Introduction
Breast cancer is the most frequent form of malignant
cancer in women worldwide (1). While
the primary tumor is often treatable, tumor recurrence remains the
most frequent cause of breast cancer mortality. Thus, the treatment
of metastatic disease is crucial for improving breast cancer
survival (2). Furthermore, designing
subsequent treatment strategies using accurate initial response
data could improve the outcome of advanced breast cancer and reduce
the use of ineffective chemotherapeutic agents (3).
One of the most popular criteria used to evaluate
therapeutic strategies is referred to as Response Evaluation
Criteria in Solid Tumors (RECIST) (4). According to RECIST 1.1, a patient's
therapeutic response can be classified into four conditions:
Complete response (CR), partial response (PR), stable disease (SD)
and progressive disease (PD). In SD patients receiving
chemotherapy, certain patients would develop PD over time, while
other patients would maintain SD or even experience remission.
Previous evidence indicates that there are a number
of metastatic forms of breast cancer that are not adequately
assessed by RECIST (4), including
pleural/pericardial effusion, ascites, the majority of bone
metastases and lesions resected by surgery. In particular, ~50% of
breast cancer patients develop bone metastases (5), and a large number of these patients
would be non-assessable by RECIST. Various alternative methods have
been proposed to assesses the therapeutic response of bone
metastases, including 18F-fluorodeoxyglucose-positron
emission tomography (6) and
bone-specific biochemical markers such as N-terminal telopeptide
(7,8).
However, these indicators are not applicable to other
non-assessable lesions.
Previous studies suggest that carcinoembryonic
antigen (CEA) and carbohydrate antigen 15–3 (CA15-3) are predictive
markers of radiological response in metastatic breast cancer
(9). Thus, these markers may be
useful for monitoring the therapeutic response of metastatic breast
cancer patients. Despite extensive study of CEA and CA15-3, their
utility as breast cancer markers remains unclear (10). The majority of tumor markers are used
for early diagnosis, determining prognosis, monitoring therapeutic
efficacy and follow-up subsequent to therapy (11–16).
However, CEA and CA15-3 are unsuitable for early detection due to
their low expression and lack of sensitivity in breast cancer
(11,17). While CEA and CA15-3 have been used to
assess the follow-up of patients with breast cancer (18), their clinical value has not been
assessed (11).
Although tumor markers alone are insufficient to
evaluate therapeutic response (19),
several studies suggest that tumor marker levels correlate with
treatment response (3,20–23). For
example, Robertson et al (3)
reported that changes in the levels of tumor markers correlated
with patients' therapeutic response, as assessed by imaging methods
(3). Furthermore, reduction in CEA
and CA15-3 levels predicted a positive response to systemic therapy
in metastatic breast cancer patients (23). However, to date, no studies have
assessed the correlation between CEA and CA15-3 levels and
therapeutic response in patients with non-assessable lesions or
SD.
In order to assess the predictive efficacy of CEA
and CA15-3 in metastatic breast cancer, CEA and CA15-3 levels were
compared with radiological response in a group of patients
classified as non-assessable or SD by RECIST 1.1. In addition, it
was analyzed which factors are associated with pre-treatment levels
of CEA and CA15-3, including progression-free survival (PFS). The
present study should clarify the prognostic value of CEA and CA15-3
as tumor markers in metastatic breast cancer.
Patients and methods
Patients
All data were retrospectively collected from 232
female breast cancer patients in the Affiliated Tumor Hospital of
Harbin Medical University (Harbin, China). Patients were included
in the study if they underwent radical mastectomy but experienced
subsequent tumor recurrence or metastasis. Patients were excluded
from the study if their CEA or CA15-3 levels were not measured at
the time of the initial relapse or did not undergo therapeutic
intervention. Patients' age ranged from 25 to 76 years, and all
patients received first-line treatment between July 2001 and
February 2013 along with systemic therapies, including
chemotherapy, trastuzumab, endocrine therapy and bisphosphonate
treatment for bone metastases.
From the 232 enrolled subjects, patients with ≥1
measurable lesion according to RECIST were grouped as assessable
patients, while those with lesions resected by surgery or
non-measurable lesions (pleural/pericardial effusion, ascites and
bone metastases) were grouped as non-assessable patients. At the
first-line therapeutic cycle, 60 individuals classified with SD
were selected to study the predictive value of CEA and CA15-3
levels in evaluating the therapeutic response. Patients classified
as non-assessable by RECIST (76 patients) who had available CEA and
CA15-3 data were selected to study the predictive value of the
levels of these markers in assessing the therapeutic response in
patients with non-assessable lesions.
Determination of tumor markers
Serum CEA concentrations were determined using an
Enzyme Immunoassay kit (Dinabot, Tokyo, Japan), while serum CA15-3
levels were determined using a radioimmunoassay kit (Roche
Diagnostics, Indianapolis, IN, USA). A threshold of 0–5 ng/ml CEA
and 0–25 U/ml CA15-3 was used to determine the ‘normal’ levels of
the respective markers. Levels >5 ng/ml CEA or >25 U/ml
CA15-3 were considered elevated in patients.
Assessment
CEA and CA15-3 levels were determined within 1 week
prior to the initiation of systemic therapy, and were then
evaluated every 3 weeks during the course of therapy.
Non-assessable patients and patients receiving chemotherapy or
trastuzumab therapy underwent radiological examination, which was
performed every 6 weeks during treatment.
To study the association between tumor markers
levels and PFS, all 232 patients were divided into two groups based
on their CEA and CA15-3 levels at the time of relapse (normal and
elevated). Patients were subsequently divided into two groups based
on the relative changes in CEA and CA15-3 levels at the end of the
second therapy cycle: An increased group (an increment of >2
ng/ml CEA or 15 U/ml CA15-3 relative to pre-treatment levels) and a
non-increase group (an increment of <2 ng/ml CEA or 15 U/ml
CA15-3, or any decrease relative to pre-treatment levels).
The therapeutic response in patients with assessable
lesions was classified using RECIST into four categories: CR, PR,
SD and PD. For the 60 individuals classified as SD, the clinical
therapeutic response following the second cycle of therapy was
classified as the final clinical response. Final clinical response
was divided into two categories: PD and disease controlled (DC). DC
was defined as the lack of PD following all chemotherapy cycles.
For patients with non-assessable lesions, PD was defined as the
appearance at ≥1 new lesion and/or progression of the existing
lesions. All the patients participated in follow-up treatment and
testing until May 2013. The median follow-up time of the patients
was 11.78 months. Recurrent disease was confirmed by biopsy or, in
cases of multiple metastases, by radiological examination.
Statistical analysis
All statistical analysis were carried out using SPSS
19.0 (IBM SPSS, Armonk, NY, USA). Statistical analysis of the
differences among patient groups was performed using the t-test and
Mann-Whitney U-test for quantitative results, while the
Kruskal-Wallis test for qualitative results. The clinical response
between PD and DC was analyzed using a cross-tabulation table and
the Fisher's exact test. PFS was calculated using the Kaplan-Meier
method and the log-rank test. P<0.05 was considered to indicate
a statistically significant difference.
Results
The correlation between the clinical characteristics
of all 232 enrolled breast cancer patients and the CEA and CA15-3
levels at the time of relapse was initially analyzed (Table I). In particular, it was observed that
CA15-3 levels were highly correlated with hormone receptor (HR)
status. Significantly higher levels of CA15-3 were detected in
estrogen receptor (ER)-positive, progesterone receptor-positive and
HR-positive groups (P<0.001, P=0.001 and P<0.001,
respectively), whereas CEA did not appear to be correlated with any
HR. While there was no correlation between human epidermal growth
factor receptor-2 (HER-2) status and either CEA or CA15-3 levels,
both markers were negatively correlated with triple-negative breast
cancer (CEA, P=0.021; and CA15-3, P<0.001).
 | Table I.Correlation between patients'
characteristics and initial tumor marker levels at the first
relapse (n=232). |
Table I.
Correlation between patients'
characteristics and initial tumor marker levels at the first
relapse (n=232).
|
|
| CEA levels
(ng/ml) | CA15-3 levels
(U/ml) |
|---|
|
|
|
|
|
|---|
| Characteristic | n | Mean/median | SE | P-value | Mean/median | SE | P-value |
|---|
| Age, years |
|
|
| NSb |
|
| NSa |
|
≤35 | 37 |
9.77 |
4.04 |
| 20.96 |
7.45 |
|
|
>35 | 195 | 18.48 | 58.96 |
| 21.42 |
5.85 |
|
| BMI |
|
|
| NSb |
|
| NSb |
|
<23.9 | 108 | 13.61 |
3.83 |
| 49.14 |
6.43 |
|
|
≥23.9 | 124 | 20.12 |
5.88 |
| 59.12 |
7.66 |
|
| Pathological
type |
|
|
| NSa |
|
| NSa |
|
IDC | 188 |
2.77 | 12.43 |
| 51.84 |
5.37 |
|
|
Others | 44 |
2.87 | 15.90 |
| 65.74 | 13.74 |
|
| Histological
grade |
|
|
| NSb |
|
| NSb |
|
Well/moderate | 84 | 16.50 |
4.24 |
| 49.69 |
7.47 |
|
|
Poor | 20 | 12.97 |
9.56 |
| 50.52 | 23.31 |
|
|
Unknown | 128 |
|
|
|
|
|
|
| Tumor size |
|
|
| NSb |
|
| NSb |
| T1 | 58 | 15.87 |
4.41 |
| 45.12 |
9.88 |
|
|
T2-T4 | 122 | 19.98 |
6.38 |
| 63.47 |
7.56 |
|
|
Unknown | 52 |
|
|
|
|
|
|
| Nodal status |
|
|
| NSa |
|
| NSb |
|
N0-N1 | 127 |
2.71 |
2.36 |
| 52.46 |
6.50 |
|
|
N2-N3 | 91 |
3.00 |
8.52 |
| 61.31 |
9.10 |
|
|
Unknown | 14 |
|
|
|
|
|
|
| Ki-67, % |
|
|
| NSb |
|
| NSb |
|
≤14 | 50 | 21.72 |
5.48 |
| 60.83 | 11.31 |
|
|
>14 | 58 | 13.80 |
5.40 |
| 54.15 | 10.82 |
|
|
Unknown | 124 |
|
|
|
|
|
|
| ER expression |
|
|
| NSb |
|
|
<0.001a |
|
Negative | 66 | 10.35 |
3.23 |
| 15.89 |
6.89 |
|
|
Positive | 133 | 20.78 |
6.00 |
| 27.05 |
7.24 |
|
|
Unknown | 33 |
|
|
|
|
|
|
| PR expression |
|
|
| NSb |
|
| 0.001a |
|
Negative | 75 | 14.50 |
5.00 |
| 16.45 |
6.66 |
|
|
Positive | 124 | 19.03 |
5.95 |
| 27.41 |
7.59 |
|
|
Unknown | 33 |
|
|
|
|
|
|
| HER-2
expression |
|
|
| NSb |
|
| NSb |
|
Negative | 151 | 16.16 |
4.95 |
| 49.55 |
5.59 |
|
|
Positive | 27 | 24.13 | 11.67 |
| 45.56 | 12.02 |
|
|
Unknown | 54 |
|
|
|
|
|
|
| HR expression |
|
|
| NSa |
|
|
<0.001a |
|
Negativec | 56 |
2.29 |
2.51 |
| 15.89 |
7.14 |
|
|
Positived | 143 |
3.00 |
5.67 |
| 27.05 |
6.90 |
|
|
Unknown | 33 |
|
|
|
|
|
|
| Triple
negative |
|
|
| 0.021a |
|
|
<0.001a |
|
Yes | 36 |
2.00 |
1.10 |
| 11.93 |
5.43 |
|
| No | 158 |
2.92 |
5.19 |
| 25.79 |
6.32 |
|
|
Unknown | 38 |
|
|
|
|
|
|
| Metastatic sites,
n |
|
|
| NSb |
|
|
<0.001a |
| 1 | 134 |
2.77 |
2.55 |
| 18.18 |
5.24 |
|
| ≥2 | 98 |
2.82 |
7.80 |
| 33.08 |
9.36 |
|
| Sites of
metastases |
|
|
|
|
|
|
|
|
Bone |
|
|
|
<0.001a |
|
|
<0.001a |
|
Yes | 112 |
4.35 | 7.22 |
| 39.14 |
9.07 |
|
|
No | 120 |
2.21 | 1.26 |
| 17.17 |
3.48 |
|
|
Lung |
|
|
| NSa |
|
| NSb |
|
Yes | 82 |
9.24 | 9.26 |
| 65.58 |
9.16 |
|
|
No | 150 |
2.73 | 2.34 |
| 48.40 |
6.00 |
|
|
Liver |
|
|
| NSb |
|
| 0.009a |
|
Yes | 33 | 17.94 | 5.01 |
| 37.23 | 16.85 |
|
|
No | 199 | 16.95 | 4.13 |
| 20.15 |
5.17 |
|
|
Brain |
|
|
| NSb |
|
| NSb |
|
Yes | 7 |
8.48 | 5.06 |
| 36.27 |
7.47 |
|
|
No | 225 | 17.36 | 3.72 |
| 55.04 |
5.22 |
|
| Local
site |
|
|
| NSa |
|
| 0.039a |
|
Yes | 54 |
2.29 | 2.38 |
| 16.58 |
7.58 |
|
|
No | 178 |
3.04 | 4.64 |
| 23.87 |
6.16 |
|
|
Regional lymph node |
|
|
| 0.002a |
|
| 0.039a |
|
Yes | 62 |
1.99 | 1.42 |
| 18.54 |
8.56 |
|
|
No | 170 |
3.11 | 4.86 |
| 25.07 |
6.13 |
|
|
Non-regional lymph node |
|
|
| NSb |
|
| NSb |
|
Yes | 35 |
7.70 | 2.63 |
| 50.01 | 13.11 |
|
|
No | 197 | 18.76 | 4.22 |
| 55.27 |
5.51 |
|
In addition, it was noticed that the serum levels of
CEA and CA15-3 were highly correlated with the location and number
of metastatic sites in breast cancer patients. In particular,
patients with multiple metastases had significantly higher CA15-3
levels than patients with a single metastatic site (P<0.001).
However, CEA concentration did not appear to be correlated with the
number of metastases (P>0.05). Similarly, increased levels of
CA15-3, but not of CEA, were observed in patients with liver
metastases (P=0.009).
The serum levels of CEA and CA15-3 were both
elevated in patients with bone metastases relative to those without
bone metastases (CEA, P<0.001; and CA15-3, P<0.001). By
contrast, patients with localized invasion had reduced CA15-3
levels (P=0.039), while patients with regional lymph node
metastases had reduced levels of both CEA (P=0.002) and CA15-3
(P=0.039) compared with patients without lymph node metastases.
Neither CEA nor CA15-3 was correlated with lung or brain metastasis
in breast cancer patients (all P>0.05).
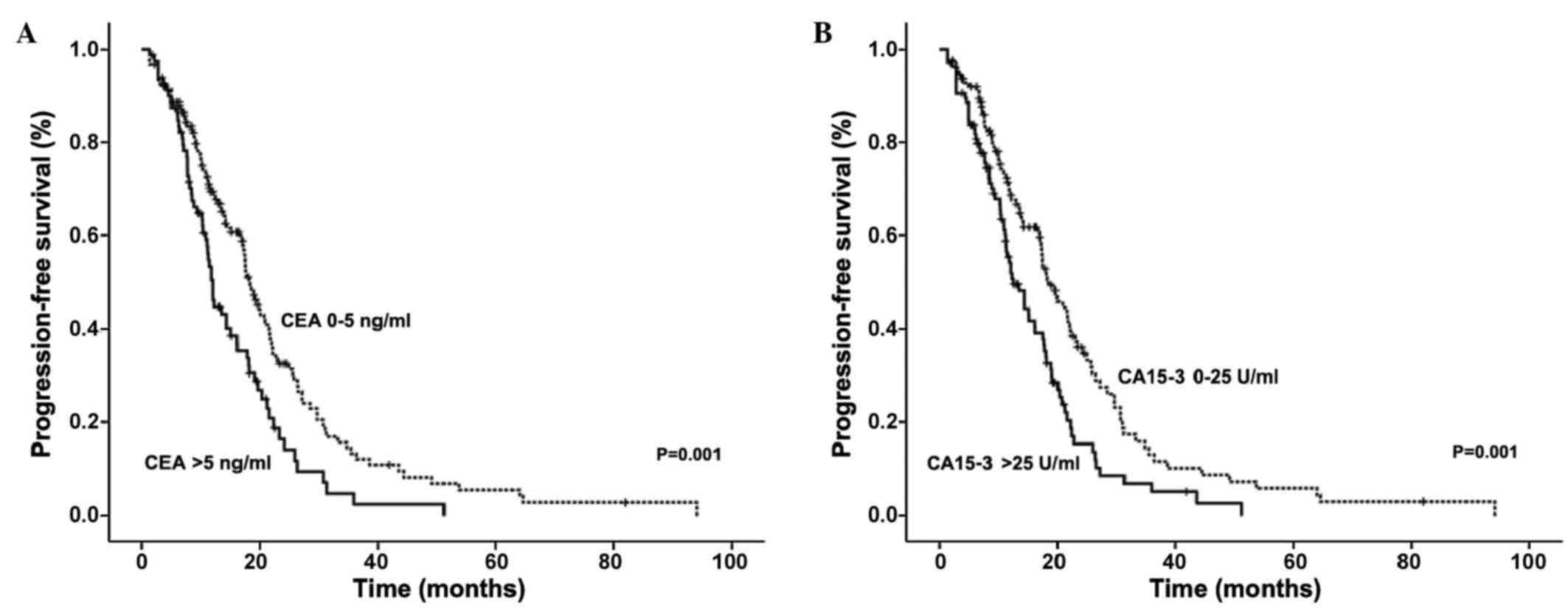
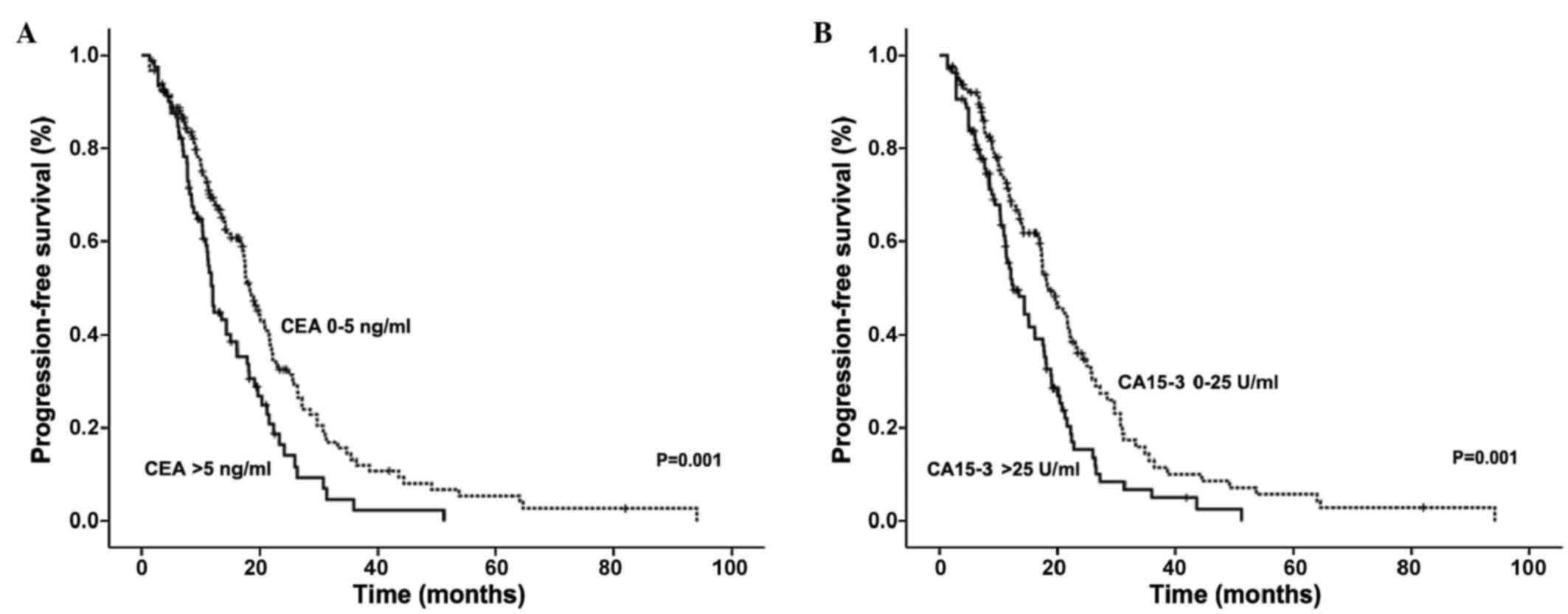
It was observed that the serum levels of CEA and
CA15-3 correlated with shorter PFS in advanced breast cancer
patients (Fig. 1). Patients with
elevated CEA had a median PFS time of 12.10 months, compared with a
PFS time of 18.33 months in patients with normal levels (P=0.001).
Similarly, patients with elevated CA15-3 levels had a median PFS
time of 12.50 months compared with 18.53 months in patients with
normal CA15-3 levels (P=0.001).
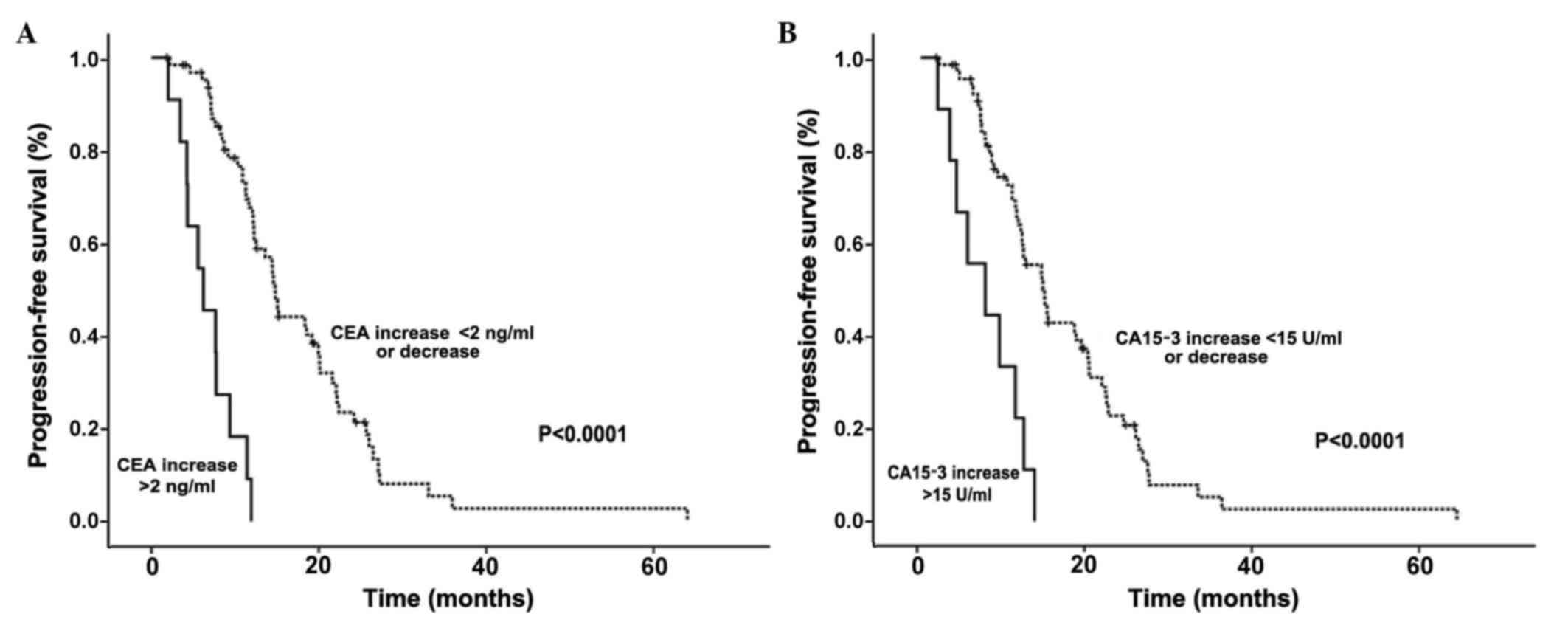
CEA and CA15-3 levels following the second therapy
cycle were also predictors of PFS in breast cancer patients with
non-assessable lesions. Patients with increased CEA levels (an
increment of >2 ng/ml relative to pre-treatment levels) had
significantly shorter PFS than patients with no increased CEA
levels subsequent to therapy (6.72 vs. 17.74 months, respectively;
P<0.001). Similarly, patients with increased CA15-3 levels (an
increment of >15 U/ml relative to pre-treatment levels)
following therapy had shorter PFS than those with no increased
CA15-3 levels (7.71 vs. 17.26 months, respectively; P<0.0001;
Fig. 2).
To assess the predictive value of CEA and CA15-3 in
patients with SD, the correlations between the serum levels of
these markers, PFS and final clinical outcome were analyzed in 60
patients classified as SD by RECIST subsequent to the second
treatment cycle. An increase in CEA or CA15-3 levels correlated
with a significantly shorter PFS (Fig.
3). Furthermore, this increase in CEA or CA15-3 levels was also
negatively correlated with achievement of a CD state (Table II). These data indicate that, even in
patients with SD, elevated CEA and CA15-3 levels correlate with a
poor prognosis. Taken together, these data suggest that CEA and
CA15-3 are predictive of PFS at both the early stages of relapse
and throughout the treatment, particularly in non-assessable
patients and in those with SD.
 | Table II.Correlation analysis of CEA and
CA15-3 levels and final clinical response in patients classified
with stable disease following the second chemotherapy cycle. |
Table II.
Correlation analysis of CEA and
CA15-3 levels and final clinical response in patients classified
with stable disease following the second chemotherapy cycle.
| Changes in
markers | PDa, n | DCb, n |
P-valuec |
|---|
| CEA (ng/ml) |
|
| 0.011 |
|
Increase of ≤2 or
decrease | 6 | 47 |
|
|
Increase of >2 | 4 | 3 |
|
| CA15-3 (U/ml) |
|
| 0.034 |
|
Increase of ≤15 or
decrease | 6 | 45 |
|
|
Increase of >15 | 4 | 5 |
|
Discussion
Predicting a patient's therapeutic response is
critical to avoid side effects from unnecessary and ineffective
drugs. Few studies have analyzed predictive factors for therapeutic
response in advanced breast cancer patients classified as SD by
RECIST or in patients with lesions that are not-assessable by
RECIST. A major reason for this is that the therapeutic response of
such non-assessable lesions (e.g. ascites) cannot be adequately
measured by radiological methods (4).
In such cases, PFS is the only criteria to assess the therapeutic
response in patients, making difficult to predict the patient's
response during treatment.
Previously established models used to predict cancer
patients' response to chemotherapy are complex and not applicable
to patients with surgically resected lesions (24). Furthermore, due to the low sensitivity
of the currently available imaging techniques, it is difficult to
detect small changes in the tumor burden (25), particularly in patients with SD.
Therefore, it is necessary to develop alternative methods to
predict therapeutic results in patients with SD or non-assessable
lesions.
CA15-3 (also known as mucin 1) is overexpressed in
>90% of human breast cancers and in their subsequent metastases
(26). CA15-3 promotes tumor invasion
and metastasis through activation of the mitogen-activated protein
kinase signaling pathway (26) and
downregulation of E-cadherin (27).
Thus, elevated levels of CA15-3 would predict a poor prognosis with
an increased risk of metastasis (28). Consistent with this, CA15-3 levels
were observed to negatively correlate with PFS (29). Similarly, CEA has also been observed
to correlate with treatment response (21,23,25,30–32).
These reports support the results of our study, suggesting that CEA
and CA15-3 may be useful markers for predicting patient prognosis
and therapeutic response.
Our data suggest that CEA and CA15-3 can predict PFS
and final clinical outcome in patients with either SD or
non-assessable lesions. An increase of >2 ng/ml CEA or >15
U/ml CA15-3 following the second therapeutic cycle predicted a
shorter PFS. Furthermore, elevation of CEA and CA15-3 following the
second cycle of chemotherapy correlated with a poor final clinical
response in SD patients.
To date, few studies have analyzed the predictive
potential of CEA and CA15-3 in advanced breast cancer patients who
are not assessable by RECIST and in those with SD. These patient
populations require an alternative determination of therapeutic
response, as current imaging-based methods are not capable of
accurately evaluating the therapeutic response of metastatic
lesions (5). Monitoring the serum
concentrations of CEA and CA15-3 provides a simple and
cost-effective method to predict the therapeutic response of these
patients, thus improving the design of therapeutic strategies and
minimizing unnecessary side effects due to ineffective
treatments.
In our study, serum CEA and CA15-3 concentrations
were predictive of HR status, number of metastases and location of
metastatic lesions. Specifically, CA15-3 was strongly associated
with liver metastasis and the presence of multiple metastatic
lesions, while both CEA and CA15-3 were associated with bone
metastasis. By contrast, lower levels of CEA and CA15-3 were
identified in patients with triple-negative tumors and with
regional lymph node recurrence. In addition, lower CA15-3 levels
were identified in patients with localized invasion. These results
are consistent with previous studies that link CEA and CA15-3
levels with breast cancer prognosis (33,34). Taken
together, these data indicate that elevated levels of CEA and
CA15-3 may be predictive of increased tumor burden in breast cancer
patients.
Elevated serum levels of CEA and CA15-3 prior to
therapy predicted shorter PFS in our patient groups. While this
finding is consistent with several reports (33–35), one
study suggested that elevated CA15-3 concentrations correlated with
longer overall breast cancer patient survival (36). This positive correlation between
CA15-3 and survival could be explained by the association between
CA15-3 levels and ER status observed in our study, as ER is
commonly used to predict a better prognosis (37,38). One
potential explanation for this discrepancy may be the relatively
low ratio of HR-positive patients in the previous study compared
with our study. It is possible that the predictive value of CEA and
CA15-3 may be dependent on the HR status of breast cancer patients.
Further research is required to better understand the role of CEA
and CA15-3 in distinct breast cancer subtypes.
To conclude, our study demonstrates the utility of
CEA and CA15-3 as markers predicting the therapeutic response of
advanced breast cancer patients. These markers may be particularly
useful in patients with non-assessable lesions or in those with SD,
as defined by RECIST. Additionally, our data indicate that
determining the serum concentrations of CEA and CA15-3 provides a
simple yet robust method to predict a patient's therapeutic
response. However, since our results are based on a retrospective
analysis, other tumor markers such as HER-2, epidermal growth
factor receptor or tissue polypeptide antigen (25,39–41) were
not included in our analysis. Analyzing these markers in addition
to CEA and CA15-3 could potentially provide even more accurate
predictions of therapeutic response than those reported in the
present study. In conclusion, the determination of CEA and CA15-3
levels can provide a powerful tool to complement RECIST in
assessing and predicting the therapeutic response of advanced
breast cancer patients.
Acknowledgements
Financial support for the present study was provided
by the Bureau of Technology and Science of Harbin (Harbin, China;
grant number 2009RFXXS017 awarded to L.C.) and the Natural Science
Foundation of Heilongjiang Province (Harbin, China; grant number
LC2012C08 awarded to Q.M.).
Glossary
Abbreviations
Abbreviations:
|
RECIST
|
Response Evaluation Criteria In Solid
Tumors
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
CEA
|
carcinoembryonic antigen
|
|
CA15-3
|
carbohydrate antigen 15-3
|
|
PFS
|
progression free-survival
|
|
RIA
|
radioimmunoassay
|
|
DC
|
disease controlled
|
|
ER
|
estrogen receptor
|
|
HR
|
hormone receptor
|
|
HER-2
|
human epidermal growth factor
receptor-2
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esteva FJ, Valero V, Pusztai L,
Boehnke-Michaud L, Buzdar AU and Hortobagyi GN: Chemotherapy of
metastatic breast cancer: What to expect in 2001 and beyond.
Oncologist. 6:133–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robertson JF, Jaeger W, Syzmendera JJ,
Selby C, Coleman R, Howell A, Winstanley J, Jonssen PE, Bombardieri
E, Sainsbury JR, et al: European Group for Serum Tumour Markers in
Breast Cancer: The objective measurement of remission and
progression in metastatic breast cancer by use of serum tumour
markers. Eur J Cancer. 35:47–53. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe H, Okada M, Kaji Y, Satouchi M,
Sato Y, Yamabe Y, Onaya H, Endo M, Sone M and Arai Y: New response
evaluation criteria in solid tumours-revised RECIST guideline
(version 1.1). Gan To Kagaku Ryoho. 36:2495–2501. 2009.(In
Japanese). PubMed/NCBI
|
|
5
|
Clamp A, Danson S, Nguyen H, Cole D and
Clemons M: Assessment of therapeutic response in patients with
metastatic bone disease. Lancet Oncol. 5:607–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stafford SE, Gralow JR, Schubert EK, Rinn
KJ, Dunnwald LK, Livingston RB and Mankoff DA: Use of serial FDG
PET to measure the response of bone-dominant breast cancer to
therapy. Acad Radiol. 9:913–921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costa L, Demers LM, Gouveia-Oliveira A,
Schaller J, Costa EB, de Moura MC and Lipton A: Prospective
evaluation of the peptide-bound collagen type I cross-links
N-telopeptide and C-telopeptide in predicting bone metastases
status. J Clin Oncol. 20:850–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipton A, Demers L, Curley E, Chinchilli
V, Gaydos L, Hortobagyi G, Theriault R, Clemens D, Costa L, Seaman
J, et al: Markers of bone resorption in patients treated with
pamidronate. Eur J Cancer. 34:2021–2026. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massacesi C, Rocchi MB, Marcucci F, Pilone
A, Galeazzi M and Bonsignori M: Serum tumor markers may precede
instrumental response to chemotherapy in patients with metastatic
cancer. Int J Biol Markers. 18:295–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molina R, Auge JM, Farrus B, Zanón G,
Pahisa J, Muñoz M, Torne A, Filella X, Escudero JM, Fernandez P, et
al: Prospective evaluation of carcinoembryonic antigen (CEA) and
carbohydrate antigen 15.3 (CA 15.3) in patients with primary
locoregional breast cancer. Clin Chem. 56:1148–1157. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duffy MJ: Serum tumor markers in breast
cancer: Are they of clinical value? Clin Chem. 52:345–351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H and Luo RC: Diagnostic value of
combined detection of TPS, CA153 and CEA in breast cancer. Di Yi
Jun Yi Da Xue Xue Bao. 25(1293–1294): 12982005.(In Chinese).
|
|
13
|
Ebeling FG, Stieber P, Untch M, Nagel D,
Konecny GE, Schmitt UM, Fateh-Moghadam A and Seidel D: Serum CEA
and CA 15-3 as prognostic factors in primary breast cancer. Br J
Cancer. 86:1217–1222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marić P, Ozretić P, Levanat S, Oresković
S, Antunac K and Beketić-Oreskovic L: Tumor markers in breast
cancer - evaluation of their clinical usefulness. Coll Antropol.
35:241–247. 2011.
|
|
15
|
Agrawal AK, Jelen M, Rudnicki J,
Grzebieniak Z, Zyśko D, Kielan W, Słonina J and Marek G: The
importance of preoperative elevated serum levels of CEA and CA15-3
in patients with breast cancer in predicting its histological type.
Folia Histochem Cytobiol. 48:26–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parker C: Active surveillance: Towards a
new paradigm in the management of early prostate cancer. Lancet
Oncol. 5:101–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Gioia D, Heinemann V, Nagel D, Untch M,
Kahlert S, Bauerfeind I, Koehnke T and Stieber P: Kinetics of CEA
and CA15-3 correlate with treatment response in patients undergoing
chemotherapy for metastatic breast cancer (MBC). Tumour Biol.
32:777–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariani L, Miceli R, Michilin S and Gion
M: Serial determination of CEA and CA 15.3 in breast cancer
follow-up: An assessment of their diagnostic accuracy for the
detection of tumour recurrences. Biomarkers. 14:130–136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr: American
Society of Clinical Oncology: American Society of Clinical Oncology
2007 update of recommendations for the use of tumor markers in
breast cancer. J Clin Oncol. 25:5287–5312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams MR, Turkes A, Pearson D,
Griffiths K and Blamey RW: An objective biochemical assessment of
therapeutic response in metastatic breast cancer: A study with
external review of clinical data. Br J Cancer. 61:126–132. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robertson JF, Pearson D, Price MR, Selby
C, Blamey RW and Howell A: Objective measurement of therapeutic
response in breast cancer using tumour markers. Br J Cancer.
64:757–763. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixon AR, Jackson L, Chan SY, Badley RA
and Blamey RW: Continuous chemotherapy in responsive metastatic
breast cancer: A role for tumour markers? Br J Cancer. 68:181–185.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurebayashi J, Nishimura R, Tanaka K,
Kohno N, Kurosumi M, Moriya T, Ogawa Y and Taguchi T: Significance
of serum tumor markers in monitoring advanced breast cancer
patients treated with systemic therapy: A prospective study. Breast
Cancer. 11:389–395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashimoto K, Yonemori K, Katsumata N,
Shimizu C, Hirakawa A, Hirata T, Kouno T, Tamura K, Ando M and
Fujiwara Y: Prediction of progressive disease using tumor markers
in metastatic breast cancer patients without target lesions in
first-line chemotherapy. Ann Oncol. 21:2195–2200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sölétormos G, Nielsen D, Schiøler V,
Mouridsen H and Dombernowsky P: Monitoring different stages of
breast cancer using tumour markers CA 15-3, CEA and TPA. Eur J
Cancer. 40:481–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schroeder JA, Thompson MC, Gardner MM and
Gendler SJ: Transgenic MUC1 interacts with epidermal growth factor
receptor and correlates with mitogen-activated protein kinase
activation in the mouse mammary gland. J Biol Chem.
276:13057–13064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka M, Kitajima Y, Sato S and Miyazaki
K: Combined evaluation of mucin antigen and E-cadherin expression
may help select patients with gastric cancer suitable for minimally
invasive therapy. Br J Surg. 90:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahn JJ, Dabbagh L, Pasdar M and Hugh JC:
The importance of MUC1 cellular localization in patients with
breast carcinoma: An immunohistologic study of 71 patients and
review of the literature. Cancer. 91:1973–1982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng JP, Yan Y, Wang XY, Lu YL, Yuan YH,
Jia J and Ren J: MUC1-positive circulating tumor cells and MUC1
protein predict chemotherapeutic efficacy in the treatment of
metastatic breast cancer. Chin J Cancer. 30:54–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sölétormos G, Nielsen D, Schiøler V,
Skovsgaard T and Dombernowsky P: Tumor markers cancer antigen 15.3,
carcinoembryonic antigen, and tissue polypeptide antigen for
monitoring metastatic breast cancer during first-line chemotherapy
and follow-up. Clin Chem. 42:564–575. 1996.PubMed/NCBI
|
|
31
|
Cheung KL, Graves CR and Robertson JF:
Tumour marker measurements in the diagnosis and monitoring of
breast cancer. Cancer Treat Rev. 26:91–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tondini C, Hayes DF, Gelman R, Henderson
IC and Kufe DW: Comparison of CA15-3 and carcinoembryonic antigen
in monitoring the clinical course of patients with metastatic
breast cancer. Cancer Res. 48:4107–4112. 1988.PubMed/NCBI
|
|
33
|
Lee JS, Park S, Park JM, Cho JH, Kim SI
and Park BW: Elevated levels of serum tumor markers CA 15-3 and CEA
are prognostic factors for diagnosis of metastatic breast cancers.
Breast Cancer Res Treat. 141:477–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yerushalmi R, Tyldesley S, Kennecke H,
Speers C, Woods R, Knight B and Gelmon KA: Tumor markers in
metastatic breast cancer subtypes: Frequency of elevation and
correlation with outcome. Ann Oncol. 23:338–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bidard FC, Hajage D, Bachelot T, Delaloge
S, Brain E, Campone M, Cottu P, Beuzeboc P, Rolland E, Mathiot C,
et al: Assessment of circulating tumor cells and serum markers for
progression-free survival prediction in metastatic breast cancer: A
prospective observational study. Breast Cancer Res. 14:R292012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishimura R, Nagao K, Miyayama H, Matsuda
M, Baba K, Matsuoka Y and Yamashita H: Elevated serum CA15-3 levels
correlate with positive estrogen receptor and initial favorable
outcome in patients who died from recurrent breast cancer. Breast
Cancer. 10:220–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:pp. 10869–10874. 2001, View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakshatri H, Srour EF and Badve S: Breast
cancer stem cells and intrinsic subtypes: Controversies rage on.
Curr Stem Cell Res Ther. 4:50–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Molina R, Augé JM, Escudero JM, Filella X,
Zanon G, Pahisa J, Farrus B, Muñoz M and Velasco M: Evaluation of
tumor markers (HER-2/neu oncoprotein, CEA, and CA 15.3) in patients
with locoregional breast cancer: Prognostic value. Tumour Biol.
31:171–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pedersen AC, Sorensen PD, Jacobsen EH,
Madsen JS and Brandslund I: Sensitivity of CA 15-3, CEA and serum
HER2 in the early detection of recurrence of breast cancer. Clin
Chem Lab Med. 51:1511–1519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bao H, Yu D, Wang J, Qiu T, Yang J and
Wang L: Predictive value of serum anti-p53 antibodies,
carcino-embryonic antigen, carbohydrate antigen 15-3, estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor-2 in taxane-based and anthracycline-based neoadjuvant
chemotherapy in locally advanced breast cancer patients. Anticancer
Drugs. 19:317–323. 2008. View Article : Google Scholar : PubMed/NCBI
|