigel exhibit a bidirectional cross-modulation
of β1-integrin and epidermal growth factor receptor signaling via
the mitogen-activated protein kinase signaling pathway, but this
reciprocal modulation does not occur in monolayer culture (75). These different signal pathways
included transforming growth factor β family (76) and phosphatase and tensin
homolog/platelet-derived growth factor signaling network (77), which are relevant with chemotherapy
and radiotherapy resistance. CSC research using Matrigel has also
been performed. For example, CD271+ uveal melanoma stem cells may
undergo vasculogenic mimicry in 3D Matrigel culture (78). Another previous study revealed that
nicastrin, a novel typeItransmembrane glycoprotein, is associated
with breast cancer stem cell properties using Matrigel culture
(79). The defects of Matrigel are
that it is expensive, has complex compositions and uncertain
proportions between different batches of ingredients (80).
To date, novel gel techniques emerge constantly.
Other new commercial gel materials include PathClear Grade Basement
Membrane gel (AMS Biotechnology, Ltd., Abingdon, UK), ECM gel
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), ECL Cell
Attachment Matrix (Merck KGaA) and Geltrex (Invitrogen, Thermo
Fisher Scientific, Inc., Waltham, MA, USA). In the future,
corresponding ECM components may be extracted from different
tissues to prepare the gel according to tumor type, in order to
improve mimicry of the tumor microenvironments in vivo. The
potential drawback of the gel embedding culture approach is that
gel lacks the cross-linked network system for the mechanical
support of tumor cell growth, meaning that it is difficult to grow
tumor cells diffusely.
Scaffold culture
3D scaffolding is a product of tissue engineering
developments. It may act as a surrogate for the missing ECM,
representing the available space of tumor tissue, providing the
physical support for cell growth, adhesion and proliferation, and
causing the cells to form an appropriate spatial distribution and
cell-cell or cell-ECM interaction. There are a number of
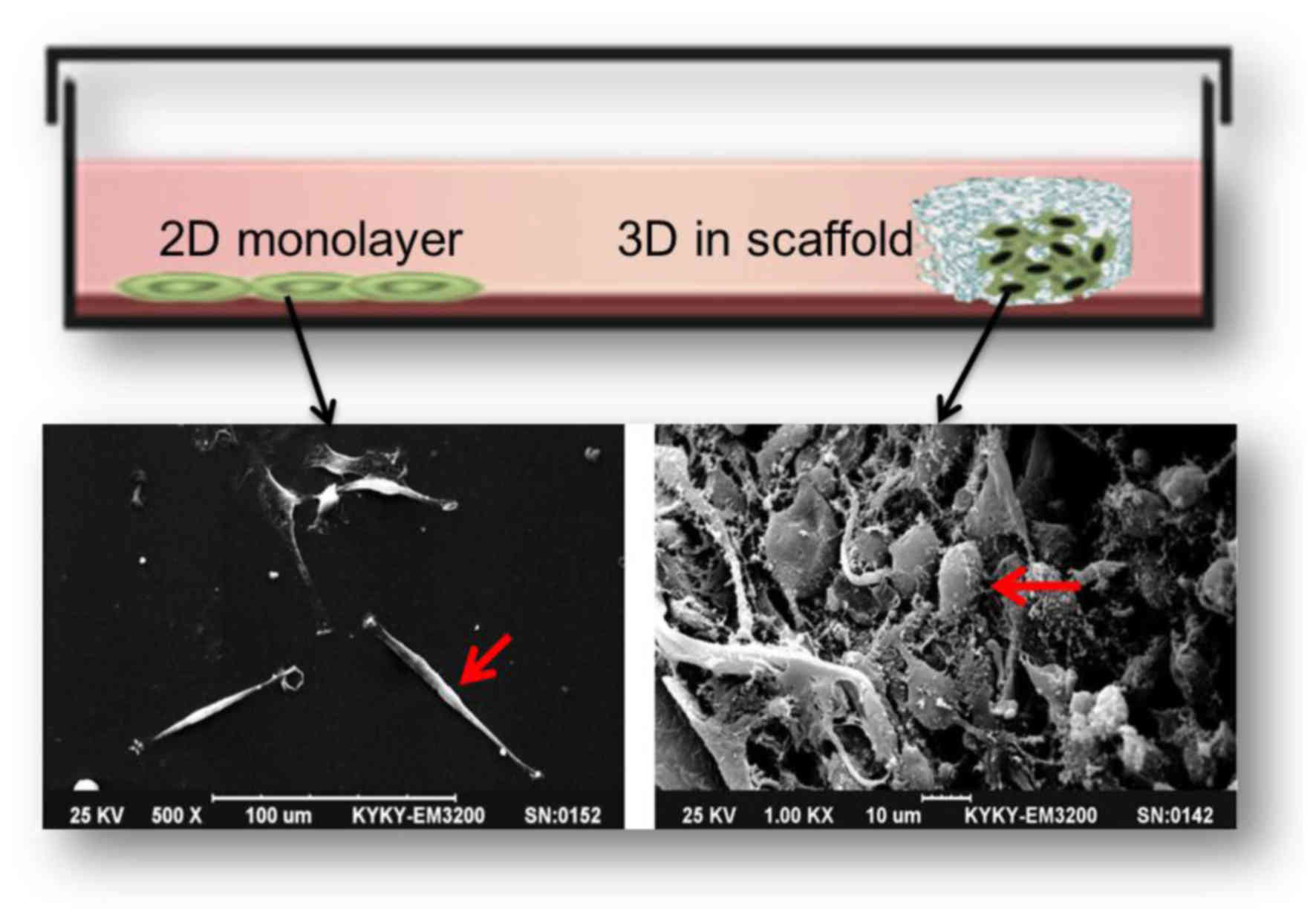
differences between 2D and 3D scaffold culture. A notable
comparison is that tumor cells cultured in 3D scaffolds exhibit
morphological similarities to tumor cells cultured in human tumor
tissues (Fig. 2). For example,
glioblastoma U87 cells are fusiform, flat and epithelioid in 2D
culture, but grew as small, round or ovoid cells and formed complex
structures with cilia or microvilli on their surface in 3D scaffold
culture (32).
In general, the procedures for preparing the
scaffolds fall into one of two major categories: 1, natural
polymers derived from natural polymer materials, including
collagen, chitosan, glycosaminoglycans (mainly hyaluronic acid),
fibroin, agarose, alginate, and starch (mainly used as additives);
and 2, synthetic polymers, containing polyglycolic acid, polylactic
acid, polyorthoester and their copolymers or blends, as well as the
aliphatic polyester polycaprolactone (81). Natural polymers have improved
biocompatibility and lower toxicity, while the artificial synthetic
polymers have higher versatility, reproducibility, enhanced
workability, and in a majority of cases may be processed more
easily than the former, but are not bioactive (82). The processing techniques of
scaffolding preparation are much more complicated than gel
embedding. For providing a more well-defined architecture for tumor
cell growth and improved repeatability, the porosity, mechanical
strength, structural stability and degradation kinetics of the
scaffold needs to be controlled. A wide range of techniques are
used in generating different scaffolds, including solvent
casting/particulate leaching, freeze-drying, phase inversion,
electrospinning, stereolithography, selective laser sintering,
shape deposition manufacturing, 3D printing, robotic microassembly
and fused deposition modeling (81).
Among these techniques, solvent casting, freeze drying, phase
inversion and fiber electrospinning are used a majority of the
time. But the latter three techniques may become the main methods
of preparing precise micro-scaffolds in the future.
A number of highly influential studies regarding 3D
scaffolding culture in drug research and CSCs have been reported.
Dhiman et al (7) studied the
growth of breast cancer MCF-7 cells on porous chitosan scaffolds,
and revealed that the response of cancer cells to tamoxifen was a
10-fold increase in drug resistance compared with those in
monolayer culture. Fong et al (8) established an in vitro 3D Ewing
sarcoma model with porous 3D polycaprolactone (PCL) scaffolds. The
results revealed that the tumor cells on scaffolds were not only
more resistant to traditional cytotoxic drugs but also exhibited
remarkable differences in the expression pattern of the
insulin-like growth factor-1 receptor/mammalian target of rapamycin
pathway. Furthermore, Chen et al (6) studied breast cancer cell growth on a
porous collagen scaffold, and revealed that the porous scaffolds
not only induced the diversification of cell morphologies but also
extended cell proliferation and notably increased the expression of
vascular endothelial growth factor (VEGF) and matrix
metalloproteinases. Additionally, 3D collagen scaffolding
upregulated a subpopulation of breast cancer stem cells, and
xenografts with 3D cells formed larger tumors. These results
indicate that 3D collagen scaffolds may provide a useful platform
for anti-cancer therapeutics and CSC research. Furthermore, breast
cancer stem cell expansion in PCL scaffolds (83), ovarian cancer stem cell behavior and
drug resistance investigated in 3D basement membrane extract
scaffolds (84) and glioma stem cells
proliferating in 3D chitosan-alginate scaffolds (85) were observed in these studies,
sequentially.
Future trends and conclusions
Different approaches offer different advantages and
disadvantages, as summarized in Table
II. Due to the inherent differences in complexity and
functionality, the choice of model is usually dependent on the
application. Additionally, advances in tumor cell biology, tissue
engineering, biomaterials, micro-fabrication and microfluidics have
enabled the rapid development of 3D tumor cell culture. Future
prospects include ensuring that the main features of culture
substrate are controllable and adjustable with more advanced
materials and processing technologies. Depending on the different
tumor types and research purposes, different biomaterials are of
varying suitability for 3D culture in each specific study. For
example, biomedical polymer scaffolds with low degradation rates
should be manufactured for the research of bone cancer metastases.
Additionally, scaffolds with added growth factors including VEGF
may potentially promote the study of vascular mimicry. In addition,
combining with different types of bioreactors, for example gel
embedding or scaffolds used in conjunction with a micro-fluid
bioreactor, may resolve the problem of cell metabolites or drugs
diffusing limitedly. Furthermore, a co-culture system with two or
more types of cells established under the 3D culture platform,
including the co-culture of cancer cells, immune cells and
endothelial cells, may mimic real tumor niches more effectively and
help to clarify the effect of interactions among cancer cells, ECM
and other neighboring cells (86).
Automated quantification of 3D cultures is required in order to
achieve high throughput drug screening programs (87). In addition, CSCs are novel therapeutic
targets (88), and 3D tumor cell
culture ought to be a useful technique in this area due to its
CSC-enriching function. Greater focus on exploring appropriate ECM
components in a 3D model is also critically important for
monitoring tumor cell responses to exogenous cues, including growth
factor activation or chemotherapy (32,33).
 | Table II.Comparison of various methods of 3D
tumor cell culture. |
Table II.
Comparison of various methods of 3D
tumor cell culture.
| Method | Advantages | Disadvantages |
|---|
| Static suspension
culture | Relatively simple,
low cost, spheroid production is easily accessible,
reproducible | Continuous passage
culture difficult, relatively labor intensive, only autocrine
ECM |
| Hanging drop | Control of spheroid
size, low cost if using standard 96-well plate, uniform spheroid
size, homogenous spheroids, suitable for high-throughput
testing | Expensive if using
specialized plates, long-term culture difficult, small culture
volume, medium exchange difficult |
| Magnetic
levitation | Relatively simple
tool, efficient, does not require any specialized media | Colors the 3D
culture brown and limit certain applications, numerous cells also
adhere to the bottom of the plate |
| Spinner/rotational
based approaches | Mass production,
long-time culture, relatively simple | Require specialized
equipment, not possible to control uniformity of spheroid (size,
composition), exposed to high shear force |
| Microfluidic
device | Suitable for
control of spheroid (size, parameters), continuous perfusion for
faster spheroid formation | Difficulty
collecting cells for analysis, require expense and complicated
equipment |
| Gel embedding | Resemblance to the
in vivo conditions, gel provides rich network of ECM
signals | Poor mechanical
properties, certain components of natural gel are variable and
undefined, lacks vasculature |
| Scaffolds | Maximum resemblance
to the in vivo conditions, composition and it is possible to
precisely control ECM presentation, possible to combine with
preferred growth factors | Expensive for
large-scale production, trouble in cells dissociation from
scaffold, complex operation |
In summary, the biological influence of the 3D
microenvironment on tumor cell differentiation, progression,
metastasis and chemotherapy-resistance has gained increased
recognition. 3D culture, which ranges from the simple cell spheroid
model to complex tissue-engineered constructs, serves an
increasingly important function in tumor cell biology research. Gel
embedding and scaffolds have a number of advantages in simulating
the 3D structure of tumor cells in vivo compared with
diverse suspension culture methods, because they are more accurate
at modelling the full range of microenvironmental cues, including
3D cell-cell and cell-ECM interactions. In multiple cancer research
areas, particularly in the direction of drug discovery and CSC
enrichment, 3D culture has unique advantages. An optimized 3D tumor
model for the research of drug discovery in vitro will
require cancer biologists and tissue engineers to increase efforts
in this field.
Acknowledgements
The present study was supported the Key Health
Project of the Nanjing Military Region (grant no. 12MA031).
References
|
1
|
Abbott A: Cell culture: Biology's new
dimension. Nature. 424:870–872. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weigelt B, Ghajar CM and Bissell MJ: The
need for complex 3D culture models to unravel novel pathways and
identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev.
69–70:42–51. 2014. View Article : Google Scholar
|
|
3
|
Jacks T and Weinberg RA: Taking the study
of cancer cell survival to a new dimension. Cell. 111:923–925.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breslin S and O'Driscoll L:
Three-dimensional cell culture: The missing link in drug discovery.
Drug Discov Today. 18:240–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griffith LG and Swartz MA: Capturing
complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol.
7:211–224. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su
G, Chen B and Dai J: The enhancement of cancer stem cell properties
of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and
anti-cancer drugs. Biomaterials. 33:1437–1444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhiman HK, Ray AR and Panda AK:
Three-dimensional chitosan scaffold-based MCF-7 cell culture for
the determination of the cytotoxicity of tamoxifen. Biomaterials.
26:979–986. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fong EL, Lamhamedi-Cherradi SE, Burdett E,
Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra
D, Demicco EG, Menegaz BA, et al: Modeling Ewing sarcoma tumors in
vitro with 3D scaffolds. Proc Natl Acad Sci USA. 110:pp. 6500–6505.
2013, View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wästfelt M, Fadeel B and Henter JI: A
journey of hope: Lessons learned from studies on rare diseases and
orphan drugs. J Intern Med. 260:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hutmacher DW: Biomaterials offer cancer
research the third dimension. Nat Mater. 9:90–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Desrochers TM, Palma E and Kaplan DL:
Tissue-engineered kidney disease models. Adv Drug Deliv Rev.
69–70:67–80. 2014. View Article : Google Scholar
|
|
13
|
Cree IA, Glaysher S and Harvey AL:
Efficacy of anti-cancer agents in cell lines versus human primary
tumour tissue. Curr Opin Pharmacol. 10:375–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uchida Y, Tanaka S, Aihara A, Adikrisna R,
Yoshitake K, Matsumura S, Mitsunori Y, Murakata A, Noguchi N, Irie
T, et al: Analogy between sphere forming ability and stemness of
human hepatoma cells. Oncol Rep. 24:1147–1151. 2010.PubMed/NCBI
|
|
15
|
Stevens JL and Baker TK: The future of
drug safety testing: Expanding the view and narrowing the focus.
Drug Discov Today. 14:162–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huh D, Matthews BD, Mammoto A,
Montoya-Zavala M, Hsin HY and Ingber DE: Reconstituting organ-level
lung functions on a chip. Science. 328:1662–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell WMS and Burch RL: The principles
of humane experimental technique. Methuen; London: 1959
|
|
18
|
Talukdar S and Kundu SC: A non-mulberry
silk fibroin protein based 3d in vitro tumor model for evaluation
of anticancer drug activity. Adv Funct Mat. 22:4778–4788. 2012.
View Article : Google Scholar
|
|
19
|
Dunne LW, Huang Z, Meng W, Fan X, Zhang N,
Zhang Q and An Z: Human decellularized adipose tissue scaffold as a
model for breast cancer cell growth and drug treatments.
Biomaterials. 35:4940–4949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maguire SL, Peck B, Wai PT, Campbell J,
Barker H, Gulati A, Daley F, Vyse S, Huang P, Lord CJ, et al:
Three-dimensional modelling identifies novel genetic dependencies
associated with breast cancer progression in the isogenic MCF10
model. J Pathol. 240:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W,
Chen J, Chen G, Huang L, Blair AM, et al: Tumor-penetrating peptide
fused EGFR single-domain antibody enhances cancer drug penetration
into 3D multicellular spheroids and facilitates effective gastric
cancer therapy. J Control Release. 200:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kundu B, Saha P, Datta K and Kundu SC: A
silk fibroin based hepatocarcinoma model and the assessment of the
drug response in hyaluronan-binding protein 1 overexpressed HepG2
cells. Biomaterials. 34:9462–9474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Z, Gao Y, Hao Y, Li E, Wang Y, Zhang J,
Wang W, Gao Z and Wang Q: Application of a microfluidic chip-based
3D co-culture to test drug sensitivity for individualized treatment
of lung cancer. Biomaterials. 34:4109–4117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon KA, Mosadegh B, Minn KT, Lockett MR,
Mohammady MR, Boucher DM, Hall AB, Hillier SM, Udagawa T, Eustace
BK and Whitesides GM: Metabolic response of lung cancer cells to
radiation in a paper-based 3D cell culture system. Biomaterials.
95:47–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stratmann AT, Fecher D, Wangorsch G,
Göttlich C, Walles T, Walles H, Dandekar T, Dandekar G and Nietzer
SL: Establishment of a human 3D lung cancer model based on a
biological tissue matrix combined with a Boolean in silico model.
Mol Oncol. 8:351–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JM, Mhawech-Fauceglia P, Lee N,
Parsanian LC, Lin YG, Gayther SA and Lawrenson K: A
three-dimensional microenvironment alters protein expression and
chemosensitivity of epithelial ovarian cancer cells in vitro. Lab
Invest. 93:528–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin CS, Kwak B, Han B and Park K:
Development of an in vitro 3D tumor model to study therapeutic
efficiency of an anticancer drug. Mol Pharm. 10:2167–2175. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loessner D, Rizzi SC, Stok KS, Fuehrmann
T, Hollier B, Magdolen V, Hutmacher DW and Clements JA: A
bioengineered 3D ovarian cancer model for the assessment of
peptidase-mediated enhancement of spheroid growth and
intraperitoneal spread. Biomaterials. 34:7389–7400. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z and Zhao X: A 3D model of ovarian
cancer cell lines on peptide nanofiber scaffold to explore the
cell-scaffold interaction and chemotherapeutic resistance of
anticancer drugs. Int J Nanomedicine. 6:303–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fitzgerald KA, Guo J, Tierney EG, Curtin
CM, Malhotra M, Darcy R, O'Brien FJ and O'Driscoll CM: The use of
collagen-based scaffolds to simulate prostate cancer bone
metastases with potential for evaluating delivery of
nanoparticulate gene therapeutics. Biomaterials. 66:53–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Sabanayagam CR, Harrington DA,
Farach-Carson MC and Jia X: A hydrogel-based tumor model for the
evaluation of nanoparticle-based cancer therapeutics. Biomaterials.
35:3319–3330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv D, Yu SC, Ping YF, Wu H, Zhao X, Zhang
H, Cui Y, Chen B, Zhang X, Dai J, et al: A three-dimensional
collagen scaffold cell culture system for screening anti-glioma
therapeutics. Oncotarget. 7:56904–56914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma NK, Lim JK, Leong MF, Sandanaraj E, Ang
BT, Tang C and Wan AC: Collaboration of 3D context and
extracellular matrix in the development of glioma stemness in a 3D
model. Biomaterials. 78:62–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pedron S, Becka E and Harley BA:
Regulation of glioma cell phenotype in 3D matrices by hyaluronic
acid. Biomaterials. 34:7408–7417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Munson JM, Bellamkonda RV and Swartz MA:
Interstitial flow in a 3D microenvironment increases glioma
invasion by a CXCR4-dependent mechanism. Cancer Res. 73:1536–1546.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fennema E, Rivron N, Rouwkema J, van
Blitterswijk C and de Boer J: Spheroid culture as a tool for
creating 3D complex tissues. Trends Biotechnol. 31:108–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid-based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH,
Yao XH, Gao L, Wang JM and Bian XW: Isolation and characterization
of cancer stem cells from a human glioblastoma cell line U87.
Cancer Lett. 265:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuhas JM, Li AP, Martinez AO and Ladman
AJ: A simplified method for production and growth of multicellular
tumor spheroids. Cancer Res. 37:3639–3643. 1977.PubMed/NCBI
|
|
41
|
Lawlor ER, Scheel C, Irving J and Sorensen
PH: Anchorage-independent multi-cellular spheroids as an in vitro
model of growth signaling in Ewing tumors. Oncogene. 21:307–318.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin RZ and Chang HY: Recent advances in
three-dimensional multicellular spheroid culture for biomedical
research. Biotechnol J. 3:1172–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pang R, Law WL, Chu AC, Poon JT, Lam CS,
Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al: A subpopulation of
CD26+ cancer stem cells with metastatic capacity in human
colorectal cancer. Cell Stem Cell. 6:603–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ivascu A and Kubbies M: Rapid generation
of single-tumor spheroids for high-throughput cell function and
toxicity analysis. J Biomol Screen. 11:922–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Louis SA, Rietze RL, Deleyrolle L, Wagey
RE, Thomas TE, Eaves AC and Reynolds BA: Enumeration of neural stem
and progenitor cells in the neural colony-forming cell assay. Stem
Cells. 26:988–996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ng KW, Leong DT and Hutmacher DW: The
challenge to measure cell proliferation in two and three
dimensions. Tissue Eng. 11:182–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tung YC, Hsiao AY, Allen SG, Torisawa YS,
Ho M and Takayama S: High-throughput 3D spheroid culture and drug
testing using a 384 hanging drop array. Analyst. 136:473–478. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zanetta M, Quirici N, Demarosi F, Tanzi
MC, Rimondini L and Farè S: Ability of polyurethane foams to
support cell proliferation and the differentiation of MSCs into
osteoblasts. Acta Biomater. 5:1126–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Souza GR, Molina JR, Raphael RM, Ozawa MG,
Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu MM,
et al: Three-dimensional tissue culture based on magnetic cell
levitation. Nat Nanotechnol. 5:291–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Su J, Zhang L, Zhang W, Choi DS, Wen J,
Jiang B, Chang CC and Zhou X: Targeting the biophysical properties
of the myeloma initiating cell niches: A pharmaceutical synergism
analysis using multi-scale agent-based modeling. PLoS One.
9:e850592014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Durand RE and Sutherland RM: Effects of
intercellular contact on repair of radiation damage. Exp Cell Res.
71:75–80. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goodwin TJ, Prewett TL, Wolf DA and
Spaulding GF: Reduced shear stress: A major component in the
ability of mammalian tissues to form three-dimensional assemblies
in simulated microgravity. J Cell Biochem. 51:301–311. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen X, Xu H, Wan C, McCaigue M and Li G:
Bioreactor expansion of human adult bone marrow-derived mesenchymal
stem cells. Stem Cells. 24:2052–2059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luni C, Feldman HC, Pozzobon M, De Coppi
P, Meinhart CD and Elvassore N: Microliter-bioreactor array with
buoyancy-driven stirring for human hematopoietic stem cell culture.
Biomicrofluidics. 4:pii: 034105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chang TT and Hughes-Fulford M: Monolayer
and spheroid culture of human liver hepatocellular carcinoma cell
line cells demonstrate distinct global gene expression patterns and
functional phenotypes. Tissue Eng Part A. 15:559–567. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Redden RA and Doolin EJ: Microgravity
assay of neuroblastoma: In vitro aggregation kinetics and organoid
morphology correlate with MYCN expression. In Vitro Cell Dev Biol
Anim. 47:312–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kaur P, Ward B, Saha B, Young L, Groshen
S, Techy G, Lu Y, Atkinson R, Taylor CR, Ingram M and Imam SA:
Human breast cancer histoid: An in vitro 3-dimensional co-culture
model that mimics breast cancer tissue. J Histochem Cytochem.
59:1087–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marrero B, Messina JL and Heller R:
Generation of a tumor spheroid in a microgravity environment as a
3D model of melanoma. In Vitro Cell Dev Biol Anim. 45:523–534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Z and Nagrath S: Microfluidics and
cancer: Are we there yet? Biomed Microdevices. 15:595–609. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gill BJ and West JL: Modeling the tumor
extracellular matrix: Tissue engineering tools repurposed towards
new frontiers in cancer biology. J Biomech. 47:1969–1978. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Loessner D, Stok KS, Lutolf MP, Hutmacher
DW, Clements JA and Rizzi SC: Bioengineered 3D platform to explore
cell-ECM interactions and drug resistance of epithelial ovarian
cancer cells. Biomaterials. 31:8494–8506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nirmalanandhan VS, Duren A, Hendricks P,
Vielhauer G and Sittampalam GS: Activity of anticancer agents in a
three-dimensional cell culture model. Assay Drug Dev Technol.
8:581–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Freeman AE and Hoffman RM: In vivo-like
growth of human tumors in vitro. Proc Natl Acad Sci USA. 83:pp.
2694–2698. 1986, View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Takamura Y, Kobayashi H, Taguchi T,
Motomura K, Inaji H and Noguchi S: Prediction of chemotherapeutic
response by collagen gel droplet embedded culture-drug sensitivity
test in human breast cancers. Int J Cancer. 98:450–455. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang J, Richards J, Bowman P, Guzman R,
Enami J, McCormick K, Hamamoto S, Pitelka D and Nandi S: Sustained
growth and three-dimensional organization of primary mammary tumor
epithelial cells embedded in collagen gels. Proc Natl Acad Sci USA.
76:pp. 3401–3405. 1979, View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rowehl RA, Burke S, Bialkowska AB, Pettet
DW III, Rowehl L, Li E, Antoniou E, Zhang Y, Bergamaschi R, Shroyer
KR, et al: Establishment of highly tumorigenic human colorectal
cancer cell line (CR4) with properties of putative cancer stem
cells. PLoS One. 9:e990912014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yip D and Cho CH: A multicellular 3D
heterospheroid model of liver tumor and stromal cells in collagen
gel for anti-cancer drug testing. Biochem Biophys Res Commun.
433:327–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rao SS, Dejesus J, Short AR, Otero JJ,
Sarkar A and Winter JO: Glioblastoma behaviors in three-dimensional
collagen-hyaluronan composite hydrogels. ACS Appl Mater Interfaces.
5:9276–9284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dvir-Ginzberg M, Gamlieli-Bonshtein I,
Agbaria R and Cohen S: Liver tissue engineering within alginate
scaffolds: Effects of cell-seeding density on hepatocyte viability,
morphology, and function. Tissue Eng. 9:757–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang X, Wang W, Yu W, Xie Y, Zhang Y and
Ma X: Development of an in vitro multicellular tumor spheroid model
using microencapsulation and its application in anticancer drug
screening and testing. Biotechnol Prog. 21:1289–1296. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu XX, Liu C, Liu Y, Yang L, Li N, Guo X,
Sun GW and Ma XJ: Enrichment of cancer stem cell-like cells by
culture in alginate gel beads. J Biotechnol. 177:1–12. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kleinman HK and Martin GR: Matrigel:
Basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang F, Weaver VM, Petersen OW, Larabell
CA, Dedhar S, Briand P, Lupu R and Bissell MJ: Reciprocal
interactions between beta1-integrin and epidermal growth factor
receptor in three-dimensional basement membrane breast cultures: A
different perspective in epithelial biology. Proc Natl Acad Sci
USA. 95:pp. 14821–14826. 1998, View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ampuja M, Jokimäki R, Juuti-Uusitalo K,
Rodriguez-Martinez A, Alarmo EL and Kallioniemi A: BMP4 inhibits
the proliferation of breast cancer cells and induces an
MMP-dependent migratory phenotype in MDA-MB-231 cells in 3D
environment. BMC Cancer. 13:4292013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Christensen M, Najy AJ, Snyder M, Movilla
LS and Kim HR: A critical role of the PTEN/PDGF signaling network
for the regulation of radiosensitivity in adenocarcinoma of the
prostate. Int J Radiat Oncol Biol Phys. 88:151–158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Valyi-Nagy K, Kormos B, Ali M, Shukla D
and Valyi-Nagy T: Stem cell marker CD271 is expressed by
vasculogenic mimicry-forming uveal melanoma cells in
three-dimensional cultures. Mol Vis. 18:588–592. 2012.PubMed/NCBI
|
|
79
|
Lombardo Y, Filipović A, Molyneux G,
Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yagüe E, Michel L
and Coombes RC: Nicastrin regulates breast cancer stem cell
properties and tumor growth in vitro and in vivo. Proc Natl Acad
Sci USA. 109:pp. 16558–16563. 2012, View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sodunke TR, Turner KK, Caldwell SA,
McBride KW, Reginato MJ and Noh HM: Micropatterns of Matrigel for
three-dimensional epithelial cultures. Biomaterials. 28:4006–4016.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Carletti E, Motta A and Migliaresi C:
Scaffolds for tissue engineering and 3D cell culture. Methods Mol
Biol. 695:17–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu X, Holzwarth JM and Ma PX:
Functionalized synthetic biodegradable polymer scaffolds for tissue
engineering. Macromol Biosci. 12:911–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Feng S, Duan X, Lo PK, Liu S, Liu X, Chen
H and Wang Q: Expansion of breast cancer stem cells with fibrous
scaffolds. Integr Biol (Camb). 5:768–777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen J, Wang J, Zhang Y, Chen D, Yang C,
Kai C, Wang X, Shi F and Dou J: Observation of ovarian cancer stem
cell behavior and investigation of potential mechanisms of drug
resistance in three-dimensional cell culture. J Biosci Bioeng.
118:214–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kievit FM, Florczyk SJ, Leung MC, Wang K,
Wu JD, Silber JR, Ellenbogen RG, Lee JS and Zhang M: Proliferation
and enrichment of CD133(+) glioblastoma cancer stem cells on 3D
chitosan-alginate scaffolds. Biomaterials. 35:9137–9143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Amann A, Zwierzina M, Gamerith G, Bitsche
M, Huber JM, Vogel GF, Blumer M, Koeck S, Pechriggl EJ, Kelm JM, et
al: Development of an innovative 3D cell culture system to study
tumour-stroma interactions in non-small cell lung cancer cells.
PLoS One. 9:e925112014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hoque MT, Windus LC, Lovitt CJ and Avery
VM: PCaAnalyser: A 2D-image analysis based module for effective
determination of prostate cancer progression in 3D culture. PLoS
One. 8:e798652013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ciurea ME, Georgescu AM, Purcaru SO,
Artene SA, Emami GH, Boldeanu MV, Tache DE and Dricu A: Cancer stem
cells: Biological functions and therapeutically targeting. Int J
Mol Sci. 15:8169–8185. 2014. View Article : Google Scholar : PubMed/NCBI
|